Abstract
Loss of the RNA-binding protein fragile X mental retardation protein (FMRP) represents the most common form of inherited intellectual disability. Studies with heterologous expression systems indicate that FMRP interacts directly with Slack Na+-activated K+ channels (KNa), producing an enhancement of channel activity. We have now used Aplysia bag cell (BC) neurons, which regulate reproductive behaviors, to examine the effects of Slack and FMRP on excitability. FMRP and Slack immunoreactivity were colocalized at the periphery of isolated BC neurons, and the two proteins could be reciprocally coimmunoprecipitated. Intracellular injection of FMRP lacking its mRNA binding domain rapidly induced a biphasic outward current, with an early transient tetrodotoxin-sensitive component followed by a slowly activating sustained component. The properties of this current matched that of the native Slack potassium current, which was identified using an siRNA approach. Addition of FMRP to inside-out patches containing native Aplysia Slack channels increased channel opening and, in current-clamp recordings, produced narrowing of action potentials. Suppression of Slack expression did not alter the ability of BC neurons to undergo a characteristic prolonged discharge in response to synaptic stimulation, but prevented recovery from a prolonged inhibitory period that normally follows the discharge. Recovery from the inhibited period was also inhibited by the protein synthesis inhibitor anisomycin. Our studies indicate that, in BC neurons, Slack channels are required for prolonged changes in neuronal excitability that require new protein synthesis, and raise the possibility that channel–FMRP interactions may link changes in neuronal firing to changes in protein translation.
Introduction
The FMR1 gene encodes the fragile X mental retardation protein (FMRP), an RNA-binding protein (Willemsen et al., 2003) that is expressed in many tissues and at high levels in neurons (Devys et al., 1993; Fähling et al., 2009). FMRP binds a subset of mRNAs and regulates their translation at synapses in response to activation of group 1 metabotropic glutamate receptors and other stimuli (Bassell and Warren, 2008). Loss of FMRP leads to altered synaptic function and loss of protein synthesis-dependent plasticity (Bassell and Warren, 2008; Li et al., 2009). In humans, absence of FMRP causes fragile X syndrome, the most common form of inherited intellectual disability (Willemsen et al., 2003; Bear et al., 2004; Bagni and Greenough, 2005). FMRP interacts with a range of mRNA targets and a variety of intracellular protein partners. Recent studies suggest that one of these FMRP-interacting proteins is the Na+-activated K+ channel (KNa) Slack (Brown et al., 2010). Biochemical experiments indicate that FMRP interacts with the cytoplasmic C-terminal domain of Slack. Direct application of FMRP to the cytoplasmic face of excised patches containing Slack channels enhances channel activity. Whether the protein–protein interactions of FMRP with ion channels directly influence the excitability of neurons is, however, unknown.
A model system for investigation of changes in neuronal excitability is that of the bag cell (BC) neurons of the marine mollusk, Aplysia californica. These neurons control a series of reproductive behavior culminating in egg laying. Although these peptidergic neurons display no spontaneous firing, they undergo a ∼30 min afterdischarge of actions potentials in response to brief synaptic stimulation, triggering the release of several neuroactive peptides (Conn and Kaczmarek, 1989). The electrical characteristics of BC neurons, including their resting potential, input resistance, and action potential height and width have been shown to be potently modulated by several signaling pathways including the cAMP-dependent protein kinase (Kaczmarek et al., 1980), protein kinase C (White et al., 1998), and tyrosine kinases/phosphatases (Wilson and Kaczmarek, 1993).
KNa channels are widely expressed and have been recorded in neurons of a variety of species in which they contribute to the afterhyperpolarization that follows one or more evoked action potentials (Bader et al., 1985; Wallén et al., 2007; Nanou et al., 2008). KNa channels generally have a large unitary conductance with various degrees of voltage dependency and are gated by increases in Na+ and Cl− (Joiner et al., 1998; Bhattacharjee et al., 2003; Yuan et al., 2003; Bhattacharjee and Kaczmarek, 2005; Santi et al., 2006; Chen et al., 2009). In this study, we have characterized the native KNa channels in Aplysia BC neurons and provide evidence that they are encoded by the Slack gene. We have also demonstrated that Slack exists in a protein–protein complex with FMRP in these neurons, and that, as predicted by mammalian studies, intracellular injection of FMRP acutely increases KNa current and alters their firing response to depolarizing current pulses. Furthermore, we show that Slack channels are required for a prolonged change in the excitability of BC neurons that requires new protein synthesis.
Materials and Methods
Animals and BC neuron culture.
Adult A. californica weighing 150–200 g were obtained from Marine Specimens Unlimited or Marinus. Primary cultures of isolated BC neurons were prepared as described previously (White et al., 1998; Magoski et al., 2002) and were maintained in normal artificial sea water (nASW) containing the following (in mm): 460 NaCl, 10.4 KCl, 11 CaCl2, 55 MgCl2, and 15 HEPES; plus 100 U ml−1 penicillin and 0.1 mg l-1 streptomycin; pH 7.8.
Cloning of Aplysia Slack.
We identified an Aplysia homolog of Slack based on a tblastn search of the Aplysia genome trace archive and amplified partial Slack cDNA by PCR from an Aplysia BC neuron cDNA library. PCR products were purified and cloned into the T/A cloning vector pCR2.1 (Invitrogen). The products were sequenced by the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale.
RNA interference.
Predesigned silencer select siRNAs (Aplysia Slack, Shab, and scrambled siRNA) were purchased from Ambion. For each gene siRNA, we targeted two different sites, and then mixed the two different siRNA products for treatment of neurons. The target sequences were chosen from Aplysia Slack sequence between the start codon and the S6 transmembrane domain: sense, GGUCUAGUCUCAACCUACATT and GGUCAGAUAUCAACUGGUATT; for Aplysia Shab, the target sequences were as follows: sense, GGAUAGUGUUUAUCAAGCATT and GGAUUUUAAUGCGAAGUUUTT. Commercially available scrambled siRNA (Ambion), which has previously been reported as a negative control in Aplysia neurons (Paganoni and Ferreira, 2005; Jordan et al., 2007), was used as the nontargeting sequence. All siRNAs were obtained in annealed and desalted form and were dissolved in siRNA buffer (Ambion). Isolated neurons were microinjected using pipettes containing 1 μm scrambled siRNA, Slack siRNA, Shab siRNA in 200 mm KCl. Intact clusters of neurons were treated with 20 μm Slack siRNA or scrambled siRNA in nASW. Immunocytochemistry, Western blotting, single-cell electrophysiology, and discharge experiments were performed 4–6 d after onset of siRNA treatment.
Immunocytochemistry.
