Abstract
Tumor-associated antigens are weakly immunogenic. Human carcinoembryonic antigen (CEA) is overexpressed on a wide range of human carcinomas and represents an attractive target for cancer immunotherapy. This study analyzes the ability of a Saccharomyces cerevisiae vector containing the transgene encoding CEA (yeast-CEA) to activate human dendritic cells (DCs) and stimulate CEA-specific T-cell responses. We demonstrate for the first time that treatment with yeast-CEA can activate human DCs, resulting in increases in surface expression of CD80, CD83, CD54, CD58, and MHC class II, and increased production by DCs of IL-12p70, TNF-α, IFN-γ, IL-8, IL-2, IL-13, IL-10, and IL-1β. We also show that human DCs treated with yeast-CEA can activate CEA-specific T-cell lines and can act as antigen-presenting cells (APCs) to generate CEA-specific T-cell lines capable of lysing CEA+ human tumor cells. Gene expression profiles of human DCs treated with yeast-CEA show increased expression of numerous genes involved in the production of chemokines and cytokines and their receptors, and genes related to antigen uptake, antigen presentation, and signal transduction.
Keywords: Saccharomyces cerevisiae, yeast-CEA, human dendritic cell
1. Introduction
Tumor-associated antigens (TAAs) are weakly immunogenic or nonimmunogenic in the tumor-bearing host. Various strategies are being investigated to generate dendritic cells (DCs)—the most potent antigen-presenting cells (APCs) [1]—and enhance their ability to activate tumor antigen-specific T cells. T-cell activation is dependent on at least 2 signals provided by APCs [2,3]. The first signal is antigen-specific and is mediated via the T-cell receptor (TCR) by a peptide/MHC complex on the APC. At least one more signal is required for T-cell activation and proliferation and subsequent production of cytokines. This second signal is mediated by the interaction of costimulatory molecules such as CD80, CD54, and CD58 expressed on APCs and their corresponding ligands on the surfaces of T cells. These costimulatory molecules work by activating signal transduction pathways [4,5].
In preclinical and clinical studies, liposomal plasmid DNA [6,7], adenoviruses [8], poxvirus vectors [9–13], and retroviruses [14,15] have been used to infect or transfect human DCs to present a variety of antigens. Stubbs et al. demonstrated that antigens can be engineered for heterologous expression in Saccharomyces cerevisiae yeast by cloning the gene encoding the target antigen into a yeast-expression plasmid, under the control of an inducible or constitutive promoter [16]. In this process, the recombinant DNA plasmid is transfected into S. cerevisiae. Whole recombinant yeasts are produced by culturing yeast cells and inducing expression of heterologous target antigens, then heat-inactivating the cultured cells [16]. Preclinical studies have used yeast vectors encoding antigens such as chicken ovalbumin, HIV Gag, HCV NS3-Core, and mutant Ras [16–18].
It has been demonstrated that whole recombinant S. cerevisiae vaccine can activate human DCs and induce phenotypic maturation of human DCs derived from monocytes, and increase surface expression of CD80, CD86, CD40, CD54, and MHC class II [17]. Yeast-treated human DCs produce inflammatory cytokines such as IL-12 and TNF-α and stimulate strong alloreactive T-cell proliferation [17]. Human monocyte-derived DCs have been shown to internalize recombinant yeast expressing HIV-1 Gag protein and stimulate Gag-specific CD8+ cells in vitro [17]. Saccharomyces cerevisiae expressing full-length influenza matrix protein has been shown to be a versatile vehicle for presenting antigen to human DCs, which are able to uptake, process, and cross-present influenza matrix protein-derived peptides [19]. DCs recognize S. cerevisiae and Candida albicans through dectin, mannose-fucose receptors and Toll-like receptors (TLRs) such as TLR-2 and TLR-4, and induce immunostimulation through cell-surface molecules such as glucan and mannan, that act like pathogen-associated molecular patterns (PAMPs) [20]. The extracellular domain of human TLR-4 is associated with the second protein, MD2, which is required for optimal lipopolysaccharide (LPS)-induced signaling [21]. Human TLR-2 and TLR-4 recruit and activate IL-1 receptor-associated kinase in response to a variety of PAMPs, resulting in downstream activation of NFκB and c-Jun NH2-terminal kinase and secretion of IL-8 and IL-12 [22,23]. In sum, S. cerevisiae is a promising vector for delivering antigens for immunotherapy because it is nonpathogenic and is able to activate DCs and stimulate immune responses.
Human carcinoembryonic antigen (CEA) is overexpressed on a wide range of human carcinomas, including GI, lung, breast, and prostate carcinoma, and thus represents an attractive target for human active immunotherapy. T-cell epitopes of human CEA have previously been identified, and subsequent studies have identified a T-cell enhancer agonist epitope (designated CAP1-6D) of the native CEA epitope CAP-1 [24,25]. We report here the analysis of S. cerevisiae vectors containing the CEA transgene, encoding either the native peptide (yeast-CEA) or the agonist epitope (yeast-CEA-6D). We demonstrate that (a) yeast-CEA can be used to activate human DCs and thus increase expression of chemokines and cytokines and their receptors, and gene expression profiles showed upregulation of genes related to antigen uptake, antigen presentation, and signal transduction, (b) human DCs treated with yeast-CEA can activate CEA-specific T-cell lines, and (c) human DCs treated with yeast-CEA or with yeast-CEA-6D can function as APCs to generate CEA-specific T-cell lines capable of lysing CEA+ human tumor cells.
2. Materials and methods
2.1. Cell cultures
The human pancreatic cancer cell lines CF-PAC-1 (HLA-A2+/CEA+) and AsPC-1 (HLA-A2−/CEA+) were purchased from American Type Culture Collection (Manassas, VA). These cell lines were mycoplasma-free and maintained in complete medium consisting of RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies). The V8T T-cell line, a CD8+ cytotoxic T lymphocyte (CTL) line directed against the CEA CAP-1 epitope [26], was established from a patient with metastatic colon cancer who had received a CEA-based vaccine. The T-VLG-CEA cell line, a CD8+ CTL line directed against the CEA CAP1-6D epitope, was generated from peripheral blood mononuclear cells (PBMCs) from a patient with colon cancer vaccinated with a CEA-based vaccine [27]. The V8T and T-VLG-CEA cell lines were cultured as previously described [28].
