Abstract
Both resting state functional magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) are increasingly popular techniques that can be used to non-invasively measure brain connectivity in human subjects. TMS shows additional promise as a method to manipulate brain connectivity. In this review we discuss how these two complimentary tools can be combined to optimally study brain connectivity and manipulate distributed brain networks. Important clinical applications include using resting state fcMRI to guide target selection for TMS and using TMS to modulate pathological network interactions identified with resting state fcMRI. The combination of TMS and resting state fcMRI has the potential to accelerate the translation of both techniques into the clinical realm and promises a new approach to the diagnosis and treatment of neurological and psychiatric diseases that demonstrate network pathology.
Keywords: spontaneous brain activity, non-invasive brain stimulation, neuro-navigation, anticorrelation, fMRI
Introduction
It is becoming increasingly recognized that many behavioral manifestations of neurological and psychiatric disease are not solely the result of abnormality in one isolated brain region but represent alterations in brain networks and connectivity. Examples include spatial neglect with imbalance in intraparietal sulcus activity (Corbetta, Kincade et al. 2005; He, Snyder et al. 2007), hemiparesis worsened by transcallosal inhibition (Murase, Duque et al. 2004; Duque, Hummel et al. 2005; Grefkes, Nowak et al. 2008; Carter, Astafiev et al. 2010), memory deficits in Alzheimer’s due to distributed network pathology (Buckner, Snyder et al. 2005), and depression associated with limbic hyperactivity and prefrontal hypoactivity (Mayberg 2007; Mayberg 2009; Padberg and George 2009). As such, much neuroscience research has shifted from focusing on the properties of individual brain regions to the interactions and connections between regions.
Brain connectivity has been non-invasively assessed in human subjects using techniques focused on three general network properties: anatomical connectivity, functional connectivity, and response to perturbation / stimulation. The first of these, anatomical connectivity, has relied predominantly on diffusion tensor imaging (DTI), a technique which measures the asymmetric diffusion of water molecules along white matter fiber tracks (Assaf and Pasternak 2008). The second network property, functional connectivity, is defined as a correlation between remote neuro-physiological events in the temporal domain (Friston, Frith et al. 1993; Horwitz 2003) and has been assessed using a wide variety of techniques including electro- and magnetoencephalography (EEG/MEG), positron emission tomography (PET), near infrared spectroscopy (NIRS), and functional magnetic resonance imaging (fMRI). Given the variety of approaches used to assess functional connectivity it is important to remember that this is a broad term with some inherent ambiguity (Horwitz 2003; Rogers, Morgan et al. 2007). Derivations of functional connectivity include effective connectivity, which uses a priori models to assume directional influence (Stephan and Friston 2010), and Granger causality, which uses data driven methods to determine whether signals in one region can be predicted by preceding signals in another (Roebroeck, Formisano et al. 2005). Finally, the third network property which has served as a basis for non-invasive assessment of human brain connectivity is the brain’s response to perturbation / stimulation. This approach utilizes techniques such as transcranial magnetic stimulation (TMS), focused pulsed ultrasound (Bystritsky, Korb et al. 2011), and transcranial direct current stimulation (TDCS) which can be used alone or in combination with other modalities to measure distributed brain changes occurring as a result of focal brain manipulation.
In this review we focus on two of these techniques for assessing human brain connectivity, namely resting state functional connectivity MRI (fcMRI) and TMS. This focus is motivated by the fact that resting state fcMRI is rapidly becoming the most popular of the correlational techniques for assessing functional connectivity, TMS is the most widely used perturbation approach, and the combination of the two techniques holds great promise for addressing several important clinical issues. Individual reviews have recently been written on both resting state fcMRI (Fox and Raichle 2007; van den Heuvel and Hulshoff Pol 2010; Deco, Jirsa et al. 2011) and connectivity assessed with TMS (Hampson and Hoffman 2010; Reithler, Peters et al. 2011). Therefore the focus of the current review is on the overlap between the two techniques and the ways in which they can be combined. First we review how resting state fcMRI and TMS have been used individually to measure brain connectivity, including a discussion of their limitations. Second, we highlight some important similarities and differences in connectivity measured using the two techniques. Third we discuss the promise of using connectivity including resting state fcMRI to guide TMS target selection. Finally, we review evidence that TMS can be used to manipulate connectivity and discuss the potential of TMS to correct resting state fcMRI abnormalities in neurological and psychiatric disease.
Measuring connectivity with resting state fcMRI
Resting state fcMRI examines correlations in spontaneous fluctuations in the blood oxygen level dependent (BOLD) signal (for recent reviews see (Fox and Raichle 2007; van den Heuvel and Hulshoff Pol 2010; Deco, Jirsa et al. 2011)). In contrast to traditional task-based fMRI studies, resting state functional connectivity (fcMRI) studies examine BOLD fluctuations in the absence of any explicit input or output, while subjects simply rest in the scanner. A consistent observation is that regions with similar functional properties, such as the left and right somatomotor cortices, exhibit coherent BOLD fluctuations even in the absence of movement under resting conditions (Biswal, Yetkin et al. 1995; Lowe, Mock et al. 1998; Cordes, Haughton et al. 2000; De Luca, Smith et al. 2005; Fox, Snyder et al. 2006) (Figure 1A). Similar findings have been reported in multiple other brain networks including visual, auditory, language, default mode, and corticothalamic networks (Fox and Raichle 2007). Anticorrelations between regions with apparent opposing functional properties have also been observed (Greicius, Krasnow et al. 2003; Fox, Snyder et al. 2005; Fransson 2005; Chang and Glover 2009; Fox, Zhang et al. 2009) (Figure 2D), although some debate exists surrounding the appropriate interpretation of these findings (Fox, Zhang et al. 2009; Murphy, Birn et al. 2009; Anderson, Druzgal et al. 2010). Spontaneous BOLD fluctuations can predict the task-response properties of brain regions (De Luca, Smith et al. 2005; Vincent, Snyder et al. 2006), identify subjects’ aptitude for different cognitive tasks (Hampson, Driesen et al. 2006; Seeley, Menon et al. 2007; van den Heuvel, Stam et al. 2009; Koyama, Di Martino et al. 2011; Zhu, Zhang et al. 2011; Baldassarre, Lewis et al. 2012), facilitate refinement of neuro-anatomical models (Fox, Corbetta et al. 2006; Dosenbach, Fair et al. 2007), and account for trial-to-trial variability in behavior (Fox, Snyder et al. 2007; Sadaghiani, Hesselmann et al. 2010). Resting state fcMRI correlation patterns are very robust and can be observed under sleep (Fukunaga, Horovitz et al. 2006; Horovitz, Braun et al. 2009; Larson-Prior, Zempel et al. 2009) and sedation (Kiviniemi, Kantola et al. 2003; Peltier, Kerssens et al. 2005; Vincent, Patel et al. 2007; Greicius, Kiviniemi et al. 2008) allowing for comparisons across development (Fair, Dosenbach et al. 2007; Dosenbach, Nardos et al. 2010) and even species (Vincent, Patel et al. 2007).