Staining of 1–2 d cultured BC neurons was performed on coverslips coated with 1 μg/ml poly-d-lysine, and after fixation with 4% paraformaldehyde in 400 mm sucrose/nASW. Coverslips were washed twice with PBS and blocked with 5% goat serum/PBS before incubation with primary antibodies. The anti-Slack antibody we used for immunostaining (N3/26 NeuroMab produced by University of California Davis/NIH NeuroMab Facility, Davis, CA) was raised against an antigen sequence that has 30% identity and 62% similarity compared with Aplysia Slack sequence. Rabbit anti-FMRP antibody was obtained from Abcam. The coverslips were inverted on 100 μl of primary antibody solution (anti-Slack antibody, 1:1000 dilution; anti-FMRP antibody, 5 μg/ml in 5% goat serum/PBS) and placed in a humidified chamber at 4°C overnight, washed extensively with PBS, and then incubated for 2 h at room temperature with CY3-conjugated goat anti-mouse IgG and fluorescence-conjugated anti-rabbit IgG secondary antibodies. The coverslips were washed and mounted on glass slides using mounting medium. The stained slides were viewed and photographed using AxioVision software (Zeiss).
Staining of Aplysia FMRP in transiently transfected CHO cells.
To test whether anti-FMRP antibodies raised in rabbits recognize Aplysia FMRP, we expressed Aplysia Fmr1 in CHO cells. We subcloned Aplysia Fmr1 (GenBank accession number AAQ18136) from pNEX3 into the pcDNA3 vector, and then transfected it into CHO cells using Lipofectamine. The transiently transfected cells were grown on glass coverslips to 60–70% confluence and fixed in PBS containing 4% paraformaldehyde for 10 min. After washing with PBS, cells were permeabilized in PBS containing 1% bovine serum albumin and 0.2% Triton X-100 for 10 min. Primary rabbit anti-FMRP antibody (Abcam; 5 μg/ml in 5% goat serum/PBS) was added to the coverslips and incubated for 1 h, washed three times, and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (1:500) for 30 min, washed again, and mounted on glass slides with Citifluor mounting medium (Ted Pella) for fluorescence microscopy.
Coimmunoprecipitation experiments.
BC neuron clusters were dissected from 150–200 g animals. The clusters were homogenized in 100 μl of homogenization buffer (Zhang et al., 2008a). The samples were then centrifuged at 5000 × g for 20 min. The supernatants were transferred to 1.5 ml Eppendorf tubes. Tubes were incubated at 4°C with rotation overnight after addition of 1 μg of purified mouse anti-FMRP antibody or rabbit anti-Slack antibody. After adding 30 μl of protein A-Sepharose beads [50% (v/v) in Triton X-100 buffer; Sigma-Aldrich], the samples were rotated at 4°C for 2 h. The immunoprecipitates were collected by centrifugation at 3000 × g for 1 min and washed five times in 800 μl of Triton X-100 buffer. After washes, the beads were incubated in sample buffer at room temperature for 30 min. The samples were applied to SDS-acrylamide gels. For immunoblotting, the SDS gel-electrophoresed proteins were transferred to an immunoblot polyvinylidene difluoride membrane (Bio-Rad). Nonspecific binding was blocked by incubating the blot in Tris-buffered saline Tween 20 (TBST) buffer with 5% milk for 1 h at room temperature. The blots were incubated overnight at 4°C with one of the following primary antibodies: rabbit anti-Slack antibody (1:500; Biosynthesis); or rabbit anti-Slack antibody preincubated with excess antigen obtained from rat Slack purified protein; or mouse anti-FMRP antibody (1 μg/ml; EMD Millipore); or mouse anti-FMRP antibody preincubated with excess recombinant FMRP antigen (Novus Biologicals). Next, the blots were washed four times for 30 min each, followed by application of secondary horseradish peroxidase-linked anti-rabbit or anti-mouse antibodies at 1:10,000 dilution for 1 h at room temperature. After three washes of 30 min each in TBST, immunoreactive proteins were visualized with detection reagent (GE Healthcare). In parallel experiments, we used anti-rabbit IgG or anti-mouse IgG (The Jackson Laboratory) as controls for immunoprecipitation.
Microinjection.
Current-clamp and voltage-clamp recordings were made from BC neurons using an AxonClamp 2B (Molecular Devices). For current-clamp recordings, the single-sharp-electrode, bridge balance method was used. For voltage-clamp analysis, the discontinuous single-electrode approach was used, in which the membrane potential is changed using a high-frequency train of current pulses and measurements of membrane potential are made immediately before each current pulse. The discontinuous single-electrode voltage-clamp amplifier was run at a switching frequency of 1–2 kHz. To ensure adequate voltage control, the individual pulses were monitored on an oscilloscope and adjusted so that the period of voltage sampling corresponded to at least nine electrode time constants. Microelectrodes were pulled from borosilicate glass capillaries (1.2 mm inner diameter; TW 120F-4; World Precision Instruments) and had tip diameters of <1 μm with resistances of ∼2 MΩ when filled with 3 m KCl. Microinjections were carried out using a Picospritzer that delivered 14 psi pressure pulses of 100 ms duration. Recordings were filtered at 3 kHz using the Axonclamp built-in Bessel filter and sampled at 2 kHz using a Digidata 1200 analog-to-digital (A/D) converter (Molecular Devices) using Clampex software (version 8.2; Molecular Devices).
Recombinant FMRP(1–298) was obtained from Novus Biologicals. For intracellular injections of FMRP, the pipette solution contained 530 mm K+ acetate, 11 mm Tris-HCl, pH 7.2, and 100 nm recombinant FMRP(1–298), and the external solution contained the following (in mm): 460 NaCl, 10.4 KCl, 11 CaCl2, 55 MgCl2, and 15 HEPES, pH 7.8, with modifications as described in the text.
Excised patch-clamp recordings.
BC neurons were cultured in nASW. Electrodes had a resistance of 5–10 MΩ for single-channel recordings. Excised inside-out patch recordings were performed using symmetrical K solutions: 470 mm KCl, 66 mm MgCl2, 15 mm HEPES, 2 mm EGTA. The cytoplasmic face of the membrane was perfused with bath solution supplemented with Na+ to 0, 120, or 200 mm free Na+. Data were acquired with an EPC-7 amplifier (HEKA Electronik), a Digidata 1200 A/D converter (Molecular Devices), and the Clampex acquisition program of pClamp (version 8.2; Molecular Devices). Current was sampled at 10 kHz and filtered at 1 kHz with a Bessel filter (Frequency Devices). Data were gathered in 1–3 min intervals while holding the patch at potentials between −50 and +50 mV.
Extracellular recording.