2.2. Peptides
This study employed CAP1-6D (YLSGADLNL) (American Peptide Company, Sunnyvale, CA) [24], an HLA-A2-binding CEA agonist peptide that was > 90% pure.
2.3. Yeast vectors
This study used 2 recombinant S. cerevisiae constructs—yeast-CEA (GI-6206) and yeast-CEA-6D (GI-6207) [26]—and an antigen-free S. cerevisiae construct (control yeast). All were engineered as previously described (GlobeImmune, Louisville, CO) [16].
2.4. Culture of DCs from PBMCs
HLA-A2 PBMCs were obtained from heparinized blood from a healthy donor. PBMCs were separated by lymphocyte separation medium gradient (MP Biomedicals, Aurora, OH) according to the manufacturer’s instructions. DCs were prepared using a modification of the procedure described by Sallusto et al. [29]. PBMCs (1.5 × 108) were resuspended in AIM-V medium containing 2 mM glutamine, 50 μg/ml streptomycin, and 10 μg/ml gentamycin (Invitrogen Life Technologies) and allowed to adhere in a 6-well plate. After 2 h at 37°C, nonadherent cells were removed with a gentle rinse. Adherent cells were cultured for 6 to 7 days in AIM-V medium containing 100 ng/ml of recombinant human (rh) GM-CSF and 20 ng/ml of rhIL-4. The culture medium was replenished every 3 days. For maturing DCs, 1 μg/ml CD40L (Axxora, San Diego, CA) was added to the culture 24 h prior to harvesting.
2.5. Tetramer staining
Streptavidin-phycoerythrin (PE)-labeled CEA-tetramer (YLSGADLNL-tetramer) was obtained from Beckman Coulter (Fullerton, CA). PBMCs (1 × 106) were stained with 10 μl of tetramer and anti-CD8-FITC antibody (BD Biosciences, San Jose, CA) for 30 min at room temperature in the dark, washed twice with FACS buffer, then fixed in PBS with 0.5% formaldehyde. Cells were analyzed using a FACScan (Becton-Dickinson, Franklin Lakes, NJ) and CellQuest software (BD Biosciences). Results were generated from data gathered from 100,000 cells.
2.6. Flow cytometric analysis
DCs were analyzed by dual-color flow cytometric analysis using the following antibody combinations: anti-class I FITC/anti-CD80 PE; anti-class I FITC/anti-CD83 PE; anti-class I FITC/anti-class II PE; anti-class I FITC/anti-CD58 PE; anti-class I FITC/anti-CD54 PE; and anti-IgG1-FITC/anti-IgG2a-PE (isotype controls). Antibody to MHC class II was purchased from Serotec (Oxford, UK); other antibodies were purchased from BD Biosciences. Cells were stained simultaneously for 1 h at 4°C, then washed three times with cold Ca2+- and Mg2+-free PBS, resuspended in the same buffer, and immediately analyzed using a FACScan (Becton-Dickinson) and CellQuest software (BD Biosciences). Results were generated from data gathered from 10,000 cells and expressed as %-positive cells and mean fluorescence intensity (MFI). The MFI value was collected in log scale and used to express levels of fluorescence determined by measuring the average for all cells in the fluorescence dot plot.
2.7. Intracellular staining for IFN-γ
Intracellular cytokine flow cytometry assays were done following the method described by Maecker et al. [30]. Briefly, V8T cells were stimulated with DCs treated with yeast-CEA, yeast-CEA-6D, and control yeast for 2 hours. Monensin (BioLegend, San Diego, CA) was added at 1X concentration and incubated for an additional 4 hours at 37°C. Cells were then harvested and stained for IFN-γ–FITC/CD69-PE/CD3PerCP/CD8-APC with a BD FasImmune CD8 intracellular cytokine detection kit (BD Biosciences). Samples were analyzed in an LSR II with FACSDiVa software (BD Biosciences). Results were expressed as percentage of CD3+/CD8+/CD69+ T cells that were IFN-γ+
2.8. Generation of T-cell lines
CEA-specific CTLs were generated by a modification of the protocol described by Tsang et al. [28]. The T-JMB-CEA T-cell line was generated from a breast carcinoma patient vaccinated with a CEA-based vaccine [27]. Immature autologous DCs generated as previously described were exposed to yeast-CEA (GI-6202) at a DC:yeast-CEA ratio of 1:5 for 48 h and used as APCs. Autologous nonadherent cells were added to the APCs at an effector:APC ratio of 10:1. Cultures were incubated for 3 days at 37°C in a humidified atmosphere containing 5% CO2, then supplemented with 20 units/ml rhIL-2 for 7 days. The IL-2-containing medium was replenished every 3 days. The 3-day incubation and 7-day supplementation together constituted one in vitro stimulation (IVS) cycle. The second IVS cycle began on day 11, when T-JMB-CEA cells were restimulated with yeast-CEA-treated autologous DCs as APCs. After a third IVS cycle, irradiated (23,000 rads) autologous EBV-transformed B cells were used as APCs. The EBV-transformed B cells were pulsed with 10 μg/ml of CEA peptide and used for restimulation at an effector:APC ratio of 1:3. Cultures were then incubated for 3 days at 37°C in a humidified atmosphere containing 5% CO2. The peptide-containing medium was removed and cultures were supplemented with 20 units/ml rhIL-2 for 7 days.
