Abstract
OBJECTIVE
To characterize the effect of corticosteroid exposure on clinical outcomes in children hospitalized with new-onset Henoch-Schönlein purpura (HSP).
PATIENTS AND METHODS
We conducted a retrospective cohort study of children discharged with an International Classification of Diseases, Clinical Modification code of HSP between 2000 and 2007 by using inpatient administrative data from 36 tertiary care children’s hospitals. We used stratified Cox proportional hazards regression models to estimate the relative effect of time-varying corticosteroid exposure on the risks of clinical outcomes that occur during hospitalization for acute HSP.
RESULTS
During the 8-year study period, there were 1895 hospitalizations for new-onset HSP. After multivariable regression modeling adjustment, early corticosteroid exposure significantly reduced the hazard ratios for abdominal surgery (0.39 [95% confidence interval (CI): 0.17– 0.91]), endoscopy (0.27 [95% CI: 0.13– 0.55]), and abdominal imaging (0.50 [95% CI: 0.29 – 0.88]) during hospitalization.
CONCLUSIONS
In the hospital setting, early corticosteroid exposure was associated with benefits for several clinically relevant HSP outcomes, specifically those related to the gastrointestinal manifestations of the disease.
Keywords: cohort, corticosteroids, adolescents, and epidemiology
Henoch-Schönlein purpura (HSP) is the most common vasculitis of childhood; it affects 8 to 20 per 100 000 children each year and accounts for half of all childhood vasculitides in the United States.1,2 HSP is often regarded as a benign disease; however, a subset of children requires hospitalization for acute manifestations including nephritis, gastrointestinal hemorrhage, severe musculoskeletal pain, abdominal colic, and intussusception.
Despite being the most common pediatric vasculitis, no consensus exists regarding the treatment of acute HSP. Because HSP vascular injury and necrosis are thought to result from leukocyte infiltration and immunoglobulin A deposition, and because corticosteroids inhibit inflammation, treatment with corticosteroids has long been postulated to be beneficial.3–7 Yet, although the first anecdotal reports of corticosteroid use for HSP were published in the 1950s, uncertainty remains more than half a century later about the role of corticosteroids for this common pediatric vasculitis.3,5,8 The lack of treatment consensus has resulted in significant variation in the approach to children hospitalized with HSP.9 Results of a recent study indicated substantial variation in the use of medications, including corticosteroids, for children admitted with acute HSP to 36 different children’s hospitals and that this variation was attributable not to the patient case mix but instead represented an important characteristic or proclivity of the hospitals themselves.9
For this study we used a retrospective cohort study design to analyze the large Pediatric Health Information System (PHIS) database, which contains clinical and daily pharmacy data regarding all children admitted to 36 US children’s hospitals, to evaluate the impact of corticosteroids in children who likely had similar clinical presentations but received different treatments primarily on the basis of the hospital to which they were admitted. The combination in this data set of a large sample size and hospital-based treatment variation enabled this design to estimate the effect of inpatient corticosteroid exposure on subsequent inpatient complications of acute HSP.
PATIENTS AND METHODS
Human Subjects Protections
The protocol for this study was approved and reviewed by the University of Pennsylvania and the Children’s Hospital of Philadelphia Committee for the Protection of Human Subjects.
Study Design
For this retrospective cohort study we used the PHIS database, an administrative database developed by the Child Health Corporation of America, to compare the outcomes of corticosteroid-exposed and -unexposed subjects with HSP with adjustment for prespecified variables and clustering within hospitals. The cohort consisted of patients with an International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) discharge diagnosis code of 287.0, which indicates HSP.
