Abstract
Introduction
Naturally occurring IgM antileukocyte antoantibodies (IgM-ALA) are present from birth and increase during inflammatory processes of diverse etiologies. The clinical observation demonstrating a significant correlation (P<01) between lack of acute rejections and presence of high levels of IgM-ALA in recipients of kidney allografts prompted us to study if IgM-ALA alters T-cell function and leukocyte chemotaxis.
Methods
In-vitro functional assays were performed using leucocytes isolated from human peripheral blood. In-vivo studies were performed in C57BL6 mice.
Result
Human studies revealed that IgM-ALA consist of several different IgM, each with specificities for a different leukocyte receptor, e.g., CD3, CD4, CCR5, and CXCR4. We show that IgM inhibits T-cell activation, proliferation, and chemotaxis. Data on in vivo murine models of ischemia-reperfusion injury and cardiac transplantation support our hypothesis.
Conclusion
The innate anti-inflammatory mechanism of IgM-ALA can be exploited by using purified normal IgM to inhibit inflammatory states or by a vaccine approach to increase in vivo production of IgM-ALA (e.g., prior to a transplant).
Keywords: Naturally occurring autoantibodies, IgM anti-leukocyte, chemotaxis, T-cell activation
Introduction
Naturally occurring antibodies, which are produced in the absence of deliberate immunization or exposure to foreign antigens, are detectable at birth, but the full repertoire develops by early childhood and is maintained throughout life [1-3]. These autoantibodies are produced by a subset of B cells (B1 Cells), which produce antibodies independently of T cell help [4]. Additionally, such naturally occurring antibodies are encoded by minimally or nonmutated germline genes and hence are characteristically polyreactive with low-binding affinity, and, therefore, differ from disease-producing autoantibodies in that the latter are predominantly of the IgG isotype and bind with high affinity and specificity to the autoantigen [3, 5].
We have been interested in the naturally occurring antileukocyte autoantibodies, which are predominantly of the IgM isotype; these antibodies were initially discovered because of their binding reactivity to lymphocytes [6, 7]. Such antibodies will be referred to as IgM-anti-leukocyte autoantibodies (IgM-ALA). These IgM-ALA are present at low levels in normal individuals and increase during inflammatory disorders and various infections including a subset of HIV-1 patients and end-stage renal dialysis patients (ESRD) [8-10]. Previous studies have demonstrated that IgM-ALA are a heterogeneous group of several antibodies that are reactive to autologous and allogeneic cells and have specificities for different, largely undefined membrane receptors including receptors with phospholipids and glycolipids [11-13]. These antibodies are not cytolytic in the presence of complement at 37°C but are cytolytic at colder temperatures such as 18-20°C [7, 14, 15]. Human kidney and heart transplants performed in the subset (approximately 30%) of patients having high levels of IgM-ALA have a lower incidence of acute rejections and of less severity, thus permitting better graft survival [14-16].
The protective effect of IgM-ALA in allograft transplantation together with the observation that IgM-ALA nonspecifically increase in various inflammatory and infective states led us to hypothesize whether the increased production of IgM-ALA has a role in inhibiting T-cell activation and attenuating the inflammatory response by binding to certain cell membrane receptors important in the inflammatory process; these include receptors that activate T lymphocytes and those that are important for chemotaxis.
Methods
These have been described in a prior publication; for details, refer to [17].