2.9. Cytotoxic assay
Targeted tumor cells were labeled with 50 μCi of 111Indium-labeled oxyquinoline (Medi-Physics Inc., Arlington, IL) for 15 min at room temperature. Target cells (0.3 × 104) in 100 μl of RPMI-1640 complete medium were added to 96-well flat-bottomed assay plates. Effector cells suspended in 100 μl of RPMI-1640 complete medium supplemented with 10% pooled human AB serum were added to the target cells. The plates were then incubated at 37°C in 5% CO2 for 16 h. Supernatant was harvested for gamma counting using harvester frames (Skatron, Inc., Sterling, VA). Determinations were carried out in triplicate and SDs were calculated. Specific lysis was calculated by the following formula (all values in cpm):
Spontaneous release was determined from wells to which 100 μl of RPMI-1640 complete medium was added. Total releasable radioactivity was obtained after target cells were treated with 2.5% Triton x-100.
2.10. Detection of cytokines
Supernatants were collected 48 h after DCs were exposed to yeast-CEA and kept at −20°C until analyzed. All samples were screened for secretion of IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, IL-13, and TNF-α using a multiplex cytokine/chemokine kit (Meso Scale Discovery, Gaithersburg, MD). IL-12p70, TNF-α, CXCL-10, and CXCL-11 were also analyzed by ELISA kit (BD Biosciences). Supernatants of T cells stimulated for 24 h with peptide-pulsed or yeast-CEA-treated autologous DCs in IL-2-free medium were screened for secretion of IFN-γ by ELISA kit (BioSource International, Camarillo, CA).
2.11. RNA isolation
Total RNA from DCs was isolated using an RNeasy extraction kit (Qiagen Inc., Valencia, CA). RNA concentrations were determined by A260 nm reading, and RNA quality was evaluated by the ratio A260 nm/280 nm. Samples were stored at −80°C.
2.12. Microarray analysis
Gene expression of DCs was analyzed pre- and post-treatment with yeast-CEA by the Oligo GEArray® Human Dendritic and Antigen Presenting Cell Microarray (SuperArray Bioscience Corp., Frederick, MD). This microarray profiles expression of 260 genes, including those involved in DC activation and maturation such as chemokines and cytokines and their receptors, other cell-surface receptors, signal transduction molecules, and genes involved in antigen uptake, processing, and presentation. Hybridization procedures were conducted according to the manufacturer’s instructions. Upregulation or downregulation of gene expression was calculated as a ≥ 3.0-fold or ≤–3.0-fold change, respectively, after normalization of intensity. Experiments were repeated three times.
2.13. Statistical analysis
Statistical significance was calculated using a 2-tailed paired Student’s t test and StatView software (Abacus Concepts, Berkeley, CA).
3. Results
Studies were first carried out to evaluate the effect of yeast-CEA on human DCs by analyzing expression of the cell-surface markers CD80, CD83, CD54, CD58, MHC class I and class II. Human DCs were treated at DC:yeast-CEA ratios of 1:1, 1:5, and 1:10. After 48 h of incubation, yeast-CEA-treated DCs showed a substantial increase in the percentage of DCs expressing CD80, CD83, CD54, and CD58 at all ratios compared to untreated DCs. MFI levels of all 6 markers analyzed were also increased compared to untreated DCs. Treatment of DCs with yeast-CEA also resulted in enhanced production of IL-12p70 and TNF-α. Higher levels of IL-12p70 and TNF-α were produced at DC:yeast-CEA ratios of 1:5 and 1:10, compared to the ratio of 1:1 and untreated DCs. Subsequent studies were conducted at a ratio of 1:10; analyses of three different donors showed a range of cytokine production. For example, TNF-α production ranged from 498 pg/ml to > 10,000 pg/ml among different donors.
Studies were then undertaken to compare the effect on DC maturation following treatment with yeast-CEA vs. CD40L (Tables 1A and B). Treatment of immature DCs with control yeast, yeast-CEA, and CD40L led to substantial increases in the percentage of cells positive for CD80, CD83, CD54, CD58 and moderately enhanced levels of expression of MHC class I and class II in the percent-positive cells and/or MFI compared to untreated DCs (Table 1A). The MFI of CD54 and CD58 are minimally enhanced by treatment with control yeast and yeast-CEA. These two molecules are significantly enhanced by treatment with CD40L in MFI.
Table 1.
Phenotypic and functional analysis of the effect of recombinant yeast on human DCs.
| A. Phenotypic analysis of surface markers
| ||||||
|---|---|---|---|---|---|---|
| Treatment of DCs | CD80 | CD83 | CD54 | CD58 | Class I | Class II |
| Untreated | 18.1 (57.7) | 38.0 (85.6) | 88.3 (115.6) | 86.4 (98.1) | 98.9 (627.4) | 82.3 (558.4) |
| CD40L | 80.0 (47.8) | 86.5 (51.8) | 99.9 (434.7) | 99.9 (278.7) | 99.8 (1,403.8) | 99.9 (434.7) |
| Control yeast | 83.2 (36.8) | 81.4 (51.7) | 99.9 (155.0) | 99.3 (106.0) | 99.9 (1,232.7) | 92.7 (370.0) |
| Yeast-CEA | 74.3 (25.5) | 77.0 (42.5) | 99.9 (144.5) | 98.9 (94.1) | 99.5 (1,214.4) | 96.4 (270.9) |
| B. Production of cytokines and chemokines
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment of DCs | IL-12p70 | TNF-α | IFN-γ | IL-8 | IL-2 | IL-4 | IL-13 | IL-5 | IL-10 | IL-1β |
| Untreated | 0 | 1 | 0 | 44 | 2 | 177 | 11 | 0 | 0 | 0 |
| CD40L | 693 | 8,969 | 735 | 526 | 136 | 51 | 233 | 24 | 113 | 21 |
| Control yeast | 3,503 | >10,000 | 5,349 | 5,827 | 375 | 70 | 72 | 4 | 85 | 49 |
| Yeast-CEA | 5,914 | >10,000 | 6,718 | 7,148 | 773 | 42 | 131 | 6 | 141 | 79 |
Human DCs cultured for 48 h with media only (untreated), stimulated with CD40L (1 μg/ml), or treated with control yeast or yeast-CEA (DC:yeast ratio = 1:10). A, DCs were harvested and analyzed by flow cytometry for surface-marker expression; results are shown in %-positive cells and MFI (parentheses). B, Cultured supernatants were collected and screened for cytokine and chemokine production by multiplex cytokine/chemokine kit. Results are expressed in pg/ml.