Abdominal ganglia were placed in a recording chamber at 14°C. A wide-bore, fire-polished glass suction electrode placed at the distal end of one connective, and a recording suction electrode was placed at the rostral end of the corresponding bag cell neuron cluster (Kaczmarek et al., 1978). Current pulse trains (20 V pulses of 2.5 ms duration at 6 Hz for 10–20 s) were delivered with a Grass S88 stimulator and isolation unit, and voltage was recorded using a Warner DP-301 differential amplifier. Within 15 min of the end of a first afterdischarge, a similar pulse train was applied one or two times to ensure that the cells had entered the refractory period. To test the effect of the protein synthesis inhibitor anisomycin on the afterdischarge, 3 μm anisomycin was added to clusters at least 30 min before stimulation.
Results
Cloning of Slack from BC neurons
We identified an Aplysia ortholog of Slack based on a tblastn search of the Aplysia genome trace archive and amplified Slack cDNA by PCR from an Aplysia BC neuron cDNA library. Several Slack isoforms, differing at their 3′-ends, are found in BC neurons. The longest full sequence has a 3.531 kb coding region corresponding to six putative membrane-spanning regions (S1–S6) and a long, presumably cytoplasmic, C terminal (GenBank accession number HG413690). This Aplysia Slack shares 46% identity and 72% similarity with human SLACK (Needleman-Wunch method; BLOSUM62). A region of sequence between S6 and the first RCK domain is also highly conserved between rat and human Slack.
Slack channels and FMRP interact and are colocalized in BC neurons
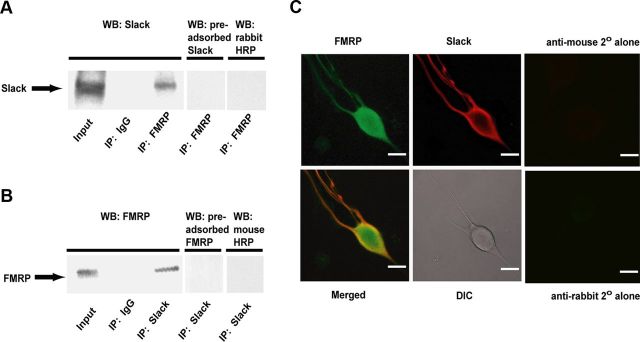
Like Slack, FMRP has been conserved throughout evolution. The Aplysia nervous system expresses a single Fmr1 gene (Moroz et al., 2006) that is 74.4% identical to that of the mouse Fmr1 at the genomic level (AlignX, Vector NTI software). FMRP contains two heterogeneous nuclear ribonucleoprotein (hnRNAP) K-homology (KH) domains and one arginine-glycine domain (RGG box) that can mediate protein/RNA interactions (Ramos et al., 2006; Bassell and Warren, 2008; Bechara et al., 2009). The major functional mRNA-binding domains of FMRP are the KH1, KH2, and RGG domains, which are 69, 83, and 83% identical between rat and Aplysia. Moreover, the sequence of the N terminus of Aplysia FMRP is 58% identical and 76% similar to that of mouse FMRP and 55% identical, 75% similar to that of human FMRP. Antibodies against this conserved N terminus of human FMRP specifically recognize a single band on Western blots of BC neuron homogenates that is of the expected size for a close ortholog (75 kDa; Fig. 1B). Recent work has shown that, in mammalian cells, Slack can form a protein complex with FMRP (Brown et al., 2010). We therefore performed coimmunoprecipitation experiments to test whether Slack interacts with FMRP in Aplysia neurons. Using extracts from the BC neurons, we found that immunoprecipitates obtained using the mouse anti-FMRP antibody contained a Slack-immunoreactive band with a molecular weight of 90 kDa, a size comparable with that reported in lamprey (Nanou et al., 2008) (Fig. 1A). The rabbit anti-Slack antibody was generated against the C terminus of rat Slack (Bhattacharjee et al., 2002) and the Slack-immunoreactive band in Aplysia neurons was completely eliminated when the anti-Slack antibodies were preadsorbed with purified rat Slack protein (Fig. 1A). Conversely, a single FMRP-immunoreactive band was detected in immunoprecipitates obtained using the anti-Slack antibodies (Fig. 1B). Again, the FMRP-immunoreactive band was eliminated when the anti-FMRP antibodies were preadsorbed with human FMRP(1–298). No bands were detected when control IgG was used for immunoprecipitation or when secondary antibody alone was used for immunoblotting (Fig. 1A,B).
Figure 1.
Slack channels and FMRP interact and are colocalized in BC neurons. A, Protein was extracted from BC clusters and used to immunoprecipitate FMRP with a mouse anti-FMRP antibody. Samples were immunoblotted with a rabbit anti-Slack antibody, and an immunoreactive band was detected at 90 kDa. There was no immunoreactive band detected in control immunoprecipitations using an anti-mouse IgG or when immunoblotting with secondary anti-rabbit antibody alone. Additionally, no immunoreactive band was detected when the rabbit anti-Slack antibody was preincubated with purified rat Slack before immunoblotting. Input samples were positive for the 90 kDa immunoreactive band. B, The reverse coimmunoprecipitation experiment showed that Slack immunoprecipitates with FMRP (immunoreactive band at 75 kDa). Control experiments were negative as described in A. C, Aplysia BC neurons are immunoreactive with both Slack and FMRP antibodies. Confocal immunofluorescence staining for FMRP (left top) (green); Slack (middle top) (red); Slack and FMRP colocalize in BC neurons (left bottom). Differential interference contrast (DIC) imaging is shown (middle bottom). Scale bar, 30 μm.
We also performed double immunocytochemistry to examine the subcellular localization of Slack and FMRP in isolated cultured BC neurons (Fig. 1C). To test the specificity of the rabbit anti-FMRP antibody we used for immunocytochemistry, we transiently transfected CHO cells with Aplysia Fmr1. On subsequent immunostaining of the transfected cells, strong staining of the transfected cells was seen using the anti-FMRP antibody, but no staining was detected in untransfected CHO cells (data not shown). In BC neurons, immunoreactivity for FMRP was diffusely distributed throughout the cells including the somata and neurites, whereas Slack immunoreactivity was highly enriched at the periphery of the neurons, consistent with localization to the plasma membrane. Merged images demonstrate that, at the light level, Slack and FMRP immunoreactivity is colocalized at the periphery of the cells and within the neurites. Overlap appeared strongest at the plasma membrane of the somata, perhaps because the ratio of FMRP to Slack is reduced in the neurites. No staining was detected when secondary antibodies alone were used (Fig. 1C).