Results and Discussion
High IgM-ALA levels are associated with minimal acute rejections and better graft survival
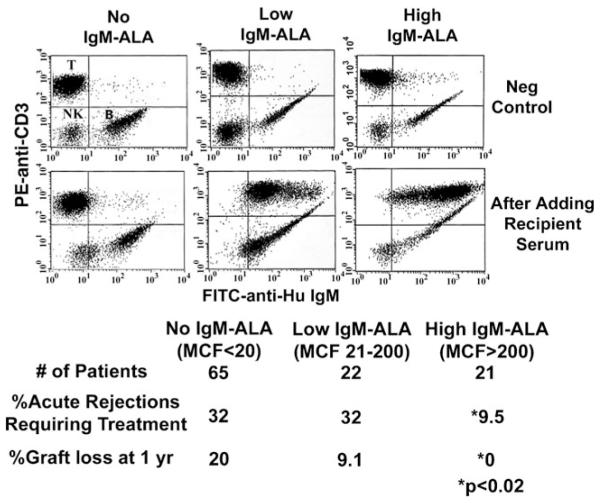
Prior to a human kidney transplant, sera from the recipient is routinely reacted with the potential donor’s lymphocytes to exclude the presence of IgG anti-HLA antibodies, which can give rise to hyper-acute or accelerated rejection and to determine if the recipient has IgM reactive to donor lymphocytes. Figure 1 depicts the incidence of biopsyproven acute rejections in kidney allograft recipients having different quantities of IgM-ALA reactive to their donor lymphocytes. Recipients with high levels of IgM-ALA reactivity had significantly less rejection episodes when compared with the other subset of patients (Fig. 1). These differences in rejection rates among the three groups of recipients did not correlate with age, sex, race, level of HLA antigen mismatch, propensity to develop anti-HLA antibodies pretransplant, and anti-rejection medications. There was no difference in total serum IgM levels among the three groups. These IgM anti-lymphocyte autoantibodies had reactivity to T, B, and natural killer (NK) cells and to CD14 positive macrophages and CD15 positive neutrophils. Prior studies also revealed reactivity of IgM to endothelial cells obtained from human kidneys and the myeloid K562 cell line lacking HLA class I and II receptors [17].
Fig. 1.
High levels of serum IgM-ALA in transplant recipients are associated with better kidney allograft survival. Upper panels (before adding serum) depict B lymphocytes that normally express membrane IgM (FITC positive). The lower panels depict differences in the level of serum IgM bound to donor T lymphocytes (IgM-ALA) after addition of pretransplant serum from different ESRD patients. The difference in percentage of acute rejections and graft loss comparing high MCF versus the no and low MCF groups was statistically significant (P<0.02), as shown by the asterisks. MCF indicates increase in mean channel fluorescence of anti-IgM staining after addition of serum. Lobo PI, Schlegel KH, Spencer CE, Okusa MD, Chisholm C, Mchedlishvili N, et al.: Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T-cell activation and chemotaxis. J. Immunol. 180:1780-1791, 2008. Copyright 2008. The American Association of Immunologists, Inc
These in vivo observations of decreased rejections and the in vitro finding that IgM bound to T cells and other leukocytes and endothelial cells prompted us to examine whether IgM mediated these beneficial effects by inhibiting T-cell activation and proliferation and the inflammatory process mediated by chemokines and chemokine receptors. Binding of IgM to chemokine receptors appeared to be an attractive possibility and could explain the widespread binding of IgM to the different leukocyte and endothelial cells.
IgM was purified by size exclusion chromatography from serum [17]. Either individual IgM (labeled #1, #2, etc.) or pooled IgM (labeled p-) was used. Purified human IgG and a human Waldenstrom monoclonal IgM that bound to an undefined leukocyte receptor were used as controls.