Data Source
The PHIS database is an administrative database that contains comprehensive inpatient data from 36 freestanding, noncompeting children’s hospitals in the United States. There are 2 types of data contained in the PHIS: level 1 and 2 data. Level 1 data contain encrypted patient identifiers, demographics, dates of admission and discharge, and ICD-9-CM diagnosis and procedure codes. Level 2 data contain information about the patient encounter including financial and utilization data, including pharmacy, supply, laboratory, imaging, and clinical services. Data are deidentified and subjected to rigorous reliability and validity checks before acceptance into the database. A total of 36 hospitals and 232 hospital-years of data were analyzed in this study.
Subjects
The source population for this study was subjects younger than 18 years admitted to a Child Health Corporation of America–participating hospital between January 1, 2000, and December 31, 2007 (N = 3 275 947). The cohort consisted of subjects with an ICD-9-CM discharge diagnosis that indicated HSP (code 287.0). To ensure that the cohort represented hospitalizations for new-onset HSP, subjects with an admission or discharge diagnosis of HSP in the 6 months before the study period were excluded. Subjects were excluded if they had a rheumatic discharge diagnosis (ie, Wegener granulomatosis, systemic lupus erythematosus, juvenile dermatomyositis, or polyarteritis nodosa) that called into question the validity of their HSP diagnosis (n = 16). Subjects who were missing an admission diagnosis were also excluded (n = 239). Subjects were followed for 30 days after their initial hospitalization for HSP. After inclusion and exclusion criteria were satisfied, 1895 hospitalizations for new-onset HSP remained for analysis.
Demographics
Age, gender, race, and Medicaid status were available for all subjects and included in the final analysis model. Race was coded as a categorical variable (white, black, Asian or American Indian, other, or missing).
Severity of Illness During the Early Hospitalization Period
To assess illness severity at the time of hospital admission, we reasoned that the level of patients’ resource utilization during the first days of hospitalization would correspond to the severity of their illness during that time period. Therefore, we tallied for each subject the charges on hospital days 1 and 2 and normalized them for each of the hospitals (to account for differences in resource expenses and utilization across hospitals). The resulting z scores were used as a measure of resource utilization during the early hospital period and, thus, a proxy for illness severity at admission. We used this approach as opposed to the all-payer refined– diagnosis-related group (APR-DRG) hospitalization severity score as a measure of illness severity, because the APR-DRG score is determined at the time of discharge by computer algorithms that account for the course of illness for the entire hospitalization, including final diagnoses, medications, and procedures.10 Consequently, the APR-DRG score is not an appropriate measure of illness severity at the time of admission.
Admission and Discharge Diagnoses of HSP
Only 1 admission diagnosis, the primary admission diagnosis, is coded for each hospitalization in the PHIS database. However, up to 21 discharge diagnoses may be coded. All subjects in the cohort had a discharge diagnosis of HSP, but not all subjects were diagnosed with HSP at the time of admission. We report results only for subjects with complete data, including an admission diagnosis.
Discharge Month and Year
Because HSP is known to have seasonal variation, discharge month was included in the analysis model. In addition, discharge year was included in the final model.
Drug Exposure(s)
Corticosteroid, antihypertensive, opioid, and nonsteroidal anti-inflammatory drug (NSAID) use was determined by using pharmacy billing data (see Appendix 1 for a list of included generic drugs). Corticosteroid exposure was characterized by day of initial dose and duration of treatment. For day of initial dose, children were coded into 1 of the following categories: (1) first dose on day 1 or 2 of hospitalization; (2) first dose on day 3 or 4 of hospitalization; or (3) first dose beyond day 4 or no receipt of drug during hospitalization. Day of corticosteroid initiation was limited to the first 4 days of hospitalization in an effort to reduce confounding by indication whereby corticosteroids were started as a reaction to worsening clinical course. Corticosteroid duration was defined as the number of consecutive days that corticosteroids were administered after the initial dose.
Measured Outcomes
The primary outcome of this study was abdominal surgery. Secondary outcomes included endoscopy, use of parenteral nutrition, abdominal imaging, initiation of antihypertensive agents, opioids, and NSAIDs, and readmission. For each outcome, the model was conditioned on whether the outcome occurred before initial corticosteroid exposure. The drugs and procedures used to define the outcomes are listed in Appendices 1 and 2.