Figure 1.
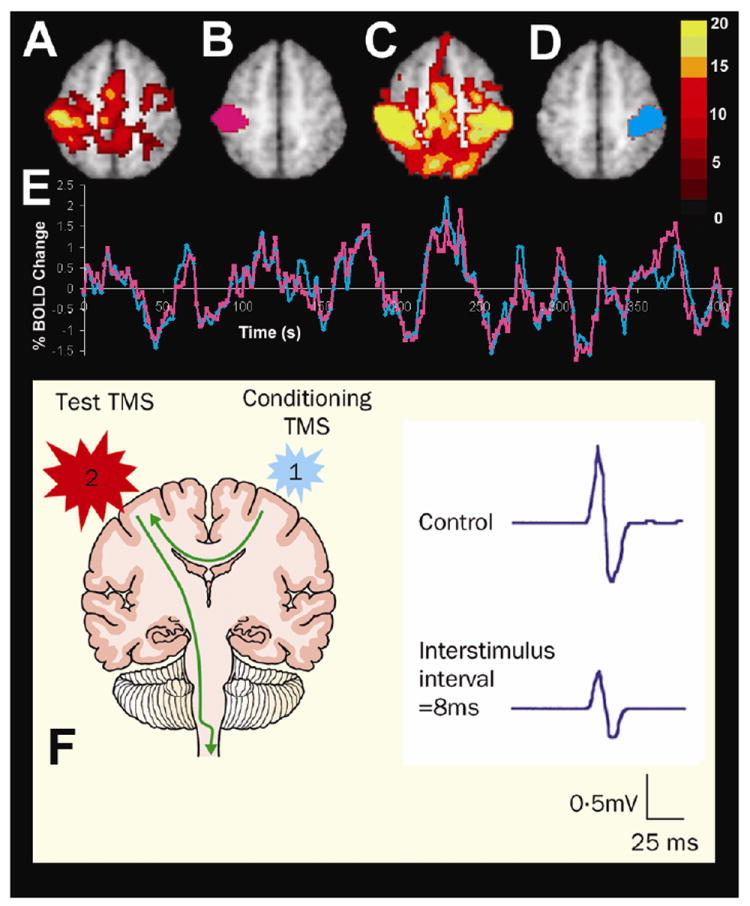
Connectivity between the motor cortices assessed with resting state functional connectivity MRI and dual-coil stimulation with TMS. The top panel shows fMRI activation in response to a right hand button press (A), a left somatomotor region of interest (B), resting state functional connectivity with this left somatomotor cortex region of interest (C), a right somatomotor cortex region of interest defined on the basis of the resting state functional connectivity (D), and spontaneous fluctuations recorded in the left (pink line) and right (blue line) somatomotor cortices during the resting state conditions showing significant interhemispheric correlation (modified with permission from (Fox, Snyder et al. 2007)). The lower panel shows the effect of transcallosal inhibition using dual-coil TMS. When a conditioning pulse is delivered to the left motor cortex 8 ms before the test pulse is delivered to the right motor cortex the motor evoked potential recorded from the left hand is significantly decreased (modified with permission from (Kobayashi and Pascual-Leone 2003)).
Figure 2.
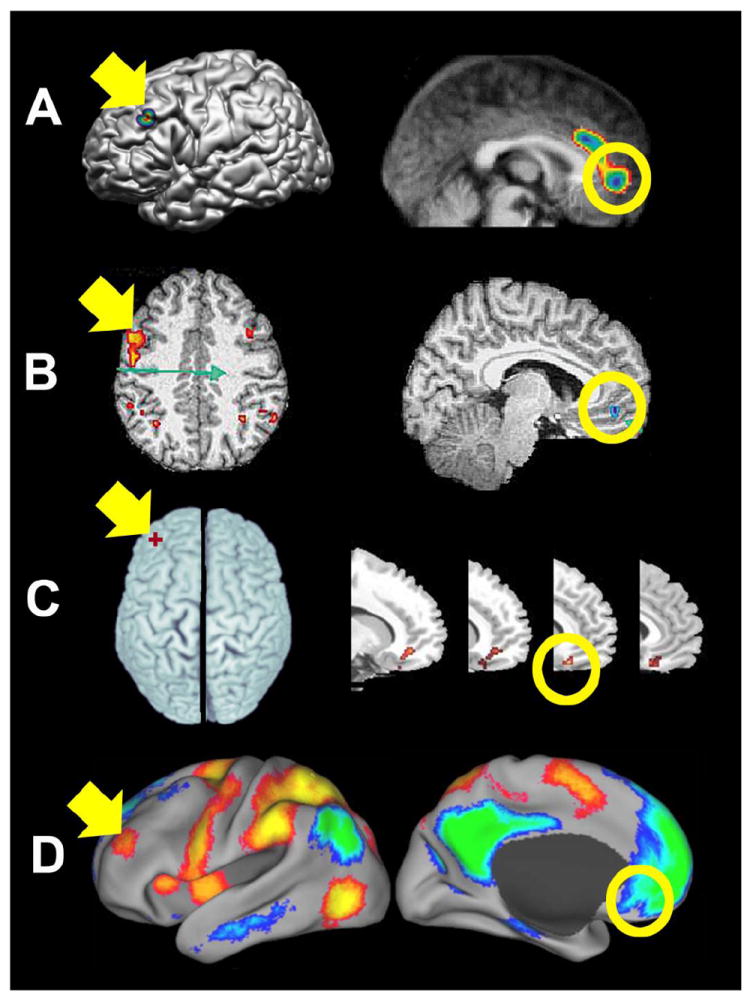
Functional connectivity between the left dorsal lateral prefrontal cotex (DLPFC, yellow arrows) and ventral medial prefrontal cortex (yellow circles) assessed with TMS/Imaging and resting state functional connectivity MRI. A) Regional CBF changes assessed with PET in response to double-pulse TMS to the left DLPFC (modified with permission from (Paus, Castro-Alamancos et al. 2001)). B) BOLD changes assessed with fMRI in response to 1 Hz TMS to the left DLPFC (modified with permission from (Li, Nahas et al. 2004)). C) Dopamine release (decreases in [11C]FLB 457 binding potential) in response to 10 Hz TMS to the left DLPFC (modified with permssion from (Cho and Strafella 2009)). D) Anticorrelated networks identified using resting state functional connectivity MRI based on correlations within a system and negative corelations between systems (modified with permission from (Fox, Snyder et al. 2005)).