Evidence to show specific binding of IgM-ALA to leukocyte receptors
Because IgM is a pentameric molecule and is encoded by nonmutated germline genes (i.e., polyreactive), it became necessary to exclude the possibility of nonspecific binding by IgM to leukocytes. To more conclusively exclude this possibility, we evaluated the binding of several different IgM obtained from a panel of IgM secreting human EBV transformed B cell clones derived from human umbilical cord blood [17]. Of the 79 supernatants containing IgM (concentration, 300-1,100 ng/mL), only eight had IgM-secreting clones with IgM-ALA reactivity. Clones secreting IgM-ALA had the following binding specificities to cell lines: two clones with only anti-T cell (Jurkat, Sup T-1) reactivity, two clones with only anti-monocyte (U937) reactivity, and four clones with reactivity to all cell lines, e.g., T cells, B cells, and U937 monocytoid cells. Two of these eight monoclonal IgM-ALA also had IgM that bound to soluble CD4 as evaluated by an enzyme-linked immunosorbent assay (ELISA) technique. All eight clones secreting IgM-ALA did not bind to IgG-coated latex beads as they lacked rheumatoid factor activity. These findings, which include the presence of IgM-ALA reactivity in 10% of IgM-secreting clones (and not the majority of these clones) and the binding of IgM-ALA to CD4 and specific cell lines (T cells or monocytes), would strongly argue against nonspecific IgM binding to leukocyte receptors. They also provide evidence indicating that IgM-ALA consists of different antibodies that have different specificities, including IgM with specificity for CD4 and another antibody with specificity for a receptor on T or monocyte cells. Furthermore, binding of IgM to all cell lines can best be explained either by polyspecificity of certain IgM autoantibodies or by binding of IgM-ALA to a leukocyte receptor that is common to all cell lines (e.g., CD45).
Naturally occurring IgG-ALA are rare in sera and in supernatants of umbilical cord B cell clones
Previous studies that demonstrated the presence of naturally occurring IgG autoantibodies, with reactivity to intracellular proteins, prompted studies to examine if IgG-ALA is also present in newborn sera, normal sera, and disease sera. In these studies, the presence of IgG-ALA was evaluated by the binding of IgG from B cell clone supernatants or sera to cell lines. No IgG-ALA was detected in 96 umbilical cord B cell clone supernatants, 12 cord sera, and 27 normal adult sera. However, six of 135 ESRD sera had IgG-ALA that bound to either B or T cells. All six ESRD IgG-ALA were autoantibodies when tested against autologous leukocytes, but none of these sera had IgG anti-HLA reactivity when tested with immobilized HLA antigens (class I and II) using the single HLA antigen flow beads (One Lambda, Canoga Park, CA, USA). None of the 19 HIV-1 sera had IgG-ALA. These studies clearly demonstrate that IgG-ALA are not present in newborn sera and in normal sera but occasionally present in ESRD and HIV-sera. Further studies are needed to evaluate whether ESRD and HIV IgG-ALA are encoded by minimal or nonmutated germline genes, which are a characteristic feature of naturally occurring autoantibodies.
In the following studies, we analyzed the effect of IgM-ALA on T cells and the chemokine receptor by using purified polyclonal IgM rather than serum. As controls for nonspecific IgM binding to leukocyte receptors, we used purified IgM obtained from different individual donors. These included normals, patients with ESRD or HIV-1, and a patient who was diagnosed with Waldenstrom’s macroglobulinemia and had a monoclonal IgM with binding reactivity to an undefined leukocyte receptor.
Purified normal, ESRD, and HIV IgM immunoprecipitate CD3, CD4, CCR5, and CXCR4 receptors
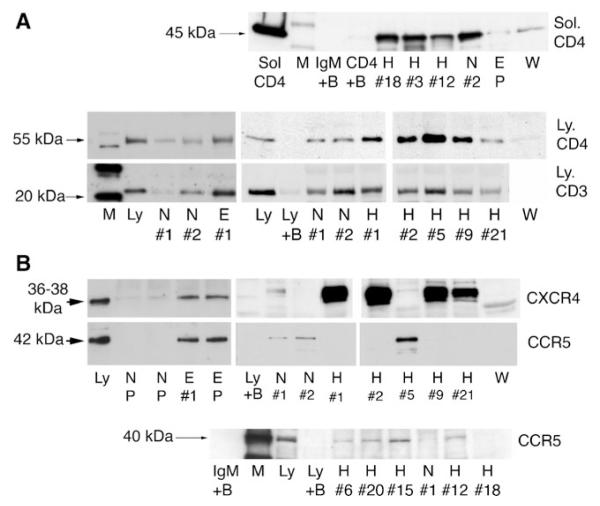
Based on our observations on allograft rejection, it became important to initially determine by immunoprecipitation assays whether IgM bound to receptors important in T-cell activation as well as to receptors involved in leukocyte chemotaxis. Our studies revealed that purified normal, ESRD, and HIV IgM immunoprecipitated CD3, CD4, CCR5, and CXCR4 receptors (Fig. 2). However, significantly more of these receptors were immunoprecipitated by HIV and ESRD IgM from whole cell lysates. Of note, IgM from several different normal, HIV, and ESRD patients failed to immunoprecipitate interleukin (IL)2-R, human leukocyte antigen (HLA)-A, and HLA-DR receptors. Normal or ESRD IgM failed to immunoprecipitate chemokines, i.e., recombinant CCL5 and CXCL12.