Levels of production of cytokines and chemokines by immature DCs treated with control yeast and yeast-CEA were compared to levels in DCs matured with CD40L. Production of IL-12p70, TNF-α, IFN-γ, IL-8, and IL-2 was enhanced in DCs treated with control yeast and yeast-CEA compared to DCs matured with CD40L and untreated DCs (Table 1B). These studies were repeated three times using DCs from three healthy donors, with similar results.
DCs matured with control yeast or CD40L were evaluated for their ability to activate CEA-specific T-cell responses when pulsed with CEA peptide (CAP1-6D). T-cell activation was monitored by IFN-γ production after 24 h of stimulation. As shown in Table 2, activation of the CEA-specific T-cell line (V8T) was seen when CAP1-6D peptide-pulsed yeast-treated DCs were used. A similar level of V8T activation with respect to production of IFN-γ was seen when CAP1-6D peptide-pulsed CD40L-treated DCs were used.
Table 2.
Specific release of IFN-γ by CEA-specific T cells stimulated by DCs treated with CEA peptide, CD40L, and/or control yeast.
| DCs | Treatment | CEA-6D peptide | CEA-specific T cells | IFN-γ |
|---|---|---|---|---|
| − | − | − | + | <15.6 |
| + | − | − | − | <15.6 |
| + | − | − | + | <15.6 |
| + | − | + | + | 47.4 |
| + | CD40L | − | − | <15.6 |
| + | CD40L | − | + | <15.6 |
| + | CD40L | + | + | 535.5 |
| + | Control yeast | − | − | <15.6 |
| + | Control yeast | − | + | <15.6 |
| + | Control yeast | + | + | 534.3 |
DCs were generated from PBMCs of healthy HLA-A2+ donors and treated at a DC:yeast ratio of 1:5.T cell:APC ratio was 10:1. DCs treated with CD40L (1 μg/ml) were cultured for 24 h, then pulsed with CEA-6D peptide at 10 μg/ml. DCs treated with control yeast were cultured for 48 h, then pulsed with CEA-6D peptide at 10 μg/ml. CEA-specific V8T cells were stimulated with peptide-pulsed or unpulsed DCs. Supernatants were harvested after 24 h and screened for production of IFN-γ by ELISA. Results are expressed in pg/ml.
Studies were then undertaken to examine the ability of yeast-CEA treated DCs to process endogenous CEA to activate CEA-specific T cells. DCs treated with yeast-CEA or yeast-CEA-6D (CEA with the agonist epitope) were used as APCs to stimulate a CEA-specific T-cell line. While DCs treated with both yeast-CEA and yeast-CEA-6D were able to activate these T cells, DCs treated with yeast-CEA-6D induced a higher level of IFN-γ than DCs treated with yeast-CEA (Table 3A). Intracellular cytokine staining was performed using a CEA-specific T-cell line (V8T). Results showed that a greater number of the T cells were CD3+/CD8+/CD69+/IFN-γ+ when T cells were stimulated with DCs treated with yeast-CEA-6D, compared to when T cells were stimulated with DCs treated with yeast-CEA. No CD3+/CD8+/CD69+/IFN-γ+ T cells were detected when T cells were stimulated with DCs treated with control yeast (Table 3B).
Table 3.
Enhanced activation of CEA-specific T cells by recombinant yeast expressing an enhancer agonist CEA epitope.
| A. Specific release of IFN-γ by CEA-specific T cells stimulated by human DCs treated with yeast-CEA and yeast-CEA-6D (agonist).
| |||
|---|---|---|---|
| DCs | Treatment | CEA-specific T cells | IFN-γ |
| − | − | + | <15.6 |
| + | Yeast-CEA | − | <15.6 |
| + | Yeast-CEA-6D | − | <15.6 |
| + | Yeast-CEA | + | 508.7 |
| + | Yeast-CEA-6D | + | 1,501.8 |
| B. Intracellular cytokine staining for IFN-γ of V8T cells stimulated with human DCs treated with yeast-CEA and yeast-CEA-6D (agonist).
| |
|---|---|
| Treatment | CD3+/CD8+/CD69+/IFN-γ+ |
| Control yeast | 0 |
| Yeast-CEA | 14.0 |
| Yeast-CEA-6D | 20.2 |
DCs were generated from PBMCs of healthy HLA-A2+ donors and treated at a DC:yeast ratio of 1:5.T cell:APC ratio was 10:1. DCs were treated with yeast-CEA or yeast-CEA-6D and cultured for 48 h, then used to stimulate a CEA-specific T-VLG cell line. Supernatants were harvested after 24 h and screened for production of IFN-γ by ELISA. Results are expressed in pg/ml.
Results are expressed in % of CD3+/CD8+/CD69+/IFN-γ+ T cells of CD8 cells. DCs were generated from PBMCs of healthy HLA-A2+ donor and treated at a DC:yeast ratio of 1:5. T cell:APC ratio was 10:1. DCs were treated with yeast-CEA or yeast-CEA-6D and cultured for 48 h, and then used to stimulate a CEA-specific V8T cell line. Intracellular cytokine staining was performed on the stimulated V8T cells (see Materials and Methods).