Importantly, resting state fcMRI may enjoy several practical and theoretical advantages over task based fMRI for clinical applications, including improved signal to noise, reduced need for patient compliance, avoidance of task performance confounds, and expanded patient populations (Fox and Greicius 2010). Leveraging these advantages, significant resting state fcMRI abnormalities have been identified across almost every major neurological and psychiatric disease (for reviews see (Greicius 2008; Fox and Greicius 2010; Zhang and Raichle 2010)). These fcMRI abnormalities have been correlated with the severity of disease in depression (Greicius, Flores et al. 2007), schizophrenia (Bluhm, Miller et al. 2007; Vercammen, Knegtering et al. 2010), neglect (He, Snyder et al. 2007; Carter, Astafiev et al. 2010), and hemiparesis (Carter, Astafiev et al. 2010), and can differentiate normal controls from patients with Alzheimer’s disease (Li, Li et al. 2002; Greicius, Srivastava et al. 2004; Wang, Jiang et al. 2006; Supekar, Menon et al. 2008) or depression (Craddock, Holtzheimer et al. 2009).
Despite its potential, there are important limitations to measuring connectivity with resting state fcMRI. First, because patients are not performing a specific task there is no clear measure of performance or mental state. Second, resting state fcMRI is purely correlational in nature, not causal, limiting the conclusions that can be drawn. Third, it is difficult to separate coincidence task-evoked modulation from true connectivity. For example if one hears a beep and sees a flash at the same time the measured correlation between the visual and auditory cortex will increase, but this does not mean the synaptic strength of the connection between the regions has changed. Finally, resting state fcMRI is purely a way to measure, not manipulate functional connectivity. As resting state fcMRI abnormalities continue to be replicated, refined, and clarified, the next step will be translating this information into practical clinical interventions. In such an effort, fcMRI can offer valuable guidance and assessment tools, but combination with methods to manipulate connectivity will be critical.
Measuring connectivity with TMS
TMS is a noninvasive technique that utilizes short, rapidly changing magnetic field pulses to induce electrical currents in underlying cortical tissue (for reviews see (Kobayashi and Pascual-Leone 2003; Hallett 2007; Wagner, Valero-Cabre et al. 2007)). Single pulses can be used to briefly disrupt or excite underlying cortical tissue while repeated pulses (rTMS) at different frequencies can be used to create changes in cortical excitability that outlast the duration of the stimulation itself. Such lasting modulation of cortical excitability depends on the stimulation parameters and can resemble long-term potentiation (when rTMS is applied in higher frequency, bursting patterns, eg in burst of at 4 stimuli at 20 Hz with inter-burst pauses of 28 s) or long-term depression (when rTMS is applied at lower frequency as a continuous train, eg 20 min of continuous 1 Hz rTMS). The duration of these changes varies depending on the duration of the rTMS train, and can be extended to 90 minutes of modulation after only a few minutes of stimulation with special stimulation protocols, such as theta-burst stimulation (triplets of stimuli at 50 Hz applied at 5 Hz frequency either continuously or intermittently (Thut and Pascual-Leone 2010)). Such rTMS-induced modulation of cortical excitability can be done safely if published recommendations and established safety standards are followed (Rossi, Hallett et al. 2009). In addition to being a powerful research tool, significant clinical effects have been observed across a wide variety of neurological and psychiatric conditions (Burt, Lisanby et al. 2002; Fregni and Pascual-Leone 2007; Hallett 2007), and Neuronetics®’ Neurostar TMS protocol has been approved by the US Food and Drug Administration (FDA) for the treatment of certain patients with medication-resistant depression (Padberg and George 2009). Accumulating evidence, from human and animal studies, suggests that TMS modulates neuronal activity beyond the site of stimulation, impacting a distributed network of brain regions (Valero-Cabre, Payne et al. 2005; Valero-Cabre, Payne et al. 2007; Ruff, Driver et al. 2009; Siebner, Bergmann et al. 2009; Ferreri, Pasqualetti et al. 2010) and that therapeutic and behavioral effects of TMS are mediated by such distributed network effects. Given that TMS effects can propagate beyond the site of stimulation, it has become a powerful tool for measuring brain connectivity.
A simple example of a TMS-based connectivity measure involves delivering a single TMS pulse to primary motor cortex then measuring the induced contralateral muscle contraction in the form of a motor evoked potential (MEP). Note that for the TMS pulse to reach muscle it must cross synapses in the anterior horn of the spinal cord and at the neuromuscular junction. By analyzing the time it takes the TMS pulse to travel this path one can derive central conduction time, a TMS connectivity measure with some clinical utility in spinal injury (Brunholzl and Claus 1994), multiple sclerosis (Hess, Mills et al. 1986), and amyotrophic lateral sclerosis (Floyd, Yu et al. 2009).
Connectivity between separate cortical areas can be measured with TMS by pairing stimulations together with two TMS coils, aptly referred to as dual-coil experiments. In the classic example, a conditioning pulse (usually subthreshold) is applied to the primary motor cortex of one hemisphere followed by a test pulse to the motor cortex of the opposite hemisphere (Figure 1B). If the MEP induced by the test stimulus changes with the addition of the conditioning stimulus this suggests a functional connection between the two sites. Both corticocortical inhibition and facilitation can be observed between motor cortices depending on the relative timing of the conditioning and test stimulus (Ferbert, Priori et al. 1992; Hanajima, Ugawa et al. 2001). Similar effects on primary motor cortex have been observed with conditioning pulses to cerebellar and frontal sites (Ugawa, Day et al. 1991; Civardi, Cantello et al. 2001). Dual coil experiments can also be used to assess connectivity with primary and extrastriate visual cortex, where a single TMS pulse can induce the perception of a brief flash of light, called a phosphene. Phosphene perception can be altered based on precisely timed conditioning pulses to other visual areas, frontal eye fields, or parietal cortex (Pascual-Leone and Walsh 2001; Silvanto, Lavie et al. 2006; Silvanto, Muggleton et al. 2009). Properly employed, dual-coil methods can be a powerful technique for probing the timing and directionality of the connectivity between cortical regions (Pascual-Leone and Walsh 2001; Silvanto, Cowey et al. 2005).