Injection of Na+ increases outward current in BC neurons
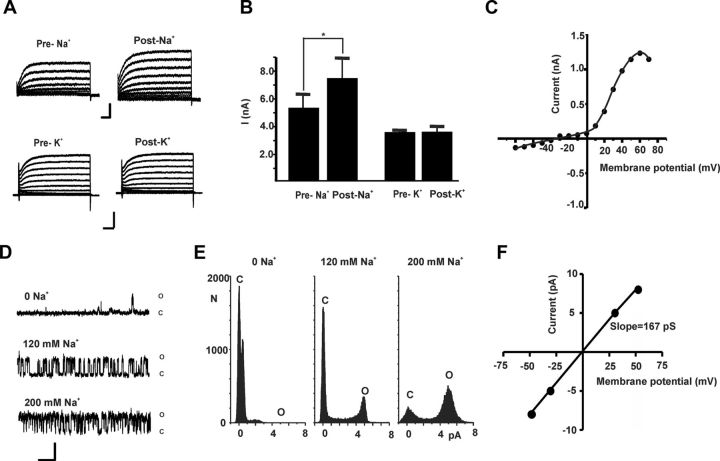
To test whether KNa channels are present in the plasma membrane of BC neurons, we first recorded outward currents from isolated cultured neurons using the sharp single-electrode voltage-clamp technique. Intracellular injections of Na+ were made using pulses of pressure (14 psi, one to two pulses, 100 ms duration) applied to the intracellular electrodes, which contained 0.5 m Na+ acetate, NaCl, or Na2SO4. We found that intracellular injections of Na+ produced a significant increase in the outward current (Fig. 2A–C; n = 6; p < 0.05). This increased current does not depend on the coinjection anion. In contrast, intracellular injections of KCl, using the same injection parameters, produced no significant change in outward currents (Fig. 2A,B; n = 3).
Figure 2.
Detection of sodium-activated potassium current in Aplysia BC neurons. A, Injection of Na+ increased outward currents while injection of K+ had no effect. Calibration: 5 nA, 50 ms. Traces show superimposed currents evoked from a holding potential of −60 mV to command potentials between −60 and +70 mV in 10 mV increments. B, Bar graphs summarizing the effects of injection of Na+ (n = 6), or K+ (n = 3) on peak currents are measured at +20 mV. Data are expressed as mean ± SEM. *Significant difference from control (p < 0.05; n = 6; paired t test). C, Current–voltage relationships for the difference current measured by subtracting peak currents before and after Na+ injection. D, Single-channel inside-out patches recordings from BC neurons shows increased open probability of KNa channels with increasing Na+ concentration (holding potential, +30 mV). Calibration: 5 pA, 100 ms. E, All-points amplitude histograms for 10 s of recording in 0, 120, or 200 mm Na+. F, KNa current–voltage (I--V plot) relationship in symmetric 470 mm KCl. Unitary conductance is ∼167 pS (n = 3).
Further evidence for the presence of Na+-activated K+ channels was obtained using excised inside-out patches from cultured BC neurons, using symmetrical 470 mm KCl recording solutions with no Ca2+ ions at the cytoplasmic face. Under these conditions, it was possible to readily identify these channels based on their large unitary conductance, voltage dependence of opening (an increase in P0 with depolarization) and sensitivity to Na+ ions. Little channel activity was detected at +30 mV in the absence of Na+ (Fig. 2D,E). When the Na+ concentration at the cytoplasmic face of the patch was raised to 120 mm, channel activity increased markedly, and further elevation of the Na+ concentration to 200 mm produced a further increase in open probability from 0.05 ± 0.01 (0 Na+) to 0.37 ± 0.02 (120 mm Na+) to 0.52 ± 0.02 (200 mm Na+). Subconductance states could sometimes also be observed in the single-channel recordings and, in amplitude histograms, produced a slight asymmetry in peaks for the closed state (Fig. 2E). When the amplitude of the KNa channels was plotted as a function of voltage, the unitary conductance was found to be ∼167 pS (Fig. 2F; n = 3).
Slack subunits contribute to KNa currents in BC neurons
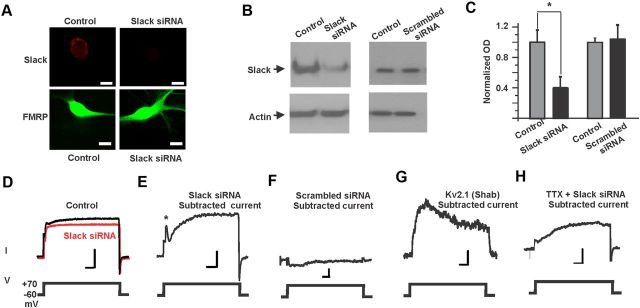
To confirm the correspondence between Slack channels and the KNa currents in BC neurons, we tested the effects of decreasing the synthesis of native KNa channels by microinjecting double-strand siRNA against Aplysia Slack into isolated cultured BC neurons. This method has been shown to inhibit gene expression specifically in various organisms including Aplysia (Lee et al., 2001). By comparing the level of immunostaining for Slack in isolated neurons injected with the Slack siRNA with that in control neurons from the same animal, we confirmed that levels of the Slack channel in BC neurons are decreased after Slack expression is knocked down using this approach (Fig. 3A). The diffuse localization of FMRP throughout somata and neurites did not appear to be altered by Slack siRNA (Fig. 3A). Intact ganglia containing clusters of BC neurons were treated with Slack siRNA or scrambled siRNA (Korneev et al., 2002). After 5–6 d, Slack protein was greatly reduced in Slack siRNA-treated cells compared with scrambled siRNA-treated cells, as determined by Western blotting (Fig. 3B,C).
Figure 3.
Slack contributes to outward K+ current in BC neurons. A, Slack staining was reduced in BC neurons injected with siRNA-Slack. Scale bar, 25 μm. B, C, Immunoblots demonstrating that Slack protein level is decreased in Slack siRNA-treated BC neurons. Data are expressed as mean ± SEM (n = 3; *p < 0.05, paired t test). Immunoblots against rabbit anti-actin antibodies are provided as a control for equivalence of protein samples. No change in Slack protein levels was observed in scrambled siRNA-treated BC neurons (n = 3; NS, paired t test). D, Averaged traces of outward current in control and Slack siRNA-treated neurons. Calibration: 5 nA, 50 ms. Subtracted currents for treatment with either Slack siRNA (E) (calibration: 0.5 nA, 50 ms), scrambled siRNA (F) (calibration: 0.5 nA, 50 ms), Shab siRNA (G) (calibration: 0.4 nA, 50 ms), and Slack siRNA pretreated with 100 μm TTX (H) (calibration: 0.5 nA, 50 ms). All currents were recorded by depolarizing the membrane from a holding potential of −60 mV to a test potential of +70 mV.