Data Analysis
Data from 36 hospitals were included in the analysis. Subject demographic variables were examined by using medians with ranges or percentages. We used Cox proportional hazards regression models with prespecified covariates (age, gender, race, Medicaid status, admission diagnosis of HSP, discharge month and year, and exposure to opioids, NSAIDs, or antihypertensive drugs on hospital day 1) and stratified according to the admitting hospital to estimate the relative effect of time-varying corticosteroid exposure on the risks of clinical outcomes. We used the Kaplan-Meier estimate of the failure function to calculate the cumulative incidence of outcomes among corticosteroid-exposure groups. All analyses were performed by using Stata 11 (Stata Corp, College Station, TX).
RESULTS
Subjects
During the 8-year study period, there were 1895 hospitalizations for new-onset HSP. The median age for the cohort was 6 years (interquartile range: 4 – 8), and 90% of the subjects were between the ages of 2 and 15 years. The median length of stay was 3 days (interquartile range: 2–5). Demographic characteristics and admission and discharge diagnoses are listed in Table 1. The distributions of age, gender, and race are consistent with results from observations in previously published studies of HSP in children.11–14
TABLE 1.
Subject Demographics and HSP Characteristics
| Index HSP Admissions (N = 1895) | |
|---|---|
| Demographic | |
| Age, median (interquartile range), y | 6 (4–8) |
| Male, n (%) | 1119 (59) |
| Race, n (%) | |
| White | 1481 (78) |
| Black | 139 (7) |
| Asian | 62 (3) |
| American Indian | 12 (1) |
| Other/Unknown | 201 (11) |
| Medicaid, n (%) | 611 (32) |
| Length of stay, median (interquartile range), d | 3 (2–5) |
| Primary admission ICD-9-CM code, n (%)a | |
| HSP | 727 (38) |
| Abdominal pain/vomiting/diarrhea | 352 (19) |
| Gastrointestinal bleeding | 58 (3) |
| Arthritis or arthralgias | 41 (2) |
| Infection | 40 (2) |
| Hematuria or proteinuria | 39 (2) |
| Nephrotic syndrome/nephritis | 37 (2) |
| Intussusception | 26 (1) |
| Purpura, not otherwise specified | 22 (1) |
| Acute renal failure | 8 (<1) |
| Hypertension | 9 (<1) |
| Other | 536 (28) |
| Discharge ICD-9-CM code, n (%)b | |
| HSP | 1895 (100) |
| Abdominal pain | 395 (21) |
| Nephrotic syndrome/nephritis | 160 (8) |
| Gastrointestinal bleeding | 148 (8) |
| Hypertension | 135 (7) |
| Hematuria or proteinuria | 156 (8) |
| Intussusception | 66 (4) |
| Arthritis or arthralgias | 81 (4) |
| Acute renal failure | 34 (2) |
Mutually exclusive (only 1 admission diagnosis is assigned per subject).
Not mutually exclusive.
Corticosteroid Use
Corticosteroid therapy (oral or intravenous) was initiated during the first 2 days of hospitalization in 42% (n = 801, early period) of the subjects and on days 3 or 4 of hospitalization in 8% (n = 158, middle period) of the subjects. Seven percent (n = 140) received an initial dose of corticosteroids beyond day 4 of hospitalization (late period). Of those who received corticosteroids at any point during hospitalization, 98% (n = 1079) received an intermediate-acting corticosteroid (prednisone, prednisolone, or methylprednisolone).
We evaluated whether, during the first 48 hours of hospitalization, the severity of illness during this early period was related to the propensity to use early-period corticosteroids. We used patients’ hospital charges during the first 2 days as a measure of their early-period resource utilization, which can be regarded as a proxy measure for early-period severity of illness. As early-period resource utilization increased, the likelihood of having received early-period corticosteroids also increased, a finding that would be consistent with a confounding by indication bias whereby patients who are more severely ill are more likely to receive a particular treatment (Fig 1). This type of bias would typically result in the treatment seeming to be associated with poorer outcomes.15
FIGURE 1.