Rather than using two TMS coils, brain connectivity can also be assessed by combining TMS with a second methodology to measure remote effects of stimulation in connected brain regions. This approach has resulted in an increasing number of TMS-EEG, TMS-PET, and TMS-fMRI experiments (Bestmann, Ruff et al. 2008; Ruff, Driver et al. 2009; Hampson and Hoffman 2010; Reithler, Peters et al. 2011). Remote effects can be measured simultaneously with TMS in an online approach, or before and after rTMS in an offline approach. While a full review of this extensive literature is beyond the scope of this paper, we highlight a few examples to illustrate the strengths of various multi-modal TMS-based connectivity approaches. For example, the temporal resolution of EEG has been utilized to time the spread of excitation to connected brain regions following focal TMS to the primary sensorimotor cortex (Ilmoniemi, Virtanen et al. 1997). The spatial resolution of PET has been used to show remote cerebral blood flow (CBF) increases in the parietal/occipital cortex in response to frontal eye field stimulation (Paus, Jech et al. 1997) and remote CBF decreases in the peri-cingulate region in response to stimulation to the dorsolateral prefrontal cortex (DLPFC) (Paus, Castro-Alamancos et al. 2001)(Figure 2A). Using PET radioligands specific to neurotransmitter binding sites, excitatory TMS to the left (but not right) DLPFC has been shown to cause dopamine release in the subgenual cingulate cortex (Cho and Strafella 2009) (Figure 2C).
Further improving on spatial resolution with fMRI, inhibitory TMS to the left dorsal premotor cortex has been shown to reduce activation in the left premotor cortex, but increase activation in the right dorsal premotor cortex and medial motor areas when subjects perform a subsequent motor task, resembling adaptive changes observed post stroke (O’Shea, Johansen-Berg et al. 2007). While technically challenging, simultaneous TMS-fMRI can provide both good spatial and temporal resolution (Bestmann, Ruff et al. 2008). For example, one can examine both the distributed activation pattern and time-course of TMS to the left DLPFC (Li, Nahas et al. 2004)(Figure 2B). Using this simultaneous approach, TMS to the frontal eye fields has been shown to increase activity in retinotopic representations of the peripheral visual field, but decrease activity in the central field, a result that matches psychophysical changes in contrast perception (Ruff, Blankenburg et al. 2006).
There are several important limitations to connectivity assessed with TMS. First, it stimulates neuronal tissue exogenously and artificially, thus connectivity revealed by TMS may be different than connectivity present under more physiological conditions. Second, TMS can only selectively target areas along the cortical surface, thus assessing connectivity to or between deep brain structures becomes difficult or impossible. Presently available ‘deep TMS coils’ such as the H-coil can enable penetration to deeper brain structures, but also stimulate surface cortex immediately under the coil and thus do not allow for selective deep stimulation (Roth, Amir et al. 2007; Deng, Peterchev et al. 2008). Eventually, multi-coil TMS arrays may offer technical solutions to this limitation. Third, connectivity measured with TMS alone (e.g. dual coil paradigms) can only be assessed in cortex with a clear TMS output effect (e.g. motor or visual cortices) and connectivity between other structures necessitates the addition of a secondary monitoring method (e.g. EEG or neuroimaging). Fourth, remote changes observed in response to TMS with EEG or neuroimaging could reflect other factors besides propagation of TMS activity along cortical connections creating some interpretive ambiguity. These factors could include associated effects of TMS (e.g. tapping sensation or clicking noise), behavioral or cognitive consequences of the TMS leading to changes in brain activity, or neuronal adaptation to the TMS perturbation. Finally, the selection of an appropriate stimulation target is an ongoing clinical problem in TMS, an issue that will be discussed further in our section on using functional connectivity to guide TMS target selection.
Does connectivity measured with fcMRI and TMS reflect the same underlying phenomenon?
TMS and resting state fcMRI are complimentary techniques that if combined might compensate for the limitations of either technique alone, providing insight into a variety of neuroscience questions and facilitating the translation of both techniques into clinical care. A first step towards combining these techniques is to determine if connectivity assessed with resting state fcMRI is the same as connectivity assessed with TMS. Unfortunately, there have been no experiments that directly compare the two connectivity measures in the same subjects. However, by comparing results across different studies some useful insights can be gained.
As one might expect, connectivity assessed using either resting state fcMRI or TMS is related to and constrained by underlying anatomical connectivity. DTI, a noninvasive measure of anatomical connectivity, has been shown to relate well to both functional connectivity measured with resting state fcMRI (Koch, Norris et al. 2002; De Luca, Beckmann et al. 2006; Lowe, Beall et al. 2008; Skudlarski, Jagannathan et al. 2008; van den Heuvel, Mandl et al. 2008; Greicius, Supekar et al. 2009; Honey, Sporns et al. 2009; van den Heuvel, Mandl et al. 2009; Zhang, Snyder et al. 2010) and connectivity as assessed with TMS (Wahl, Lauterbach-Soon et al. 2007; Voineskos, Farzan et al. 2010). Some of the strongest evidence comes from studies relating individual differences in transcallosal connectivity measured with DTI to that measured with resting state fcMRI (Lowe, Beall et al. 2008), paired pulse TMS (Wahl, Lauterbach-Soon et al. 2007; Wahl, Hubers et al. 2011), and TMS-EEG (Voineskos, Farzan et al. 2010). Surgical sectioning of the corpus callosum disrupts inter-hemispheric connectivity assessed with resting state fcMRI (Johnston, Vaishnavi et al. 2008) and individuals with agenesis of the anterior trunk of the corpus callosum show disrupted transcallosal inhibition with paired pulse TMS (Meyer, Roricht et al. 1995). It is important to note that connectivity assessed with either technique involves polysynaptic connections. For example, resting state fcMRI is present between regions in the monkey visual system with no direct anatomical connections (Vincent, Patel et al. 2007), and the simple presence of a muscle twitch after TMS to the motor cortex implies polysynaptic transmission.