Fig. 2.
Immunoprecipitation experiments to show binding of IgM to CD3, CD4, CCR5, and CXCR4 obtained from leukocytes. In panels a and b, identical quantities of individual (labeled #1, #2, etc.) or pooled (P) IgM from normal (N), HIV (H), ESRD (E), or Waldenstrom (W) were used to immunoprecipitate leukocyte receptors from equal amounts of whole cell lysates. As controls, Western blots were performed with cell lysates in the absence of agarose beads (to control for binding of primary antibody to leukocyte receptor and to determine receptor size (labeled Ly). In another control, agarose beads without IgM were added to lysate to determine whether the leukocyte receptor nonspecifically bound to the bead (B plus Ly). Note that several fold more receptors were immunoprecipitated by ESRD and HIV IgM when compared with normal IgM. In a, recombinant, glycosylated, solubilized CD4 (Sol-CD4), comprising the full-length extracellular domain was immunoprecipitated by IgM. For controls, Western blots were performed with soluble CD4 in absence of IgM and agarose beads (labeled CD4) and with Sol-CD4 added to agarose beads in absence of IgM (labeled CD4+ B). Lobo PI, Schlegel KH, Spencer CE, Okusa MD, Chisholm C, Mchedlishvili N, et al.: Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J. Immunol. 180:1780-1791, 2008. Copyright 2008. The American Association of Immunologists, Inc
IgM inhibits T-cell proliferation, cytokine production, and Zap-70 phosphorylation
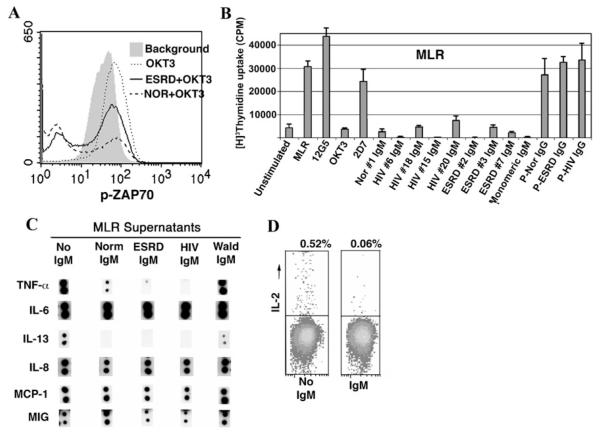
Because IgM bound to CD3 and CD4, it became important to determine whether purified IgM inhibited T-cell activation, proliferation, and cytokine production. Our data indicate that purified IgM, especially ESRD IgM, inhibits T-cell proliferation in response to alloantigens and anti-CD3 only when IgM was added to the cultures within the first 24 h of initiation (Fig. 3). The pentameric form of IgM is not essential for the inhibitory effect on mixed lymphocyte reaction (MLR) as monomeric IgM was as effective. This inhibitory effect of ESRD IgM on proliferation was not associated with increased cell death or apoptosis as determined by flow cytometry using propidium and anti-annexin reagents.
Fig. 3.