We then tested the effects of the siRNA on the electrophysiological characteristics of BC neurons. As a positive control, we used siRNA against the Aplysia voltage-dependent Shab (Kv2.1) K+ channel, which is also expressed in BC neurons but which has very different characteristics from those of Slack (Quattrocki et al., 1994; Zhang et al., 2008b). As a negative control, we used a scrambled siRNA. To test the effects of each of these siRNA reagents, BC neurons isolated from the same animal were divided into two groups. In the first group, 1 μm siRNA was injected into each neuron, while the second control group was not injected. Three days after injection of the siRNA, outward currents were recorded from both groups of cells. After normalization for capacitance, the averaged current of all the siRNA-injected cells was subtracted from the mean current in control cells. This subtracted trace corresponds to the mean change in outward currents produced by siRNA injection.
The subtracted trace for treatment with Slack siRNA revealed a biphasic outward current, with a rapid (50–100 ms) transient component that was followed by a more slowly activating component (Fig. 3D,E, n = 8). Injections of control scrambled siRNA produced no significant difference current in the subtracted traces (Fig. 3F; n = 4). Treatment with Shab siRNA resulted in an outward difference current that activated within ∼50 ms and then partially inactivated over the remainder of a 600 ms command pulse (Fig. 3G; n = 3). This slowly inactivating current very closely matches that reported for the Shab current in expression systems and in native BC neurons (Quattrocki et al., 1994).
Unlike the subtracted Slack siRNA trace, Slack currents in heterologous expression systems activate slowly with no transient component at the onset of a depolarizing pulse (Yang et al., 2006). To test the possibility that this transient component in native BC neurons reflects a transient enhanced activation of Slack channels by Na+ entry through voltage-dependent Na+ channels at the onset of the depolarizing commands, we repeated the experiments using Slack siRNA, but recorded all currents in the presence of tetrodotoxin (TTX) at a concentration (100 μm) known to block voltage-dependent Na+ channels in this species (Knox et al., 1996; Hung and Magoski, 2007). Under these conditions, the transient component was suppressed but the slowly activating component was still present (Fig. 3H; n = 6, siRNA plus TTX treated; n = 3, control). While we cannot be confident that voltage control was adequate to clamp transient Na+ currents themselves, our findings are consisted with the hypothesis that the slower transient component of K+ current reflects activation of KNa channels by Na+ entry at the onset of voltage commands.
We also tested the effects of Slack siRNA treatment on the mean resting potential of BC neurons recorded in the current-clamp mode. Reduction of Slack expression was found to produce a depolarization [mean resting potential of Slack siRNA-treated neurons, −38.2 ± 1.8 mV (n = 9) vs −44.7 ± 0.8 mV (n = 14) in controls; p < 0.002], suggesting that KNa currents contribute to the resting conductance.
Injection of FMRP increases KNa current and alters electrical properties
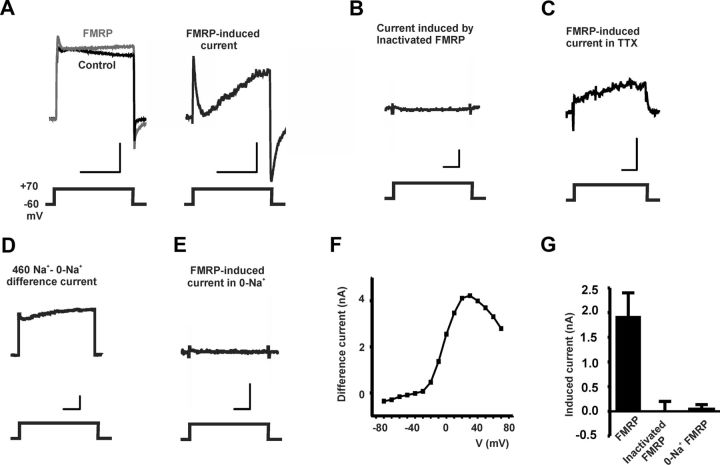
We tested the effects of recombinant FMRP that contains amino acids 1–298 [FMRP(1–298)] on macroscopic currents and on the electrical properties of isolated BC neurons. This truncated FMRP contains the N-terminal domain (NDF; amino acids 1–134) of FMRP that has been shown to serve as a platform for the majority of known protein–protein interactions of FMRP with other cellular signaling components, but lacks the C-terminal RNA-binding region (Jin et al., 2004; Darnell et al., 2005; Ramos et al., 2006). We found that injecting FMRP(1–298) into isolated BC neurons under voltage clamp produces an increase in net outward current. The increase in current began 1–5 min after injection and reached a maximum at ∼20 min. Like the subtracted Slack siRNA current, the difference currents obtained by subtracting the preinjection currents from those in the same cell after injection of FMRP(1–298) were biphasic with an early transient component lasting 50–100 ms followed by a more slowly activating component (Fig. 4A,G; n = 4). The FMRP(1–298)-induced currents progressively increased with test potentials up to approximately +30 mV, after which they declined with further depolarization (Yuan et al., 2003; Yang et al., 2006) (Fig. 4F). Injection of heat-inactivated FMRP(1–298) into the neurons maintained in a normal Na+-containing external medium had no effect on outward currents (Fig. 4B,G; n = 4)
Figure 4.
Injection of recombinant FMRP(1–298) into BC neurons increased Slack-like currents. A, Superimposed currents before and after injection of FMRP(1–298) (left, calibration: 10 nA, 250 ms) and difference currents (right, calibration: 2 nA, 250 ms). Holding potential was maintained at −60 mV, the outward current was evoked by pulses to +70 mV. B, Injection of heat-inactivated FMRP(1–298) had no effect on outward current in nASW. Calibration: 2 nA, 100 ms. C, Pretreatment of BC neurons with 100 μm TTX before injection of FMRP(1–298) eliminated the fast component of FMRP-induced outward current. Calibration: 0.5 nA, 100 ms. D, Difference current recorded on switching the external medium from Na+-containing nASW to zero Na+ ASW. Calibration: 1 nA, 100 ms. E, Injection of FMRP(1–298) had no effect when neurons were recorded in zero Na+ ASW external medium. Calibration: 2 nA, 100 ms. F, Current–voltage relationship of subtracted Slack-like current in nASW. G, Group data for steady-state difference currents produced by injecting either FMRP(1–298) in nASW, inactivated FMRP(1–298) in nASW, or FMRP(1–298) in zero Na+ ASW (n = 4 in each group; test potential, +70 mV).
The effects of FMRP(1–298) on outward current were dependent on Na+ ions. As with the Slack component of current in the siRNA-treated cells, the early transient component of current induced by FMRP(1–298) was eliminated if the cells were pretreated with sodium channel blocker TTX (100 μm) (Fig. 4C). The effects of FMRP(1–298) on the slow component of outward current were also dependent on Na+. When external Na+ was replaced by NMDG (N-methyl-d-glucamine), the total outward current was reduced (Fig. 4D). Moreover, injection of FMRP(1–298) into neurons in this Na+-free external medium was found to have no effect on either the amplitude or kinetic behavior of outward currents (Fig. 4E,G; n = 4). Overall, these findings are consistent with an acute enhancement of native Slack KNa currents by injection of FMRP(1–298).