An advantage of both fcMRI and TMS over purely anatomical connectivity measures is that they can provide information on the functional consequences of anatomical connections. Both resting state fcMRI and TMS have revealed results potentially consistent with excitatory versus inhibitory connections, however interpretation of these results and the relationship between techniques is likely to be complicated. For example, the bilateral somatomotor cortices are positively correlated when connectivity is assessed with resting state fcMRI (Figure 1A). This is consistent with inter-hemispheric facilitation using dual-coil TMS (Hanajima, Ugawa et al. 2001), changes in motor cortex excitability matching excitatory/inhibitory rTMS to the opposite side (Gorsler, Baumer et al. 2003), and some TMS-PET findings showing a contralateral increase in activity in response to excitatory M1 stimulation (Siebner, Peller et al. 2000; Ferrarelli, Haraldsson et al. 2004). However dual-coil TMS can also produce transcallosal inhibition (Ferbert, Priori et al. 1992) (figure 1b) and other TMS-PET studies have reported contralateral decreases in motor cortex activity in response to ipsilateral stimulation (Fox, Ingham et al. 1997; Fox, Narayana et al. 2006).
In a second example of how these techniques may provide insight into the functional consequences of anatomical connections, we consider the relationship between the left dorsal lateral prefrontal cortex (DLPFC) and the ventral medial prefrontal cortex (Figure 2). TMS-fMRI (Li, Nahas et al. 2004), TMS-PET measuring CBF (George, Stallings et al. 1999; Paus, Castro-Alamancos et al. 2001), TMS-PET measuring dopamine binding (Cho and Strafella 2009), and resting state fcMRI (Fox, Snyder et al. 2005) all suggest a functional connection between these two regions (Figure 2) which may have some precedence in track tracing results in monkeys (Vogt and Pandya 1987; Petrides and Pandya 1999). Interestingly, TMS-fMRI (Li, Nahas et al. 2004), TMS-PET measuring CBF (George, Stallings et al. 1999; Paus, Castro-Alamancos et al. 2001), and resting state fcMRI (Fox, Snyder et al. 2005) all suggest that this interaction may be inhibitory, such that when the DLPFC is stimulated with TMS or activity in the DLPFC increases spontaneously, activity in the ventral medial prefrontal cortex is suppressed. Obviously there is significant heterogeneity in the DLPFC and combined studies are needed before any real conclusions can be drawn, however this convergence across techniques could have important implications for network models of depression (Mayberg 2007). Further, there has been substantial debate surrounding the interpretation of anticorrelations observed with resting state fcMRI (Fox, Zhang et al. 2009; Murphy, Birn et al. 2009; Anderson, Druzgal et al. 2010), and evidence showing that stimulation to one region could causally suppresses activity in an anticorrelated region would go far in validating the functional importance of this relationship.
An important area where the relationship between resting state fcMRI and TMS is unclear is in context dependence of the measured connectivity. The idea that neuronal networks reorganize in the context of different task conditions has a strong precedent (Marder and Weimann 1991), and animal studies have shown context-dependent changes in neuronal synchrony (Engel, Fries et al. 2001; Varella, Lachaux et al. 2001). Similarly, accumulating evidence suggests that connectivity assessed with TMS depends on the task context (Koch and Rothwell 2009; Ruff, Driver et al. 2009). For example, in an elegant dual-coil TMS study connectivity was assessed between the left dorsal premotor cortex (conditioning pulse) and right primary motor cortex (test pulse) during a task in which subjects were cued to move either their right or left hand (Koch, Franca et al. 2006). A facilitatory connection was observed 75 ms after a tone indicating left hand movement (but not right hand movement), while an inhibitory connection was observed 100 ms after a tone indicating right hand movement (but not left hand movement). This shows that the strength and sign of the functional connection between these two regions varies with both time and task context. Due to its poorer temporal resolution and inability to exert causal perturbations, the context dependence of connectivity assessed with fcMRI remains less clear. Many groups have reported changes in fcMRI between rest conditions and task performance (Arfanakis, Cordes et al. 2000; Lowe, Dzemidzic et al. 2000; Hampson, Peterson et al. 2002; Hampson, Olson et al. 2004; Jiang, He et al. 2004; Morgan and Price 2004; Bartels and Zeki 2005; Fransson 2006; Nir, Hasson et al. 2006; Sun, Miller et al. 2006), generally reporting an increase in the correlation between regions similarly activated by the task and a decrease between regions not similarly activated. However, interpretation of these results is confounded by the superposition of task-evoked activity on top of resting state fluctuations (Fox, Snyder et al. 2006) and apparent context-dependent changes in connectivity can disappear after correction for task-evoked activity (Arfanakis, Cordes et al. 2000). Examining resting state fcMRI before and after tasks can circumvent this confound, an approach that has been used to document modulation of resting state functional connectivity by learning tasks (Albert, Robertson et al. 2009; Lewis, Baldassarre et al. 2009; Tambini, Ketz et al. 2010).
Finally, both techniques have identified connectivity changes across a range of altered states including neurological and psychiatric conditions with both concordant and discordant results (Burt, Lisanby et al. 2002; Fregni and Pascual-Leone 2007; Hallett 2007; Greicius 2008; Fox and Greicius 2010; Zhang and Raichle 2010). For example, both measures agree that there is a decrease in connectivity with sleep (Massimini, Ferrarelli et al. 2005; Horovitz, Braun et al. 2009), sedation (Greicius, Kiviniemi et al. 2008; Ferrarelli, Massimini et al. 2010), and across the corpus callosum in patients with multiple sclerosis (Lowe, Beall et al. 2008; Wahl, Hubers et al. 2011). However in blind subjects TMS-PET suggests increased connectivity between primary somatosensory and visual cortices (Wittenberg, Werhahn et al. 2004) while resting state fcMRI suggests that connectivity is decreased (Liu, Yu et al. 2007; Yu, Liu et al. 2008). Further work combining both measures in the same subjects and patient populations is needed to help understand the similarities and differences in these two connectivity techniques.
Using connectivity to guide TMS
The recognition that one is manipulating a network and not just a single brain region with TMS complicates an ongoing difficulty: How does one select the optimal site for stimulation? For example, clinical TMS for treatment of depression identifies the dorsal-lateral prefrontal cortex (DLPFC) stimulation site by moving 5 cm anterior to the motor cortex (George, Wassermann et al. 1996; Pascual-Leone, Rubio et al. 1996), a technique which frequently misses the DLPFC completely (Herwig, Padberg et al. 2001; Ahdab, Ayache et al. 2010) and contributes to variability in clinical response (Herbsman, Avery et al. 2009; Padberg and George 2009). TMS effects can be improved by targeting based on individual MRI anatomy (Gugino, Romero et al. 2001; Fitzgerald, Hoy et al. 2009) and even further augmented using individual fMRI derived activation foci (Sack, Cohen Kadosh et al. 2009). However, these approaches have translated into only modest clinical improvements. For example, anatomical DLPFC targeting improved depression scores more than standard targeting, but the study’s primary outcome measure failed to reach significance (Fitzgerald, Hoy et al. 2009). Similarly, three depression trials targeting TMS based on foci of hypometabolism in the prefrontal cortex failed to improve patient outcomes beyond standard targeting (Herwig, Lampe et al. 2003; Garcia-Toro, Salva et al. 2006; Paillère Martinot, Galinowski et al. 2010). One of the critical limitations of these efforts to improve TMS targeting may be that they have focused on the stimulation site alone and have not taken into account the distributed network properties of the targeted region.