Effect of purified IgM on T-cell activation, proliferation, and cytokine production. a Data depict flow cytometry histograms of intracellular phos-Zap-70 present in PBL. Note that background phos-Zap 70 (shaded gray) increases after incubating cells with immobilized anti-CD3 for 16 h (MCF increased from 29 to 54). b Effect of pooled normal, ESRD, and HIV IgM on T-cell proliferation induced by alloantigens, i.e., MLR. 15ųg of IgM (including monomeric IgM) were used for 1 × 106 cells/mL in these experiments and added at the time cells were co-cultured. This is a representative example of three separate experiments. The panel depicts MLR proliferation in the presence of individual (labeled #1, #6, etc.) or pooled (P) IgM (4.5 μg/ 0.3 ml), pooled IgG (2 μg/0.3 ml), and murine monoclonal antibodies (2 μg/0.3 mL) to CD3 (OKT3), CXCR4 (12G5), and CCR5 (2D7) when used at the initiation of the MLR. Values for T-cell proliferation assays are mean±SD of experiments performed in triplicate. Panel c. Pooled normal, ESRD, and HIV IgM but not Waldenstrom IgM significantly inhibited the increase in TNF-α and IL-13 but not that of IL-6, IL-8, MIG, and MCP-1 produced in response to alloantigen (MLR) activation of T cells. Panel d Dot plots depicting the effect of IgM on intracytoplasmic IL-2 in PBL activated for 48 h in an MLR. 15ųg of IgM were used for 1 × 106 cell/mL in these experiments. Lobo PI, Schlegel KH, Spencer CE, Okusa MD, Chisholm C, Mchedlishvili N, et al.: Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J. Immunol, 180:1780-1791, 2008. Copyright 2008. The American Association of Immunologists, Inc
Further studies were performed on supernatants from MLR cultures to determine whether the anti-proliferative effects of IgM-ALA were associated with a decrease in cytokine production. The addition of both normal and patient IgM at the initiation of the MLR culture had no discernable inhibitory effect on MLR-induced production ofIL-6, IL-8, monocyte chemotactic protein (MCP)-1, MIG, GRO, granulocytemacrophage colony-stimulating factor (GM-CSF), IL-1β, and transforming growth factor beta (TGF-β) but significantly decreased the quantity of certain other cytokines such as TNF-α, IL-13, MDC, and TARC in the MLR supernatants (Fig. 3). This decrease in cytokine level (e.g., TNF-α) in the presence of IgM was not a result of IgM neutralizing the secreted TNF-α, because the addition of IgM directly to the control MLR supernatants, obtained on day5, did not decrease the level of TNF-α detected by two assay systems (i.e., Array III and an ELISA technique). This indicates that IgM specifically inhibits the secretion or production of certain cytokines. Because the Array III kit could not detect IL-2 in MLR supernatants, we resorted to determine the effect of IgM on intracellular expression of this cytokine. Both normal and ESRD IgM caused a major decrease in the percentage of cells expressing intracytoplasmic IL-2. The combined data, with the different cytokine assay techniques, clearly demonstrate that normal and patient IgM predominantly inhibits the production of certain cytokines involved in T-cell proliferation (e.g., IL-2) and certain proinflammatory cytokines and chemokines (e.g., TNF-α, IL-13, MDC, and TARC when T cells are activated in an MLR). Conversely, IgM did not significantly inhibit other proinflammatory cytokines (e.g., IL-6, GM-CSF, IL-1β, and IL-8).
Because prior studies have shown that TcR/CD3 receptor activation leads to increased intracellular phosphorylation of Zap-70, we evaluated whether IgM, after binding to CD3 and/or CD4, will inhibit anti-CD3-mediated phosphorylation of Zap-70. Phosphorylated Zap-70 and total Zap-70 were quantitated by flow cytometry at 0, 2, and 5 min and at 16 h after immobilized anti-CD3 activation of freshly isolated human PBL. IgM had a mild inhibitory effect on anti-CD3-induced phosphorylation of Zap-70 at 2 min. However, the majority of individual normal, ESRD, and HIV IgM (but not Waldenstrom) inhibited both background phosphorylation of Zap-70 and the increase in Zap-70 phosphorylation induced by anti-CD3 at 16 h in 40-50% of T cells (Fig. 3). Importantly, IgM-mediated inhibition of phos-Zap-70 was not associated with inhibition of total Zap-70, which indicates that IgM inhibits proximal intracellular signaling. Pooled normal IgG had no effect on Zap-70 phosphorylation.