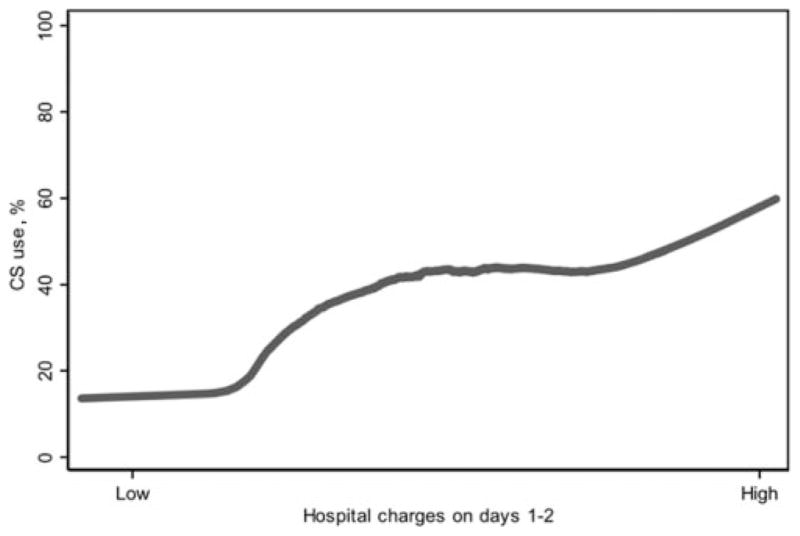
Corticosteroid use and early-period resource utilization. Corticosteroid use was defined as initial exposure to corticosteroids on day 1 or 2 of hospitalization. Hospital charges on days 1 to 2 are used as a proxy for admission severity. Hospital charges were log-transformed and then normalized for admitting hospital. CS indicates corticosteroids.
Reduced Hazard Ratios of Needing Abdominal Surgery
The most common abdominal surgery in this cohort was intraabdominal small bowel manipulation, followed by partial small bowel resection, laparoscopy, appendectomy, and reduction of intussusception (see Appendix 2 for a list of included procedures). When using a Cox proportional hazards regression model, the hazard ratio (HR) for needing abdominal surgery was significantly reduced (0.39 [95% confidence interval (CI): 0.17–0.91]) among those with exposure to early-period corticosteroids (receipt of corticosteroid on hospital days 1 or 2) compared with those with no corticosteroid exposure (Table 2).
TABLE 2.
Outcomes Associated With Corticosteroid Exposure According to Day of Corticosteroid Initiation
| Outcome During Hospitalization
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Abdominal Surgery | Endoscopy | Parenteral Nutrition | Abdominal Imaging | Initiation of NSAIDs | Initiation of Opioid Analgesics | Initiation of Antihypertensive Agents | Readmission | |
| Early CS, HR (95% CI) | 0.39 (0.17–0.91) | 0.27 (0.13–0.55) | 0.58 (0.28–1.21) | 0.50 (0.29–0.88) | 0.42 (0.34–0.52) | 0.68 (0.56–0.83) | 0.89 (0.64–1.25) | 1.19 (0.88–1.60) |
| Middle CS, HR (95% CI) | 0.41 (0.11–1.53) | 0.44 (0.17–1.10) | 0.79 (0.32–1.99) | 0.43 (0.17–1.08) | 0.24 (0.15–0.38) | 0.58 (0.40–0.84) | 0.79 (0.45–1.39) | 0.78 (0.45–1.35) |
The referent group was corticosteroids initiated after day 4 or no corticosteroid received at any time. Early CS indicates corticosteroid initiation on hospital day 1 or 2; middle CS, corticosteroid initiation on hospital day 3 or 4.