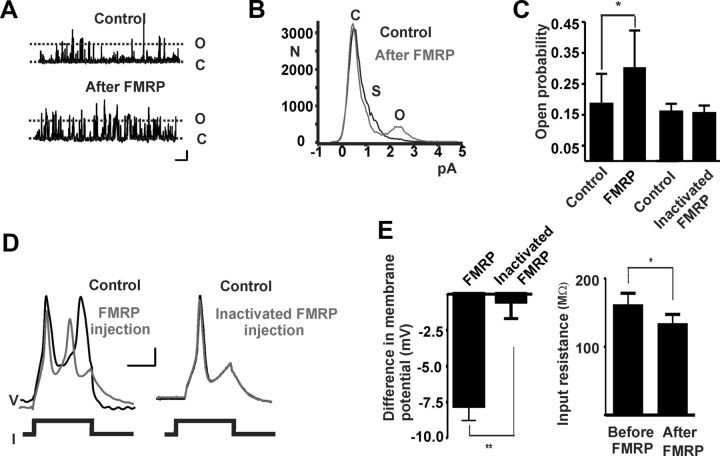
We then tested whether, as has been demonstrated in Xenopus oocytes expressing rat Slack channels, the FMRP protein acutely enhances the activity of native KNa channels in neurons. We excised inside-out patches containing KNa channels from isolated BC neurons and applied recombinant FMRP(1–298) (100 nm) to the cytoplasmic face of the patches. This produced a marked increase in channel opening, raising the channel open probability from 0.19 ± 0.09 to 0.30 ± 0.11 (Fig. 5A–C; n = 4). As has also been described for Slack channels in heterologous expression systems (Brown et al., 2010), application of FMRP suppressed the occurrence of subconductance states (Fig. 5B).
Figure 5.
Addition of FMRP(1–298) increases KNa channel open probability, hyperpolarizes membrane potential, and decreases input resistance in BC neurons. A, Representative traces before and after addition of FMRP(1–298) to an excised inside-out patch containing Na+-activated K+ channels (80 mm Na+ at cytoplasmic face; holding potential, +30 mV; calibration: 2 pA, 0.2 s; C, closed state; O, open state). B, All-points amplitude histograms before and after addition of FMRP(1–298) to an excised patch as in A (C, closed state; S, subconducance state; O, open state). C, Group data for effect of FMRP(1–298) or heat-inactivated FMRP(1–298) on channel open probability (n = 4). Data are expressed as the mean ± SEM (*p < 0.05; n = 4; paired t test). D, Injection of FMRP(1–298) hyperpolarizes membrane potential and decreases input resistance in BC neurons. Representative traces of the effect of injection of FMRP(1–298) or heat-inactivated FMRP(1–298) on action potentials evoked by injecting 0.6 nA current pulses. Calibration: 10 mV, 50 ms. E, Summary of changes in membrane potential, input resistance following injection of FMRP(1–298). Data are expressed as mean ± SEM (n = 4, **p < 0.005, unpaired t test; n = 4, *p < 0.05, paired t test).
To test the acute effects of FMRP on intrinsic electrical properties, isolated neurons were recorded in the current-clamp mode using single microelectrodes. Intracellular microinjection of FMRP(1–298) was found to decrease the input resistance and hyperpolarize the resting membrane potential (Fig. 5D,E; n = 4). In addition, the width of action potentials evoked by depolarizing currents was reduced within ∼2 min of injection. Because a change in resting potential can in itself produce changes in action potential width, we introduced a bias current to adjust the membrane potential of each neuron to its preinjection value. Under these conditions, the mean reduction in width measured at 50% spike amplitude was 4 ± 1 ms (n = 4). As with the voltage-clamp experiments, injection of heat-inactivated FMRP(1–298) had no effect on the neurons in the current-clamp mode (Fig. 5D,E; n = 4).
Suppression of Slack prevents recovery of BC neurons from a long-lasting inhibited state
In response to brief stimulation of their synaptic input, BC neurons in the abdominal ganglion of the mollusk Aplysia generate a ∼30 min discharge of action potentials, which is followed by a prolonged inhibited period during which further stimulation of the input either fails to trigger an afterdischarge or triggers discharges of reduced duration (Kupfermann and Kandel, 1970; Kauer and Kaczmarek, 1985). In vivo, this sequence of changes in excitability serves to trigger a series of reproductive behaviors culminating in egg-laying and to limit their frequency (Kupfermann and Kandel, 1970). Recovery from the inhibited period occurs gradually over ∼18 h, such that stimulation 1 d after evoking a first discharge (day 2) triggers discharges that are of the same duration as those on day 1 (Fig. 6A,C; n = 14).
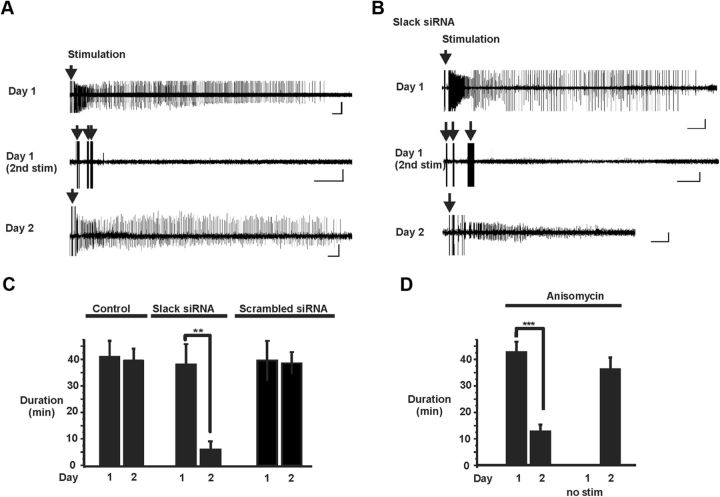
Figure 6.