Purified IgM inhibits chemokine binding and chemotaxis
Because IgM immunoprecipitated CXCR4 and CCR5 from cells, it became important to determine if purified IgM inhibited binding of chemokine to these receptors as well as inhibited chemotaxis. Both normal and ESRD IgM inhibited to a similar degree binding of biotin-labeled CCL3 (MIP-Iα) to CCR5, and binding of CXCL12 to CXCR4 which were present on two cell lines and on PBL activated for 3 days with PHA and IL-2. IgM inhibited chemokine binding in a dose-dependent manner. Incubating cells with IgM and/or chemokine at 37°C or 4°C did not change the magnitude of the inhibitory effect of IgM on chemokine binding, thus indicating that the IgM-mediated inhibitory effect was not due to IgM-induced internalization of the receptor at 37°C. Waldenstrom IgM and pooled human IgG had no inhibitory effect on chemokine binding.
In addition, IgM inhibited chemotaxis of activated PBL and T-cell lines in response to CXCL12. However, ESRD IgM had a significantly more pronounced inhibitory effect on chemotaxis, even though both normal and ESRD IgM had a similar inhibitory effect on the binding of CXCL12 to the CXCR4 receptor. These differences in inhibitory effects on chemotaxis with the T-cell lines were not due to increased apoptosis or cell death as evaluated by flow cytometry using propridium and anti-annexin. These data would suggest that ESRD IgM additionally inhibits chemotaxis through effects on other cell receptors (e.g., adhesion molecules or integrins) and/or intracellular activation pathways that are involved in both chemokinesis and chemotaxis activity. These studies indicate that purified IgM binds to chemokine receptors and through this mechanism inhibits chemokine binding, chemokine-induced chemotaxis, and receptor downregulation. Finally, we failed to demonstrate binding of IgM to chemokines using both immunoprecipitation and ELISA techniques.
The finding that IgM-ALA inhibits T-cell function and proliferation as well as leukocyte chemotaxis prompted us to evaluate whether IgM-ALA inhibited a T cell-mediated inflammatory response of allograft rejection in a murine model. Preliminary findings would support the in vitro data.
The maintenance of immune system homeostasis is essential for protecting the host against excess inflammation and autoimmunity [18]. Homeostasis maintenance by different subsets of natural and adaptive regulatory T cells (CD4+ or CD8+), NK-T cells, and dendritic cells have gained particular attention for understanding peripheral regulation of self-reactive T cells [19, 20]. Our studies would suggest yet another innate immune regulatory mechanism that involves a noncytolytic (at 37°C) naturally occurring IgM-ALA to maintain immune homeostasis and protect against autoimmunity. Potential mechanisms for IgM-ALA include inhibition of T-helper cell activation with decreased production of certain specific proinflammatory cytokines, e.g., TNF-α, IL-2. Importantly, the current data would also indicate that IgM-ALA controls the magnitude of the inflammatory response by not broadly inhibiting all proinflammatory cytokines (possibly to avert excess immunosuppression) and by regulating leukocyte chemotaxis. In addition, the marked individual variation in the repertoire of IgM with specificity to the different leukocyte receptors that we observed in both normal and disease states may play a role in contributing to the differences in the vigor or character of the inflammatory response among different individuals when exposed to the same inciting agent. This innate anti-inflammatory mechanism of IgM-ALA can be exploited by using purified normal IgM to inhibit inflammatory states or by a vaccine approach to increase in vivo production of IgM-ALA (e.g., prior to a transplant).
Acknowledgments
We would like to acknowledge the Ryan White HIV Clinic at the University of Virginia for identifying patients who were willing to donate their blood. We would like to thank Rhonda S. Hawkins for her expert assistance in preparing this manuscript.