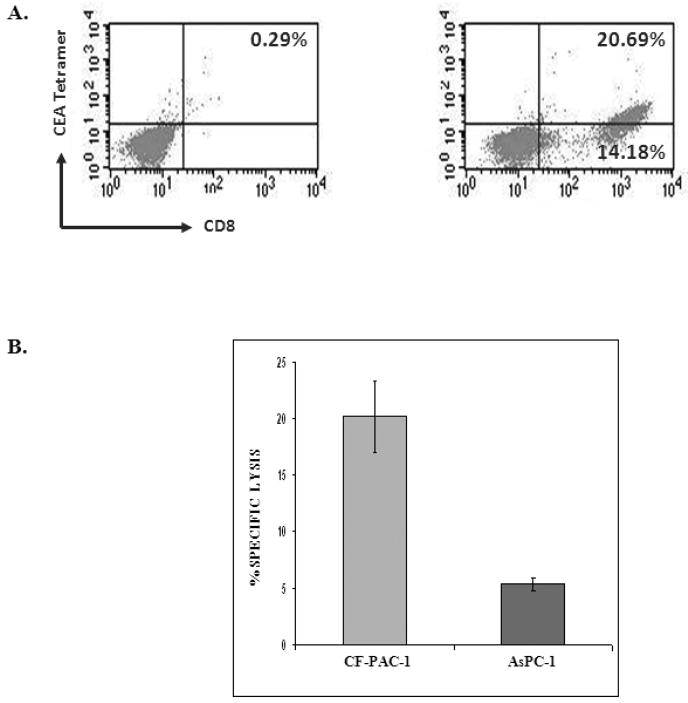
Studies were then undertaken to determine whether DCs treated with yeast-CEA could be used to actually establish a CEA-specific T-cell line (T-JMB-CEA) in vitro. PBMCs of a breast carcinoma patient vaccinated with a CEA-based vaccine [27] were used in the study. As shown in Figure 1A, T cells generated from the breast cancer patient using autologous DCs treated with yeast-CEA as APCs were CEA-specific, as determined by their ability to bind MHC-CEA-tetramer. It is uncertain whether the CEA-specific T cells were generated by precursors from the immunized patient that were amplified by IVS, or by in vitro stimulation by yeast-CEA treated DCs, or both. A total of 59.3% of CD8+ cells were MHC-CEA-tetramerpositive; the isotype control was negative (0.29%) (Fig 1A). These tetramer+ T cells were isolated by cell sorting and shown to be capable of lysing an HLA-A2+/CEA+ pancreatic cancer cell line (CF-PAC1), but not the HLA-A2−/CEA+ pancreatic cancer cell line AsPC-1, in an MHC-restricted manner (Fig. 1B).
Figure 1.
Ability of an established T-cell line (T-JMB-CEA) to lyse CEA-expressing tumor cells. DCs obtained from a patient with breast carcinoma were treated with yeast-CEA at a DC:yeast-CEA ratio of 1:5. (A) After IVS-3 using yeast-CEA-treated DCs as APCs, cells were analyzed by flow cytometry for their ability to bind CEA-tetramer. The panel at left is the isotype control. (B) A 16-h 111Indium release assay was performed using CD8+/CEA-tetramer+ cells, with CF-PAC1 (HLA-A2+/CEA+) and AsPC-1 (HLA-A2−/CEA+) pancreatic carcinoma tumor cell lines as targets. Results are expressed as percentage of specific lysis at an E:T ratio of 25:1. The % of lysis of CF-PAC1 cells was significantly higher than the lysis of AsPC-1 cells (P < 0.01, paired t test).
To examine the underlying effects of yeast-CEA on human DCs, in addition to the phenotypic and functional analysis described above, the gene profile of yeast-CEA-treated DCs was analyzed by a cDNA oligoarray for human DCs and APCs. RNA was isolated from DCs after 8 h and 24 h of incubation with yeast-CEA and compared to RNA isolated from untreated DCs. Expression of 260 genes involved in DC activation and maturation was analyzed as described in Materials and methods. As shown in Table 4, 27 genes were upregulated ≥ 3-fold after 8 h of incubation with yeast-CEA, and 35 genes were upregulated after 24 h of incubation. After the same periods of incubation with yeast-CEA, 68 genes (8 h) and 32 genes (24 h) respectively were downregulated < 1-fold (Table 4). Further analysis revealed that genes with the most significant involvement in DC activation and maturation had the strongest and most rapid upregulation (Table 4). IL-12β (cytokines group) was greatly upregulated after both 8 h (162.8-fold) and 24 h (65.5-fold), ISG20 (signal transduction group) was upregulated 96.7-fold, and CXCL10 and CXCL11 (chemokines/receptors group) were upregulated > 100-fold after treatment with yeast-CEA (Table 4).
Table 4.
Gene profile and expression of human DCs treated with yeast-CEA at 8 h and 24 h.
| Functional gene group | Fold increase*
|
||
|---|---|---|---|
| 8 h | 24 h | ||
Chemokines/receptors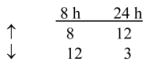
|
CCL2 | 8.5 | 41.9 |
| CCL20 | 4.8 | 8.1 | |
| CCL3L1 | / | 6.9 | |
| CCL4 | / | 32.3 | |
| CCL5 | 12.9 | 77.1 | |
| CCL8 | 19.3 | 51.9 | |
| CCR7 | / | 12.9 | |
| CXCL1 | 6.9 | / | |
| CXCL10 | 59.6 | 276.6 | |
| CXCL11 | 51.5 | 431.3 | |
| CXCL2 | / | 3.5 | |
| CXCL9 | 4.7 | 6.5 | |
| IL8 | / | 17.2 | |
|
| |||
Cytokines/receptors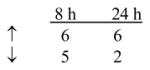
|
EBI3 | / | 3.2 |
| IFNB1 | 7.9 | / | |
| IL12A | / | 4.3 | |
| IL12B | 162.8 | 65.5 | |
| IL23A | / | 8.5 | |
| IL27 | 13.5 | / | |
| IL29 | 3.5 | / | |
| IL6 | 47.1 | 10.5 | |
| TNF | 29.9 | 17.3 | |
|
| |||
Antigen uptake
|
MARCKS | 4.0 | 10.8 |
| PNRC1 | / | 4.9 | |
| SOD2 | / | 10.9 | |
| TAP1 | 8.3 | / | |
|
| |||
Antigen presentation
|
CD80 | / | 3.3 |
| LAMP3 | 5.0 | 5.8 | |
|
| |||
Cell-surface receptors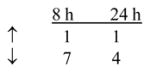
|
CD40 | 3.7 | / |
| LY75 | / | 5.1 | |
|
| |||
Signal transduction
|
BTG1 | / | 5.0 |
| GBP2 | / | 3.9 | |
| GBP3 | / | 3.5 | |
| IFI6 | 8.4 | 5.8 | |
| IFI16 | / | 3.5 | |
| IFI35 | 1.7 | 5.0 | |
| IFI44 | 6 | 5.6 | |
| IFIT1 | 9.8 | 22.5 | |
| IFIT2 | 4.0 | / | |
| IFIT3 | 7.8 | 2.9 | |
| ISG15 | 8.6 | 10.3 | |
| ISG20 | 28.7 | 96.7 | |
| LTA | 5.2 | / | |
| NFKB1 | / | 5.2 | |
Human DCs were generated from PBMCs of healthy donors and cultured for 8 h and 24 h with yeast-CEA at a DC: yeast-CEA ratio of 1:5. RNA was isolated and analyzed by Oligo GEArrayR for human DCs and APC microarray.