HRs for Secondary Clinical Outcomes
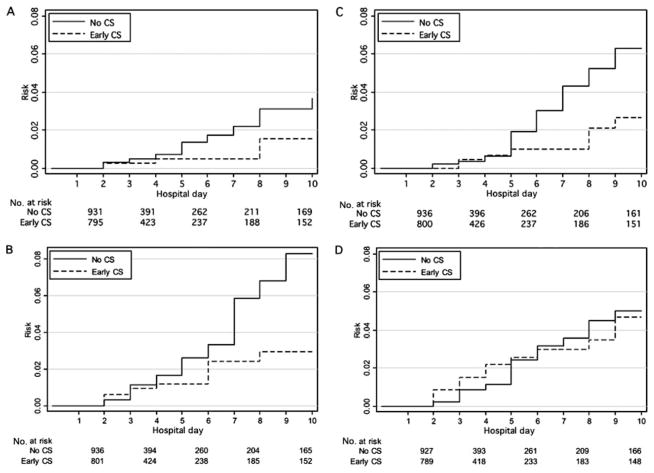
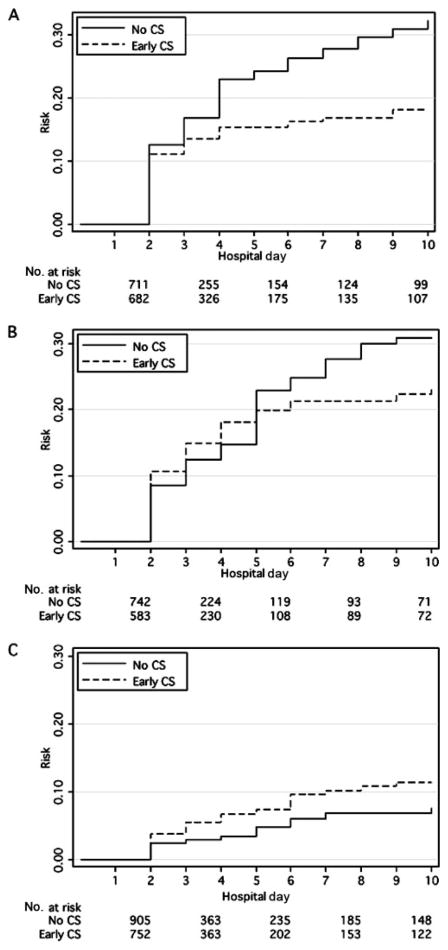
Compared with no corticosteroid exposure, early-period corticosteroid exposure (receipt of corticosteroid on hospital days 1 or 2) significantly reduced the HR for needing endoscopy (0.27 [95% CI: 0.13– 0.55]) or abdominal imaging (0.50 [95% CI: 0.29 – 0.88]) (Table 2). Figure 2 shows the Kaplan-Meier failure graphs for clinical outcomes of subjects with early corticosteroid versus no corticosteroid exposure. Early corticosteroids significantly reduced the HR for initiation of NSAIDs (0.42 [95% CI: 0.34 – 0.52]) and opioids (0.68 [95% CI: 0.56 – 0.83]) during hospitalization compared with no corticosteroid exposure (Table 2). There was no statistically significant association between early corticosteroid use and the HR for antihypertensive drug use. Figure 3 shows the Kaplan-Meier failure graphs for the initiation of NSAIDs, opioids, and antihypertensive agents in subjects with early corticosteroid versus no corticosteroid exposure. There was no statistically significant association between early corticosteroids and the HR for readmission for any reason within 30 days (Table 2).
FIGURE 2.
Clinical outcomes in corticosteroid-exposed and -unexposed subjects. Only the first 10 days of hospitalization are graphically represented, because 10 days was the 95th percentile for length of initial hospitalization. A, Surgery; B, endoscopy; C, parenteral nutrition; D, abdominal imaging. CS indicates corticosteroids; early CS, initial corticosteroid exposure on hospital day 1 or 2.