Footnotes
A longer version of this article originally appeared in the Journal of Immunology [17].
References
- 1.Hardy RR, Hayakawa K. Development and physiology of Ly-1 B and its human. Leu-1 B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 2.Ailus K, Palosuo T. IgM class autoantibodies in human cord blood. J Reprod Immunol. 1995;29:61–7. doi: 10.1016/0165-0378(95)00933-c. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix-Desmazes S, Kaveri SV, Mouthon L, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998;216:117–37. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 4.Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 5.Brezinschek HP, Foster SJ, Brenzinschek RI, Dorner T, Domiati-Saad R, Lipsky PE. Analysis of human VH gene repertoire: differential effects of selection and somatic hypermutation on human peripheral CD5+/IgM and CD5−/IgM+ B cells. J Clin Invest. 1997;99:2488–501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaki PI, Mottironi VD, Barnett EV. Cytotoxins in disease. N Engl J Med. 1970;283:724–7. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- 7.Winfield JB, Winchester RJ, Wernet P, Fu SM, Kunkle HG. Nature of cold-reactive antibodies to lymphocyte surface. Arthritis Rheum. 1975;18:1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]
- 8.Lobo PI. Nature of autolymphocytotoxins present in renal hemodialysis patients: their possible role in controlling alloantibody formation. Transplantation. 1981;32:233–7. doi: 10.1097/00007890-198109000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lobo PI, Suratt PM. Studies on the autoantibody to lymphocytes in sarcoidosis. J Clin Lab Immunol. 1979;1:283–8. [PubMed] [Google Scholar]
- 10.Dorsett B, Cronin W, Chuma V. Iochim Hl: Anti-lymphocyte antibodies in patients with the acquired immune deficiency syndrome. Am J Med. 1985;78:621–6. doi: 10.1016/0002-9343(85)90405-x. [DOI] [PubMed] [Google Scholar]
- 11.Griggi T, Bauer R, Garofalo T, et al. Autoantibodies against ganglioside GM3 represents a portion of antilymphocytic antibodies in AIDS patients. Scand J Immunol. 1994;40:77–82. doi: 10.1111/j.1365-3083.1994.tb03436.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorice M, Lenti L, Misasi R, et al. Anti-glycosphingolipid antibodies in HIV infection. AIDS. 1991;5:345–6. [PubMed] [Google Scholar]
- 13.Stimmler MM, Quismorio FPJ, McGehee WG, Boylen T, Sharma OP. Anticardiolipin antibodies in acquired immunodeficiency syndrome [see comments] Arch Intern Med. 1989;149:1833–5. [PubMed] [Google Scholar]
- 14.Lobo PI, Rudolf L, Westervelt FB. Enhanced kidney allograft survival across a positive crossmatch (Cx) arising from B-cell specific and cold reactive antibodies. Proc Dial Transp Forum. 1977;7:4–6. [PubMed] [Google Scholar]
- 15.Iwaki Y, Terasaki PI, Park MS, Billing R. Enhancement of human kidney allografts by cold B-lymphocyte cytotoxins. Lancet. 1978;1:1228–9. doi: 10.1016/s0140-6736(78)92464-9. [DOI] [PubMed] [Google Scholar]
- 16.Kerman RH, Susskind B, Buyse I, et al. Flow cytometry-detected IgG is not a contraindication to renal transplantation: IgM may be beneficial to outcome. Transplantation. 1999;68:1855–8. doi: 10.1097/00007890-199912270-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lobo PI, Schlegel KH, Spencer CE, et al. Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J Immunol. 2008;180:1780–91. doi: 10.4049/jimmunol.180.3.1780. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Chess L. Regulation of immune responses by T cells. N Eng J Med. 2006;354:1166–76. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 20.Ludewig B, Krebs P, Junt T, Bocharov G. Dendritic cell homeostasis in the regulation of self-reactivity. Curr Pharm Des. 2003;9:221–31. doi: 10.2174/1381612033392044. [DOI] [PubMed] [Google Scholar]