Results represent ≥ 3-fold increases compared to untreated DCs.
/ = no change.
↑ = number of genes upregulated. Includes only those genes upregulated ≥ 3-fold.
↓ = number of genes downregulated. Includes all downregulated genes, none of which were downregulated ≥ 1-fold.
Total genes upregulated: 8 h = 27; 24 h = 35.
Total genes downregulated: 8 h = 68; 24 h = 32.
To confirm and validate the high levels of expression of CXCL-10 and CXCL-11 obtained by cDNA oligoarray, ELISA assay was performed to analyze the production of CXCL-10 and CXCL-11 by DCs treated with yeast-CEA. As shown in Table 5, the levels of CXCL-10 and CXCL-11 produced by the yeast-CEA-treated DCs were substantially increased compared to untreated DCs at both the 8-h and 24-h time points.
Table 5.
Production of CXCL-10 and CXCL-11 by DCs treated with yeast-CEA.
| CXCL-10 (pg/ml) | CXCL-11 (pg/ml) | |||
|---|---|---|---|---|
| s | 8 h | 24 h | 8 h | 24 h |
| Media/control | < 7.8 | 82.5 | < 62.5 | < 62.5 |
| Yeast-CEA | 322.5 | > 500 | 1,199.4 | 3,050.4 |
Human DCs were generated from PBMCs of healthy donors and cultured for 8 h and 24 h with yeast-CEA at a DC:yeast-CEA ratio of 1:5. Supernatant were harvested and screened for production of CXCL-10 and CXCL-11. Results are expressed in pg/ml.
4. Discussion
The studies reported here demonstrate for the first time that recombinant yeast-CEA can efficiently mature human DCs and enhance activation of human T cells. Treating DCs with control yeast and yeast-CEA leads to an increase in the level of CD80, CD83, CD54, CD58, and MHC class I and class II (Table 1A). While previous studies [17] demonstrated that treatment of DCs with yeast upregulated MHC, ICAM, CD40, and CD86 as measured by MFI, to our knowledge, this is the first study to report both an increase in percent-positive cells and/or an increase in MFI in human DCs treated with S. cerevisiae constructs. Maturation of DCs is characterized by upregulated expression of surface MHC and costimulatory molecules, and a change in cellular function from antigen uptake to internalization of antigen and antigen processing. The yeast cell wall is composed mainly of glucan, mannan, and chitin [31], which may be partially responsible for the ability of yeast to activate DCs. Like monocytes and macrophages, DCs express cell-surface molecule pattern recognition receptors such as TLRs and mannose receptors. It has been demonstrated that TLR-2 is recruited to phagosome-containing yeasts, and that activation of TLR-2 on human DCs results in the production of IL-12 [32].
In previous studies, yeast strains such as C. albicans and Malassezia furfur, as well as S. cerevisiae, have been shown to be phagocytosed, killed, processed for antigen presentation by human DCs, and used to induce maturation of human DCs [17,33–35]. In addition, data from previous studies from our laboratory have demonstrated that vaccination of CEA-transgenic mice with heat-killed recombinant yeast constructs expressing CEA induces CEA-specific immune responses in vivo, leading to a reduction in tumor burden and increased overall survival [36].
We show here that treating human DCs with yeast-CEA leads to increased production of cytokines and chemokines, especially IL-12p70, TNF-α, IL-8, and IFN-γ (Table 1B). These studies are in agreement with previous reports that the cellular and molecular responses of DCs to yeast such as C. albicans and S. cerevisiae include production of IL-12, synthesis/release of nitric oxide, induction of TNF-α, GM-CSF, IL-1β, CD54, and MHC class I and class II [16,37]. IL-12, a major Th-1-promoting factor, is essential to the generation of Th-1 cells from naïve precursors. IL-12 can promote IFN-γ production and enhance the proliferation and cytotoxicity of CTLs. IL-12 also induces an antiangiogenic program mediated by IFN-γ–inducible genes and by lymphocyte-endothelial cell cross-talk, and provides costimulatory and antiapoptotic signals that regulate the activity of effector- memory T cells [38,39]. The minimal IL-4 production by yeast-CEA-treated human DCs (Table 1B) further highlights the Th-1 response phenotype. TNF-α is a pleiotropic inflammatory cytokine with both growth-stimulating and growth-inhibiting processes. It can induce neutrophil proliferation and augment B-cell proliferation and differentiation. It can also act as a key medium in local inflammatory immune response. IL-8 is an inflammatory mediator secreted in response to various stimuli such as LPS, TNF-α, and IL-1β. IL-8 functions as a chemoattractant and is also a potent angiogenic factor. The earliest change in protein expression detected after DCs were stimulated with LPS or CpG was shown to be increased production of IL-8. Production of IL-8 by DCs after stimulation in the presence of CpG showed a kinetic pattern similar to the upregulation of their costimulatory molecules [40]. It has also been demonstrated that activation of human DCs with PAMPs induces production of IL-8, and that production of IL-8 precedes upregulation of the activation marker CD40 [41]. In short, the findings reported here suggest that yeast treatment can initiate a full range of human DC pro-inflammatory activation responses.