Recovery from the inhibited state of BC neurons requires new protein synthesis. A, Panels shows a control discharge evoked by a first stimulus (top) and failure to trigger discharge within 1 h of the termination of the first discharge (center). The bottom panel shows a full-length discharge triggered the next day. Calibration: 50 μV and 2, 0.5, and 2 min for top to bottom panels. The arrows indicate times of stimulation. B, Treatment with Slack siRNA prevents recovery from the inhibited state. Top trace, Discharge after 5 d of Slack siRNA treatment. Center trace, Stimulation within 1 h of the termination of the first discharge. Bottom trace, Shortened discharge triggered 24 h after the first discharge in the Slack siRNA-treated neurons. Calibration: 50 μV and 2, 0.5, and 2 min for top to bottom panels, respectively. C, Discharge durations on two consecutive days after no treatment (Control) or treatment with Slack or scrambled siRNA (mean ± SEM; n = 6; **p = 0.0021, paired t test, two-tailed). D, Effects of anisomycin on mean durations of first discharges triggered on 2 consecutive days (n = 15; ***p = 0.0001, paired t tests, two-tailed). The bar graph at right shows duration on day 2 with no prior stimulation on day 1 (n = 10).
We tested the effects of suppression of Slack expression on stimulus-evoked discharges in BC neurons and on the recovery from the prolonged inhibited period. Treatment of bag cell clusters in abdominal ganglia for 5 d with Slack siRNA had no effect on the duration of a first discharge or on the subsequent entry of the neurons into the prolonged inhibited period (Fig. 6B,C; n = 6). Slack siRNA-treated BC neurons failed, however, to recover from the inhibited period (Fig. 6B,C; n = 6). In contrast, BC clusters treated with the scrambled siRNA for 6 d generated full-length discharge both on day 5 and on day 6, indicating full recovery from the inhibited state induced on day 5 (Fig. 6C; n = 6).
Previous work has shown that stimulation of BC neurons triggers an increase in synthesis of some proteins, and that this is dependent on the presence of extracellular Na+ ions (Wayne et al., 2004). We therefore tested whether recovery from the inhibited period requires new protein synthesis. As described previously (Montarolo et al., 1986), we found that pretreatment of clusters of BC neurons with the protein synthesis inhibitor anisomycin (3 μm) inhibited incorporation of [35S]methionine into new proteins by 95.4 ± 0.04% (n = 4). Anisomycin had no effect on the duration of a first discharge (Fig. 6D; n = 15) or on the subsequent inhibited state. One day later, however, stimulation could only trigger action potentials and short discharges that are identical to those in the inhibited state. This was not a nonspecific effect of protein synthesis inhibition because preincubation of BC neurons for 24 h in anisomycin without stimulation had no effect on the ability of stimulation to trigger discharges (Fig. 6D; n = 10).
Discussion
Our results indicate that the interaction between Slack KNa channels and the mRNA-binding protein FMRP, previously documented in the nervous system of mice (Brown et al., 2010), is an evolutionarily conserved one. Like their mammalian counterparts, Slack channels in Aplysia have a large unitary conductance and, upon depolarization, are activated in a biphasic manner. Rapid activation by Na+ entry through voltage-dependent Na+ channels is followed by a slower phase of activation as the depolarization is maintained. It is likely that both persistent Na+ currents and other cation channels contribute to this sustained phase (Lu et al., 2010; Hage and Salkoff, 2012). It is also possible that some activation of KNa channels by voltage steps occurs even at resting levels of internal Na+ ions, which would change only slowly on removal of external Na+.
The KNa current in BC neurons is enhanced by introduction of FMRP both in excised patches and in intact cells, leading to a hyperpolarization of the resting potential and a narrowing of action potentials. In mammalian cells, application of FMRP to KNa channels in patches from Slack-transfected cells enhances channel opening in part by suppressing the occurrence of subconductance states (Brown et al., 2010). We also observed the same effect for KNa channels in BC neurons. Nevertheless, subconductance states appeared less prominent than in transfected cells, suggesting that some binding of endogenous FMRP may occur in the patches excised from BC neurons. Regulation of the amplitude of KNa currents by FMRP is likely to be a dynamic one, subject to changes in factors such as the phosphorylation state of Slack or FMRP, which may change rapidly with neuronal stimulation.
It seems unlikely that the interaction between Slack and FMRP serves purely as a mechanism to regulate KNa current amplitude. A variety of recent studies have suggested that the gating of ion channels, triggered by depolarization or neuronal activity, may instigate cytoplasmic signaling cascades that could alter gene transcription or other biochemical events (An et al., 2000; Gomez-Ospina et al., 2006; Hegle et al., 2006; Kaczmarek, 2006). Thus, it is possible that activation of Slack channels by Na+ elevations during neuronal firing alters the ability of FMRP to participate in one or more of its functions, such as regulation of translation of its cargo mRNAs, although there is at present no direct evidence for this. FMRP is known to be required for activity-dependent increases in protein translation, most likely by suppressing translation of its cargo mRNAs until neurons become active (Li et al., 2009; Schütt et al., 2009).
A “nonconducting” function of this sort for Slack channels is consistent with the finding that suppression of Slack channels prevents recovery of BC neurons from a prolonged inhibited state that normally follow the characteristic ∼30 min discharge of these cells and that this recovery requires the synthesis of new proteins. Thus, Slack channels could comprise part of a pathway that links neuronal firing to activity-driven protein synthesis. The strongest activation of KNa channels occurs during periods of repetitive neuronal activity, during which time intracellular Na+ levels are elevated (Yang et al., 2007; Nanou et al., 2008). Moreover, it has been found that activity-dependent synthesis of a neuropeptide precursor during the discharge of BC neurons is abolished by transient removal of external Na+ ions at the onset of the discharge (Wayne et al., 2004).
While the hypothesis that Slack channel activity modulates the ability of FMRP to liberate mRNAs for translation is an attractive one, our present findings do not eliminate a variety of alternative hypotheses. For example, although suppression of Slack channels does not prevent discharges or entry into the inhibited period, changes in action potentials or resting membrane potential produced by decreased Slack levels could indirectly alter intracellular events such as calcium transients. These could, in turn, alter cell metabolism or rates of protein synthesis even if Slack channels function solely to regulate the intrinsic electrical properties of neurons.
Our findings have demonstrated that the interaction of Slack with FMRP is one that is conserved across species. Moreover, they raise the possibility that it serves to link changes in neuronal firing to changes in the translation of FMRP-associated mRNAs. In addition to the recovery from the inhibited period of BC neurons, a variety of other long-lasting changes in the excitability of neurons and in their ability to release neurotransmitter require new protein synthesis (Wang et al., 2009; Jin et al., 2011). Many such changes underlie learning, memory, and alterations in animal behavior. Definitive tests of the role of Slack channels in such events will require identification of the proteins whose synthesis is required for these prolonged changes in excitability.
Footnotes
This work was supported by National Institutes of Health Grants NS18492 and HD067517 and FRAXA (L.K.K.).
The authors declare no competing financial interests.