FIGURE 3.
Initiation of NSAIDs (A), opioids (B), and antihypertensive agents (C) in corticosteroid-exposed and -unexposed subjects. Only the first 10 days of hospitalization are graphically represented, because 10 days was the 95th percentile for length of initial hospitalization. CS indicates corticosteroids; early CS, initial corticosteroid exposure on hospital day 1 or 2.
Middle-period corticosteroid exposure (corticosteroids initiated on hospital days 3 or 4) was associated with significantly reduced HRs for NSAID use (0.24 [95% CI: 0.15– 0.38]) and opioid use (0.58 [95% CI: 0.40 –0.84]) compared with no corticosteroid exposure.
Hospitalization Day 1 and 2 Charges as a Marker of Disease Severity at Admission
To examine the possibility that the apparent effect of early-period corticosteroid exposure was attributable to confounding caused by different severity of illness among the patients, we included hospitalization charges for days 1 and 2, normalized for each admitting hospital, to the model as a marker for utilization of resources and, therefore, severity of illness during the early portion of the hospitalization. Table 3 lists the HR and 95% CI for the primary and secondary outcomes of the model including normalized charges. None of the estimates changed >10%; however, the CI for surgery increased to include 1.
TABLE 3.
Outcomes for Early-Period Corticosteroid Exposure Adjusted for Charges on Hospital Days 1 and 2
| Outcomes During Hospitalization
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Abdominal Surgery | Endoscopy | Parenteral Nutrition | Abdominal Imaging | Initiation of NSAIDs | Initiation of Opioid Analgesics | Initiation of Antihypertensives | Readmission | |
| HR (95% CI) | 0.44 (0.18–1.07) | 0.27 (0.13–0.55) | 0.59 (0.28–1.24) | 0.51 (0.29–0.89) | 0.42 (0.34–0.52) | 0.69 (0.56–0.84) | 0.90 (0.65–1.26) | 1.07 (0.79–1.46) |
Shown are the results of an analysis of outcomes associated with early-period corticosteroid exposure including total charges on hospital days 1 and 2 as a marker of disease severity at the time of admission.
DISCUSSION
This large multicenter observational study of clinical outcomes in hospitalized children with new-onset HSP revealed that early corticosteroid exposure is associated with statistically significant decreased HRs for needing abdominal surgery, endoscopy, and abdominal imaging and a decreased HR for NSAID and opioid use during hospitalization. Furthermore, the direction of our estimates was consistent when each patient’s hospitalization charges for days 1 and 2 were included in the model as a proxy for severity of illness at the time of admission.
Two additional findings warrant discussion. First, with observational studies of medical interventions, one must consider the possibility of potential confounding-by-indication bias, which arises when the patients with more severe forms of disease are more likely to both receive the intervention and experience poorer outcomes, which results in the apparent association of the intervention with poorer outcomes. In our study, if we accept the premise that patients who are more ill consume more health care resources, we do find evidence that patients who were more ill at the time of admission were more likely to receive corticosteroids, as demonstrated in Fig 1. Our analytic methods, with multivariable adjustment for patient characteristics, likely minimized but did not eliminate this confounding by indication, which raises the possibility that corticosteroids have an even greater beneficial effect on inpatient outcomes than we are able to demonstrate.
Second, in our analysis the only secondary outcome with an HR of >1 (although not to a statistically significant degree) was hospital readmission. Why this 1 possible counter-example to the otherwise apparent beneficial effects of corticosteroid? Our clinical experience suggests that children treated with corticosteroids may be (1) given too short a course of corticosteroids, (2) weaned off corticosteroids too quickly and experience rebound symptoms, or (3) more severely ill at the time of hospital admission. Unfortunately, we cannot evaluate these possibilities in the PHIS inpatient database, because it does not record whether children treated with corticosteroids as inpatients were sent home on corticosteroids, how long they were treated, or how the course of therapy was tapered and stopped.