Despite its potential, surprisingly few studies have used distributed network connectivity to guide TMS target selection. In an excellent example of how connectivity can guide TMS, diffusion tensor imaging (DTI) was used to identify subject-specific targets in the middle frontal gyrus that were connected to a particular portion of primary somatosensory cortex (Hannula, Neuvonen et al. 2010). TMS to this focus improved tactile working memory, but not TMS to non-connected portions of the middle frontal gyrus located just 18 mm away.
A few studies have used task-based fcMRI measures (as opposed to resting state fcMRI) to identify stimulation targets (Bien, Roebroeck et al. 2009; de Graaf, Jacobs et al. 2009; Zanto, Rubens et al. 2011). In perhaps the best example of this approach, functional connectivity with extrastriate visual areas (V4 and V5) during the encoding phase of a selective-attention delayed-recognition task was used to identify subject-specific targets in the inferior frontal junction (IFJ) thought to be involved in top-down modulation (Zanto, Rubens et al. 2011). Inhibitory TMS to this site disrupted both behavioral performance and EEG measures of top-down influence. Further, the magnitude of the TMS-induced change in EEG was related to the strength of functional connectivity between IFJ and V4 across subjects. Similar studies have used task-based functional connectivity to target frontal TMS targets correlated with posterior parietal cortex during a visuospatial judgment task (de Graaf, Jacobs et al. 2009) or correlated with regions involved in a set of imitation tasks (Bien, Roebroeck et al. 2009) with similar disruption in task performance. Although these studies certainly speak to the potential of functional connectivity to guide TMS target selection, an issue that complicates interpretation of these findings is the fact that the frontal targets are themselves activated by the task. It is therefore difficult to determine if it is truly the connectivity to other regions that mediates the frontal TMS effect, or if these regions could be identified just as well using traditional activation mapping. If the latter is true, the observed TMS effect could simply be the result of disrupting another region involved in the task without any clear dependence on connectivity. Further efforts linking the magnitude of TMS-induced changes to the strength of the functional connectivity between regions (Zanto, Rubens et al. 2011), or showing that TMS to a connected region not modulated by the task has an effect on task performance will be important in clarifying these issues.
Finally, a handful of studies have begun using resting state fcMRI to guide TMS target selection. Eldaief and colleagues recently used resting state fcMRI with the posterior cingulate to target rTMS to a connected region of the lateral parietal cortex in order to modulate activity within the default mode network (Eldaief, Halko et al. 2011). In an early example of using resting state fcMRI to guide therapeutic TMS, Hampson and colleagues targeted inhibitory TMS to regions correlated with Wernikes area in a small set of patients with schizophrenia and continuous auditory hallucinations (Hoffman, Hampson et al. 2007). Unfortunately rTMS to these targets did not lead to symptomatic improvement. Recently, we have examined the utility of resting state fcMRI to address the above referenced clinical problem of determining where to target rTMS in the DLPFC to improve antidepressant response (Fox, Buckner et al. 2012). We first identified DLPFC target coordinates known to be more effective versus less effective based on prior TMS clinical studies (Fitzgerald, Hoy et al. 2009) (Fig 4A). We then examined differences in fcMRI between these two targets and found that more effective sites were more negatively correlated (anticorrelated) with the subgenual cingulate cortex, a region thought to play a key role in the pathophysiology of depression and antidepressant response (Mayberg, Lozano et al. 2005; Drevets, Savitz et al. 2008; Mayberg 2009) (Fig 4B). Based on these results, we extracted the BOLD time course from the subgenual cingulate (Fig 4C) then used fcMRI to identify a theoretically optimal target site in the DLPFC (4D). While this initial analysis was performed on a population of subjects, this approach could be similarly used to identify individualized TMS targets for specific patients. Obviously, clinical trials are needed to determine the clinical utility of this approach, but this connectivity-based targeting paradigm has the potential to improve therapeutic stimulation across a range of diseases with distributed network pathology.
Figure 4.
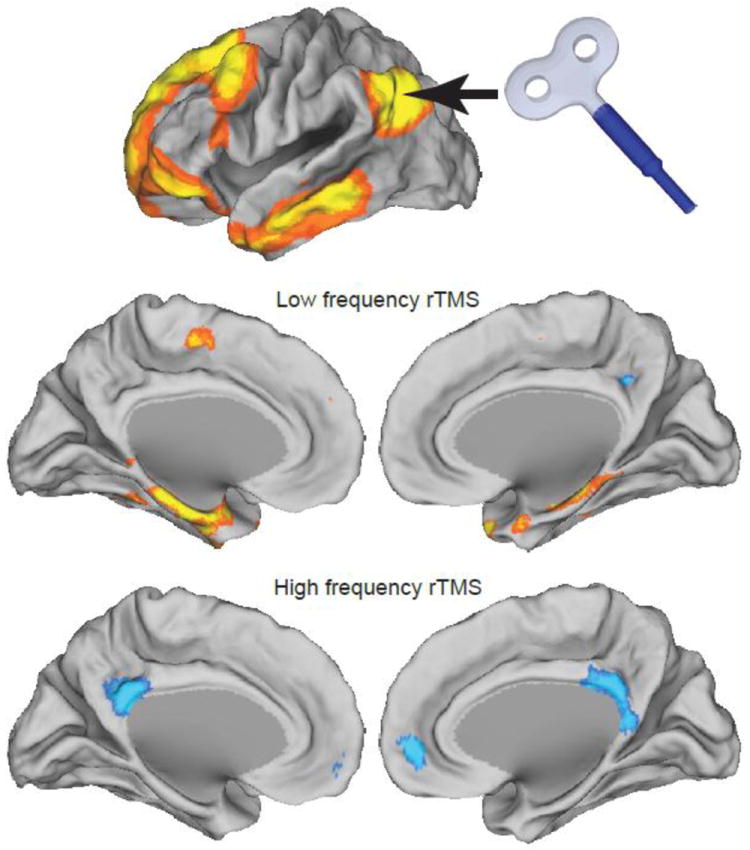
Modulating resting state functional connectivity networks using TMS. Both inhibitory and excitatory TMS were applied to the left inferior parietal lobule, part of the default mode network (top row). Inhibitory TMS resulted in pronounced increases in functional connectivity between the stimulation site and the medial temporal lobe (middle row) while excitatory TMS resulted in decreased correlation between the stimulation site and other nodes of the default mode network (bottom row). (Modified from (Eldaief, Halko et al. 2011)).