References
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317:540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P, Castets M, Pognonec P, Khandjian EW, Moine H, Bardoni B. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 2009;7:e16. doi: 10.1371/journal.pbio.1000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kronengold J, Yan Y, Gazula VR, Brown MR, Ma L, Ferreira G, Yang Y, Bhattacharjee A, Sigworth FJ, Salkoff L, Kaczmarek LK. The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels. J Neurosci. 2009;29:5654–5665. doi: 10.1523/JNEUROSCI.5978-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Kaczmarek LK. The bag cell neurons of Aplysia. A model for the study of the molecular mechanisms involved in the control of prolonged animal behaviors. Mol Neurobiol. 1989;3:237–273. doi: 10.1007/BF02740607. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19:903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Fähling M, Mrowka R, Steege A, Kirschner KM, Benko E, Förstera B, Persson PB, Thiele BJ, Meier JC, Scholz H. Translational regulation of the human achaete-scute homologue-1 by fragile X mental retardation protein. J Biol Chem. 2009;284:4255–4266. doi: 10.1074/jbc.M807354200. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel CaV1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage TA, Salkoff L. Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J Neurosci. 2012;32:2714–2721. doi: 10.1523/JNEUROSCI.5088-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc Natl Acad Sci U S A. 2006;103:2886–2891. doi: 10.1073/pnas.0505909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Magoski NS. Activity-dependent initiation of a prolonged depolarization in Aplysia bag cell neurons: role for a cation channel. J Neurophysiol. 2007;97:2465–2479. doi: 10.1152/jn.00941.2006. [DOI] [PubMed] [Google Scholar]
- Jin I, Kandel ER, Hawkins RD. Whereas short-term facilitation is presynaptic, intermediate-term facilitation involves both presynaptic and postsynaptic protein kinases and protein synthesis. Learn Mem. 2011;18:96–102. doi: 10.1101/lm.1949711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, Kaczmarek LK. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat Neurosci. 1998;1:462–469. doi: 10.1038/2176. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Khatri L, Ziff EB. Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons. Nat Neurosci. 2007;10:427–435. doi: 10.1038/nn1867. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci. 2006;7:761–771. doi: 10.1038/nrn1988. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings K, Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci U S A. 1978;75:5200–5204. doi: 10.1073/pnas.75.10.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings KR, Strumwasser F, Nairn AC, Walter U, Wilson FD, Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980;77:7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Kaczmarek LK. Peptidergic neurons of Aplysia lose their response to cyclic adenosine 3′:5′-monophosphate during a prolonged refractory period. J Neurosci. 1985;5:1339–1345. doi: 10.1523/JNEUROSCI.05-05-01339.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RJ, Jonas EA, Kao LS, Smith PJ, Connor JA, Kaczmarek LK. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J Physiol. 1996;494:627–639. doi: 10.1113/jphysiol.1996.sp021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneev SA, Kemenes I, Straub V, Staras K, Korneeva EI, Kemenes G, Benjamin PR, O'Shea M. Suppression of nitric oxide (NO)-dependent behavior by double-stranded RNA-mediated silencing of a neuronal NO synthase gene. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-11-j0003.2002. (1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Kandel ER. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol. 1970;33:865–876. doi: 10.1152/jn.1970.33.6.865. [DOI] [PubMed] [Google Scholar]
- Lee JA, Kim HK, Kim KH, Han JH, Lee YS, Lim CS, Chang DJ, Kubo T, Kaang BK. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bassell GJ, Sasaki Y. Fragile X mental retardation protein is involved in protein synthesis-dependent collapse of growth cones induced by Semaphorin-3A. Front Neural Circuits. 2009;3:11. doi: 10.3389/neuro.04.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Das P, Fadool DA, Kaczmarek LK. The slack sodium-activated potassium channel provides a major outward current in olfactory neurons of Kv1.3−/− super-smeller mice. J Neurophysiol. 2010;103:3311–3319. doi: 10.1152/jn.00607.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoski NS, Wilson GF, Kaczmarek LK. Protein kinase modulation of a neuronal cation channel requires protein-protein interactions mediated by an Src homology 3 domain. J Neurosci. 2002;22:1–9. doi: 10.1523/JNEUROSCI.22-01-00001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, et al. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Kyriakatos A, Bhattacharjee A, Kaczmarek LK, Paratcha G, El Manira A. Na+-mediated coupling between AMPA receptors and KNa channels shapes synaptic transmission. Proc Natl Acad Sci U S A. 2008;105:20941–20946. doi: 10.1073/pnas.0806403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganoni S, Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118:433–446. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki EA, Marshall J, Kaczmarek LK. A Shab potassium channel contributes to action potential broadening in peptidergic neurons. Neuron. 1994;12:73–86. doi: 10.1016/0896-6273(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Ramos A, Hollingworth D, Adinolfi S, Castets M, Kelly G, Frenkiel TA, Bardoni B, Pastore A. The structure of the N-terminal domain of the fragile X mental retardation protein: a platform for protein-protein interaction. Structure. 2006;14:21–31. doi: 10.1016/j.str.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, Salkoff L. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci. 2006;26:5059–5068. doi: 10.1523/JNEUROSCI.3372-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284:25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P, Robertson B, Cangiano L, Löw P, Bhattacharjee A, Kaczmarek LK, Grillner S. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J Physiol. 2007;585:75–90. doi: 10.1113/jphysiol.2007.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, Martin KC. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324:1536–1540. doi: 10.1126/science.1173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne NL, Lee W, Michel S, De Quintana SB. Post-afterdischarge depolarization does not stimulate prolonged neurohormone secretion but is required for activity-dependent stimulation of neurohormone biosynthesis from peptidergic neurons. Endocrinology. 2004;145:1678–1684. doi: 10.1210/en.2003-1447. [DOI] [PubMed] [Google Scholar]
- White BH, Nick TA, Carew TJ, Kaczmarek LK. Protein kinase C regulates a vesicular class of calcium channels in the bag cell neurons of Aplysia. J Neurophysiol. 1998;80:2514–2520. doi: 10.1152/jn.1998.80.5.2514. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, van Unen L, Tassone F, Hoogeveen AT, Hagerman PJ, Mientjes EJ, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- Wilson GF, Kaczmarek LK. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993;366:433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]
- Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Yang B, Desai R, Kaczmarek LK. Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Helm JS, Senatore A, Spafford JD, Kaczmarek LK, Jonas EA. PKC-induced intracellular trafficking of CaV2 precedes its rapid recruitment to the plasma membrane. J Neurosci. 2008a;28:2601–2612. doi: 10.1523/JNEUROSCI.4314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McKay SE, Bewley B, Kaczmarek LK. Repetitive firing triggers clustering of Kv2.1 potassium channels in Aplysia neurons. J Biol Chem. 2008b;283:10632–10641. doi: 10.1074/jbc.M800253200. [DOI] [PubMed] [Google Scholar]