These findings should be interpreted in the context of 4 specific limitations of our study. First, this was a study of inpatient HSP outcomes; therefore, our results are not generalizable to children with milder disease who are treated as outpatients. Second, the PHIS database does not contain outpatient data; thus, we do not know whether subjects in this study were previously diagnosed with HSP or treated with corticosteroids as an outpatient before admission. Similarly, we were not able to capture mild recurrences that did not necessitate readmission (ie, visit to a pediatrician or emergency department). Third, the results of this study reflect the impact of corticosteroid exposure in actual hospital practice and not the controlled setting of a clinical trial. Finally, the association of corticosteroids and renal disease was not examined in this study, because our outcomes of interest were limited to short-term outcomes that could be assessed during the initial hospitalization.
With these caveats kept in mind, our study findings indicate that the effect of corticosteroids on outcomes for pediatric inpatients with HSP warrants further investigation with a prospective randomized controlled clinical trial. If further studies confirm that corticosteroids are beneficial in the inpatient setting, then evidence-based practice guidelines could be established. Future studies should (1) address whether corticosteroid exposure is associated with improved clinical outcomes during hospitalization as well as subsequent long-term HSP outcomes, including kidney disease, (2) evaluate the optimal dose and duration of treatment with corticosteroids, and (3) if applicable, investigate the impact of standardized care guidelines for the management of inpatient HSP on resource utilization and outcomes of care, including kidney disease.
WHAT’S KNOWN ON THIS SUBJECT
Previous randomized controlled trials that examined the efficacy of corticosteroids for HSP have produced discrepant results. Previous retrospective studies have been limited by confounding by indication and small sample sizes. Currently, there is no consensus regarding corticosteroid use for HSP.
WHAT THIS STUDY ADDS
This study investigated the effect of corticosteroids on the risks of outcomes in children hospitalized with HSP. The results revealed that in the hospital setting, corticosteroid exposure is associated with decreased hazard ratios for surgery, endoscopy, and imaging.
Acknowledgments
Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- HSP
Henoch-Schönlein purpura
- PHIS
Pediatric Health Information System
- ICD-9-CM
International Classification of Diseases, Ninth Edition, Clinical Modification
- NSAID
nonsteroidal anti-inflammatory drug
- HR
hazard ratio
- CI
confidence interval
APPENDIX 1: GENERIC DRUGS INCLUDED IN ANALYSES
Corticosteroids
Methylprednisolone, prednisolone, prednisone, adrenal combination corticosteroids, dexamethasone, and triamcinolone.
Opioid Medications
Alfentanil HCl, butorphanol tartrate, codeine, fentanyl, hydromorphone HCl, meperidine HCl, methadone HCl, morphine sulfate, nalbuphine HCl, narcotic analgesic combinations, nonnarcotic analgesic and barbiturate combinations, oxycodone HCl, remifentanil HCl, and tramadol HCL.
Nonsteroidal Anti-inflammatory Medications
Aspirin, aspirin and other salicylate combinations, celecoxib, ibuprofen, indomethacin, ketorolac tromethamine, nabumetone, naproxen (acid) (sodium), and rofecoxib.
Antihypertensive Medications
Amlodipine, atenolol, captopril, carvedilol, clonidine HCl, diazoxide, diltiazem HCl, doxazosin mesylate, enalapril maleate, esmolol HCl, felodipine, guanfacine HCl, hydralazine HCl, isradipine, labetalol HCl, lisinopril, losartan potassium, metoprolol (succinate) (tartrate), minoxidil, nesiritide, nicardipine HCl, nifedipine, nitroglycerin, nitroprusside sodium, papaverine HCl, propranolol HCl, quinapril HCl, tolazoline HCl, valsartan, and verapamil HCl.