Moving forward, we anticipate great value in targeting TMS based on individualized connectivity with distributed brain networks, however there are a number of obstacles which must be overcome to validate the clinical utility of such a targeting approach. We delineate these obstacles here to encourage initiatives in this regard:
Identification of a remote region or network to be manipulated
Connection between the region or network one is trying to manipulate and a target on the cortical surface accessible by TMS
Spatial heterogeneity of the connectivity in the targeted region (for targeting based on connectivity to be advantageous to anatomy alone)
Subject to subject heterogeneity of the connectivity of the targeted region (for individualized targeting to be advantageous over average coordinates)
Reproducibility of individualized target identification across sessions
Manipulating Connectivity with TMS
A unique advantage of TMS compared to fcMRI, and every other noninvasive approach for assessing connectivity, is that TMS can also be used to manipulate connectivity. In fact, it is becoming apparent that some of the clinical effects of rTMS may be due more to TMS induced changes in connectivity between brain regions than local effects on the stimulated region itself (Grefkes, Nowak et al. 2010). Further, as techniques such as resting state fcMRI continue to identify reproducible pathological abnormalities in connectivity the ability of TMS to manipulate connectivity will become increasingly important.
Two different TMS-based approaches have been employed to alter connectivity, repetitive TMS (rTMS), by far the most popular approach, and paired associative stimulation (PAS), which will be discussed later. While it can be argued that the local effects of rTMS on cortical excitability are due to changes in connectivity within the stimulated region itself, the current review is focused on connectivity between brain regions. rTMS induced changes in connectivity between regions have been studied using a wide variety of connectivity measurement techniques including dual-coil TMS (Pal, Hanajima et al. 2005), TMS-PET (Paus, Castro-Alamancos et al. 2001), EEG coherence (Jing and Takigawa 2000; Strens, Oliviero et al. 2002; Oliviero, Strens et al. 2003; Fuggetta, Pavone et al. 2008; Zanto, Rubens et al. 2011), task-based effective connectivity with PET (Lee, Siebner et al. 2003), task-based effective connectivity with fMRI (Pleger, Blankenburg et al. 2006; Grefkes, Nowak et al. 2010), and finally resting state fcMRI (van der Werf, Sanz-Arigita et al. 2010; Vercammen, Knegtering et al. 2010; Eldaief, Halko et al. 2011) (TABLE 1).
Table 1.
Connectivity changes in the human brain observed in response to focal rTMS
| Connectivity Measurement | Stimulation | Connectivity Change | Comments | References |
|---|---|---|---|---|
| Dual-Coil TMS during rest | Inhibitory rTMS to primary motor cortex | Decreased inter-hemispheric inhibition with contralateral M1 | Difficult to exclude local effects (although persists when the strength of the conditioning stimulus is adjusted) | (Pal, Hanajima et al. 2005) |
| Dual-Coil TMS during rest and task | Inhibitory rTMS (continuous theta burst) to the anterior intraparietal area | Decreased connectivity between ventral premotor and M1 during grasp preparation | Effects were context dependent (not seen during rest) | (Davare, Rothwell et al. 2010) |
| Resting state EEG coherence | Excitatory rTMS to primary motor cortex | Decreased ipsilateral cortico-cortical alpha band coherence | (Oliviero, Strens et al. 2003; Fuggetta, Pavone et al. 2008) | |
| Resting state EEG coherence | Inhibitory rTMS to primary motor cortex | Increased ipsilateral cortico-cortical alpha band coherence | Effects observed up to 25 min post stimulation | (Strens, Oliviero et al. 2002) |
| Resting state EEG coherence | Excitatory rTMS to the left frontal area | Increased directed coherence from stimulated site to other cortical nodes (especially parietal) | Intra-hemispheric change more pronounced than the inter-hemispheric change | (Jing and Takigawa 2000) |
| Task-based EEG coherence | Inhibitory rTMS to the premotor area | Increase in task related coherence between motor regions | (Chen, Mima et al. 2003) | |
| Task-based EEG coherence | Inhibitory rTMS to the right inferior frontal junction | Decreased ipsilateral alpha band coherence during task | (Zanto, Rubens et al. 2011) | |
| Resting state TMS-PET | Excitatory rTMS to the left DLPFC | Increased connectivity from DLPFC to cingulate regions | Difficult to exclude local effect of rTMS on the DLPFC | (Paus, Castro-Alamancos et al. 2001). |
| Resting state functional connectivity with PET | Inhibitory rTMS to the left temporal-parietal junction | Decreased connectivity between the stimulated node and a wide variety of regions | Difficult to exclude local effect of rTMS on the TPJ, performed in patients with schizophrenia and auditory hallucinations | (Horacek, Brunovsky et al. 2007) |
| Task-based effective connectivity with PET | Inhibitory rTMS to primary motor cortex | Decreased connectivity between stimulated M1 and premotor / mesial motor areas. Increased coupling between an inferomedial portion of M1 and anterior motor areas. | (Lee, Siebner et al. 2003) | |
| Task-based effective connectivity with fMRI | Excitatory rTMS to primary sensory cortex | Increased effective connectivity from S1 to M1 | Persists up to 120 min; correlated with behavioral improvement in tactile discrimination | (Pleger, Blankenburg et al. 2006) |
| Task-based effective connectivity with fMRI | Inhibitory rTMS to contralesional M1 in stroke patients | Increased effective connectivity between ipsilesional M1 and ipsilesional SMA | Related to clinical improvement in the movement of the paretic hand | (Grefkes, Nowak et al. 2010). |
| Resting state fcMRI | Inhibitory rTMS to the left TPJ versus sham | Increased connectivity between the left TPJ and the right insula | Performed in patients with schizophrenia and auditory hallucinations | (Vercammen, Knegtering et al. 2010) |
| Resting state fcMRI | Inhibitory rTMS to the left DLPFC versus sham stimulation | Decreased connectivity between the DMN and lateral temporal cortices; trend towards decreased connectivity with the hippocampus. | (van der Werf, Sanz-Arigita et al. 2010) | |
| Resting state fcMRI | Excitatory and Inhibitory rTMS to the left inferior parietal lobule | Excitatory: Decreased connectivity within the DMN Inhibitory: Increased connectivity with hippocampus | (Eldaief, Halko et al. 2011) |
Given the variety of different connectivity measurement techniques used in the above studies, it is highly likely that rTMS can indeed be used to alter cortico-cortical connectivity. Each of these different approaches offers unique advantages and disadvantages; however taken collectively they raise several important points regarding assessing rTMS-induced connectivity changes.