In this study, CEA-specific T-cell lines were used to evaluate specific T-cell responses, using allogeneic DCs treated with yeast-CEA. No T-cell activation was observed in the absence of peptide or in untreated DCs or DCs treated with control yeast, ruling out allospecific reactions in all experiments (Tables 2 and 3A). CEA-specific V8T cells were activated when DCs were treated with control yeast and pulsed with CAP1-6D peptide. Similar results were observed when CEA-specific T-VLG cells were activated with DCs treated with yeast-CEA; DCs treated with yeast-CEA-6D (containing the agonist epitope) produced an even higher level of activation, as indicated by increased IFN-γ production. This result accords with our previously reported findings that the agonist epitope produces a higher level of T-cell stimulation than native peptide [24,42,43]. The results reported here show that DCs treated with yeast-CEA-6D can indeed process that epitope and present it to T cells.
The experiments reported here on the use of a CEA-tetramer (MHC-CAP1-6D-tetramer) demonstrate the specificity of the CEA-specific T-cell line established from a breast carcinoma patient by using yeast-CEA-treated DCs as APCs. The CEA-specific T cells isolated by the CEA-tetramer were also able to recognize the CAP-1 epitope and lyse the human pancreatic carcinoma cell line CF-PAC-1 (HLA-A2+/CEA+) but not the human pancreatic carcinoma cell line AsPC-1 (HLA-A2−/CEA+) (Fig. 1B), suggesting that tumor cells were killed in an MHC-restricted manner.
Previously, Huang et al. used oligonucleotide microarrays to compare the gene expression profiles of human monocyte-derived DCs exposed to C. albicans, Escherichia coli, and influenza virus [44]; however, to our knowledge, the study reported here is the first to analyze the gene expression of DCs exposed to S. cerevisiae constructs. The expression of 260 genes involved in DC activation and maturation after DC treatment with yeast-CEA was evaluated. Many genes were upregulated at early time points, such as those related to phagocytosis, innate immune responses and inflammation, apoptosis, signaling, T-cell regulation, and antigen presentation; more genes were upregulated at 24 h of treatment than at 8 h, and more genes were downregulated at 8 h of treatment than at 24 h (Table 4). Genes CXCL10 and CXCL11 in the chemokines/receptors group were upregulated several hundred-fold compared to controls (Table 4). These results were confirmed by ELISA assay for CXCL10 and CXCL11 (Table 5). Expression of these chemokines may allow DCs to attract activated T cells, natural killer cells, neutrophils, and monocytes. This is supported by a recent report [45] in which vaccination of mice subcutaneously with yeast-CEA resulted in rapid increases in MHC class II+ cells and total APCs in draining lymph nodes. In the same gene group, CCR-7 was upregulated 12.9-fold at 24 h. Upregulation of CCR7 can promote responses to MIP-3s/CCL19 and 6Ckine/CCL21 [46,47]. 6Ckine/CCL21, a potent chemokine for mature DCs and naïve T cells, colocalizes these 2 cell types, leading to cognate T-cell activation in secondary lymphoid organs [48]. TAP-1, a transporter-associated protein-processing gene, was upregulated 8.3-fold; TAP-1 facilitates the transfer of cytosolic peptides to endoplasmic reticulum, where they can bind to MHC class I molecules. The 5.2-fold upregulation of the transcription factor NFκB, which regulates many of the immune-response genes, may also be a factor in the enhanced response of DCs to yeast-CEA.
The results of these studies indicate that yeast-CEA can efficiently mature and activate human DCs, and therefore can enhance CEA-specific T-cell responses. These results, together with the in vitro and in vivo data [36,45] in which yeast-CEA-vaccinated CEA-transgenic mice were shown to generate CEA-specific T-cell responses and antitumor immunity, provide the rationale for the clinical evaluation of yeast-CEA constructs in patients with a range of CEA-expressing carcinomas.