APPENDIX 2: ICD-9-CM CODES AND PROCEDURES INCLUDED IN ANALYSES
Surgery
Partial small bowel resection, small-to-small bowel anastomosis, small bowel exteriorization, intraabdominal small bowel manipulation, intraabdominal large bowel manipulation, laparoscopic appendectomy, other appendectomy, laparoscopic incidental appendectomy, other incidental appendectomy, laparoscopy, laparoscopic peritoneal adhesiolysis, and reduction of intussusception of alimentary tract.
Endoscopy
Esophagoscopy, small bowel endoscopy, esophagogastroduodenoscopy with closed biopsy, colonoscopy, flexible sigmoidoscopy, and rigid proctosigmoidoscopy.
Parenteral Nutrition
Parenteral infusion of nutritious substance.
Abdominal Imaging
Upper gastrointestinal series, small bowel series, lower gastrointestinal series, computed tomography scan of abdomen, diagnostic ultrasound-digestive, diagnostic ultrasound-urinary, and diagnostic ultrasound-abdomen.
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Bowyer S, Roettcher P. Pediatric rheumatology clinic populations in the United States: results of a 3 year survey. Pediatric Rheumatology Database Research Group. J Rheumatol. 1996;23(11):1968–1974. [PubMed] [Google Scholar]
- 2.Rostoker G. Schönlein-Henoch purpura in children and adults: diagnosis, pathophysiology and management. BioDrugs. 2001;15(2):99–138. doi: 10.2165/00063030-200115020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149(2):241–247. doi: 10.1016/j.jpeds.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Reinehr T, Burk G, Berger T, Doeker B, Andler W. Steroids for prophylaxis of nephropathy in Schnlein Henoch purpura? Follow-up of 171 patients [in German] Klin Padiatr. 2000;212(3):99–102. doi: 10.1055/s-2000-9660. [DOI] [PubMed] [Google Scholar]
- 5.Huber AM, King J, McLaine P, Klassen T, Pothos M. A randomized, placebo-controlled trial of prednisone in early Henoch Schönlein purpura [ISRCTN85109383] BMC Med. 2004;2:7. doi: 10.1186/1741-7015-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saulsbury FT. Corticosteroid therapy does not prevent nephritis in Henoch-Schönlein purpura. Pediatr Nephrol. 1993;7(1):69–71. doi: 10.1007/BF00861574. [DOI] [PubMed] [Google Scholar]
- 7.Saulsbury FT. Henoch-Schönlein purpura in children: report of 100 patients and review of the literature. Medicine (Baltimore) 1999;78(6):395–409. doi: 10.1097/00005792-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mollica F, Li Volti S, Garozzo R, Russo G. Effectiveness of early prednisone treatment in preventing the development of nephropathy in anaphylactoid purpura. Eur J Pediatr. 1992;151(2):140–144. doi: 10.1007/BF01958961. [DOI] [PubMed] [Google Scholar]
- 9.Weiss PF, Klink AJ, Hexem K, et al. Variation in inpatient therapy and diagnostic evaluation of children with Henoch Schönlein purpura. J Pediatr. 2009;155(6):812.e1–818.e1. doi: 10.1016/j.jpeds.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293. doi: 10.1542/peds.2007-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang YH, Hung CF, Hsu CR, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 2005;44(5):618–622. doi: 10.1093/rheumatology/keh544. [DOI] [PubMed] [Google Scholar]
- 12.Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35(3):143–153. doi: 10.1016/j.semarthrit.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore) 2001;80(5):279–290. doi: 10.1097/00005792-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Dolezalová P, Telekesová P, Nemcová D, Hoza J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J Rheumatol. 2004;31(11):2295–2299. [PubMed] [Google Scholar]
- 15.Csizmadi I, Collet J. Bias and confounding in pharmacoepidemiology. In: Strom B, Kimmel S, editors. Textbook of Pharmacoepidemiology. Chichester, United Kingdom: Wiley; 2006. pp. 261–275. [Google Scholar]