Acknowledgments
The authors acknowledge the excellent technical assistance of Diane J. Poole and Amanda Chang, and the editorial assistance of Bonnie L. Casey and Debra Weingarten in the preparation of this manuscript. We also thank Dr. Yingnian Lu, Dr. Tom King, Dr. Deb Quick, Carol Walker, and Aline Oliver of GlobeImmune for their contributions to the characterization, process development, and assay development work for engineering and manufacturing yeast-CEA and control yeast.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 3.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8(4):531–6. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 4.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–7. [PubMed] [Google Scholar]
- 5.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144(12):4579–86. [PubMed] [Google Scholar]
- 6.Philip R, Brunette E, Ashton J, Alters S, Gadea J, Sorich M, et al. Transgene expression in dendritic cells to induce antigen-specific cytotoxic T cells in healthy donors. Cancer Gene Ther. 1998;5(4):236–46. [PubMed] [Google Scholar]
- 7.Arthur JF, Butterfield LH, Roth MD, Bui LA, Kiertscher SM, Lau R, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4(1):17–25. [PubMed] [Google Scholar]
- 8.Neering SJ, Hardy SF, Minamoto D, Spratt SK, Jordan CT. Transduction of primitive human hematopoietic cells with recombinant adenovirus vectors. Blood. 1996;88(4):1147–55. [PubMed] [Google Scholar]
- 9.Di Nicola M, Siena S, Bregni M, Longoni P, Magni M, Milanesi M, et al. Gene transfer into human dendritic antigen-presenting cells by vaccinia virus and adenovirus vectors. Cancer Gene Ther. 1998;5(6):350–6. [PubMed] [Google Scholar]
- 10.Tsang KY, Zhu M, Even J, Gulley J, Arlen P, Schlom J. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61(20):7568–76. [PubMed] [Google Scholar]
- 11.Zhu M, Terasawa H, Gulley J, Panicali D, Arlen P, Schlom J, et al. Enhanced activation of human T cells via avipox vector-mediated hyperexpression of a triad of costimulatory molecules in human dendritic cells. Cancer Res. 2001;61(9):3725–34. [PubMed] [Google Scholar]
- 12.Tsang KY, Palena C, Yokokawa J, Arlen PM, Gulley JL, Mazzara GP, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11(4):1597–607. doi: 10.1158/1078-0432.CCR-04-1609. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Tsang KY, Schlom J. Induction of higher-avidity human CTLs by vector-mediated enhanced costimulation of antigen-presenting cells. Clin Cancer Res. 2005;11(15):5603–15. doi: 10.1158/1078-0432.CCR-05-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabolcs P, Gallardo HF, Ciocon DH, Sadelain M, Young JW. Retrovirally transduced human dendritic cells express a normal phenotype and potent T-cell stimulatory capacity. Blood. 1997;90(6):2160–7. [PubMed] [Google Scholar]
- 15.Bello-Fernandez C, Matyash M, Strobl H, Pickl WF, Majdic O, Lyman SD, et al. Efficient retrovirus-mediated gene transfer of dendritic cells generated from CD34+ cord blood cells under serum-free conditions. Hum Gene Ther. 1997;8(14):1651–8. doi: 10.1089/hum.1997.8.14-1651. [DOI] [PubMed] [Google Scholar]
- 16.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7(5):625–9. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 17.Barron MA, Blyveis N, Pan SC, Wilson CC. Human dendritic cell interactions with whole recombinant yeast: implications for HIV-1 vaccine development. J Clin Immunol. 2006;26(3):251–64. doi: 10.1007/s10875-006-9020-8. [DOI] [PubMed] [Google Scholar]
- 18.Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, et al. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007;25(8):1452–63. doi: 10.1016/j.vaccine.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Wadle A, Held G, Neumann F, Kleber S, Wuellner B, Asemissen AM, et al. Cross-presentation of HLA class I epitopes from influenza matrix protein produced in Saccharomyces cerevisiae. Vaccine. 2006;24(37–39):6272–81. doi: 10.1016/j.vaccine.2006.05.096. [DOI] [PubMed] [Google Scholar]
- 20.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Korting HC. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004;12(1):44–9. doi: 10.1016/j.tim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166(1):249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275(27):20861–6. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 23.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187(12):2097–101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaremba S, Barzaga E, Zhu M, Soares N, Tsang KY, Schlom J. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997;57(20):4570–7. [PubMed] [Google Scholar]
- 25.Salazar E, Zaremba S, Arlen PM, Tsang KY, Schlom J. Agonist peptide from a cytotoxic t-lymphocyte epitope of human carcinoembryonic antigen stimulates production of tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. Int J Cancer. 2000;85(6):829–38. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Tsang KY, Zhu M, Nieroda CA, Correale P, Zaremba S, Hamilton JM, et al. Phenotypic stability of a cytotoxic T-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res. 1997;3(12 Pt 1):2439–49. [PubMed] [Google Scholar]
- 27.Gulley J, Arlen P, Dahut W, Tsang K, Jones J, Pazdur M, et al. A pilot study of a PANVAC-V and PANVAC-F in patients (pts) with metastatic carcinoma. J Clin Oncol. 2006;24(18S):2512. [Google Scholar]
- 28.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87(13):982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maecker HT, Moon J, Bhatia S, Ghanekar SA, Maino VC, Payne JK, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson RD, Shibata N, Podzorski RP, Herron MJ. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991;4(1):1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, et al. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165(7):3804–10. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 33.Pietrella D, Bistoni G, Corbucci C, Perito S, Vecchiarelli A. Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol. 2006;8(4):602–12. doi: 10.1111/j.1462-5822.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 34.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31(10):1583–93. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 35.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46(7):503–12. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 36.Wansley E, Chakraborty M, Hance K, Bernstein M, Guo Z, Quick D, et al. Vaccination of CEA-transgenic mice with a recombinant Saccharomyces cerevisiae-CEA vaccine breaks immune tolerance and elicits therapeutic antitumor responses [poster presentation]. Molecular Targets and Cancer Therapeutics; AACR-NCI-EORTC International Conference; 2007. [Google Scholar]
- 37.Buentke E, Scheynius A. Dendritic cells and fungi. APMIS. 2003;111(7–8):789–96. doi: 10.1034/j.1600-0463.2003.11107810.x. [DOI] [PubMed] [Google Scholar]
- 38.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146(7–8):423–31. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 39.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13(16):4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 40.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 41.Hellman P, Eriksson H. Early activation markers of human peripheral dendritic cells. Hum Immunol. 2007;68(5):324–33. doi: 10.1016/j.humimm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Palena C, Arlen P, Zeytin H, Greiner JW, Schlom J, Tsang KY. Enhanced expression of lymphotactin by CD8+ T cells is selectively induced by enhancer agonist peptides of tumor-associated antigens. Cytokine. 2003;24(4):128–42. doi: 10.1016/j.cyto.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Palena C, Schlom J, Tsang KY. Differential gene expression profiles in a human T-cell line stimulated with a tumor-associated self-peptide versus an enhancer agonist peptide. Clin Cancer Res. 2003;9(5):1616–27. [PubMed] [Google Scholar]
- 44.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294(5543):870–5. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, et al. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26(4):509–21. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22(4):345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 47.Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66(2):252–62. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 48.Chan VW, Kothakota S, Rohan MC, Panganiban-Lustan L, Gardner JP, Wachowicz MS, et al. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Blood. 1999;93(11):3610–6. [PubMed] [Google Scholar]