First, it is important to consider whether an observed change in connectivity actually reflects a change in connection strength between remote areas or whether it could be explained by local effects of the rTMS alone. This is particularly problematic if TMS perturbation to the area just stimulated with rTMS is part of the connectivity measure (Paus, Castro-Alamancos et al. 2001; Pal, Hanajima et al. 2005). Pal et al. showed appropriate concern for this issue in their dual-coil paradigm by adjusting the conditioning stimulus to maintain motor evoked potential amplitude; however this cannot completely exclude local effects not measured by the MEP. Even if one is not using TMS as part of the connectivity measure, differentiating changes in connectivity from purely local effects remains difficult. Studies that find a change in connectivity between remote regions that have not been stimulated make an important advance in this regard (Davare, Rothwell et al. 2010; Grefkes, Nowak et al. 2010; van der Werf, Sanz-Arigita et al. 2010). Second, when connectivity is being assessed during a task, it is important to determine if the measured change in connectivity is actually due to a change in behavior (as opposed to the change in behavior being due to a change in connectivity). Studies in which the stimulation does not change task performance are helpful in excluding this possibility (Lee, Siebner et al. 2003), but note that a change in cognitive or behavioral strategy could alter brain activity while not being captured by task performance. Third, it is important to control for as many non-specific effects as possible. An ideal study would vary stimulation frequency, stimulation site, and the networks examined to show maximal specificity of an rTMS induced connectivity change. For example, excitatory rTMS over primary motor cortex decreased ipsilateral cortico-cortical alpha band coherence (Oliviero, Strens et al. 2003) while inhibitory stimulation increased it (Strens, Oliviero et al. 2002), showing specificity of the observed connectivity change for the stimulation frequency. Finally, in the case of effective connectivity it is important to recognize that results will be constrained by the model applied. Other regions or connections not included in the model could be significantly altered and would be missed by the model-driven analysis.
Assessing rTMS induced connectivity changes with resting state fcMRI may help avoid some of the above interpretive difficulties; therefore we expect studies in this area to increase. An early study to examine such effects acquired resting state fcMRI data following low frequency rTMS to left DLPFC and sham stimulation (van der Werf, Sanz-Arigita et al. 2010). In an analysis restricted to the default mode network, they showed that rTMS resulted in a reduction in functional connectivity between the default mode network and lateral temporal cortices with a trend towards reduced functional connectivity with the bilateral hippocampus. Although sham controlled, they did not show specificity of the effect to their network of interest, stimulation site, or stimulation frequency. A recent study incorporating some of these additional controls acquired resting state fcMRI data before and after low and high frequency stimulation to the left posterior inferior parietal lobule, a node of the default mode network (Eldaief, Halko et al. 2011). Following low frequency rTMS, intrinsic correlations were increased between the stimulation site and the hippocampal formation. Following high frequency stimulation, correlations between multiple nodes of the default node network were decreased but correlations with the hippocampus were unchanged (Figure 4). No significant effects were seen in other networks such as somatomotor, visual, or auditory. While this study was again limited to one stimulation site, they showed specificity for their network of interest and stimulation frequency. Comparing results across these two rTMS - resting state fcMRI studies, low frequency stimulation appears to have opposite effects on functional connectivity between the default mode network and the hippocampus depending on the stimulation site. Interestingly, resting state fcMRI correlations observed between the two stimulation sites and the hippocampus are also opposite; the DLPFC is negatively correlated with the hippocampus while the inferior parietal lobule is positively correlated (Figure 2D)(Fox, Snyder et al. 2005). Whether this observation is anything more than coincidence will require future work.
The ability of rTMS to manipulate connectivity as measured by resting state fcMRI raises the possibility that it may be used to modify resting state fcMRI abnormalities observed in disease states that might result in behavioral gains for the patient. The above rTMS-induced manipulations of resting state fcMRI in the default mode network may prove valuable in disorders where fcMRI abnormalities in this network have been observed, including schizophrenia (Whitfield-Gabrieli, Thermenos et al. 2009), depression (Greicius, Flores et al. 2007) and Alzheimer’s disease (Greicius, Srivastava et al. 2004). To our knowledge, only one study of rTMS-induced changes in connectivity has been aimed at rectifying resting state fcMRI abnormalities in patients (Vercammen, Knegtering et al. 2010). Based on prior work relating the severity of auditory hallucinations to reduced resting state connectivity between the left temporal parietal junction (TPJ) and bilateral cingulate and amygdala (Vercammen, Knegtering et al. 2010) and evidence that inhibitory rTMS to the left TPJ could improve these symptoms (Freitas, Fregni et al. 2009), it was hypothesized that rTMS might normalize functional connectivity between these regions. In a study of 18 patients with schizophrenia there was a trend towards symptomatic benefit but no rTMS-induced change in resting state connectivity between the left TPJ and bilateral cingulate or amygdala. However there was an rTMS-induced increase in connectivity between the left TPG and right insula not seen with sham stimulation.
The above study in patients with auditory hallucinations represents an excellent example of how one might combine resting state fcMRI with TMS to identify then correct abnormalities in brain connectivity, however, it is important to realize that in the pathological brain, restoring a normal pattern of activity within a given neural network may not be the most effective way to suppress symptoms. Instead, what might need to be done is induce other changes that may prove behaviorally more adaptive. In addition, the study by Vercammen et al (Vercammen, Knegtering et al. 2010) also highlights a potentially important limitation of rTMS. While rTMS does appear to alter connectivity, it currently seems to do so in unpredictable ways, often between unexpected regions. If the goal is to selectively increase or decrease connectivity between specific brain regions in a controlled manner, advances in our understanding of rTMS or alternative approaches will likely be needed. One alternative approach that may help address this issue is termed paired associative stimulation and uses Hebbian principles of synaptic plasticity to modify connectivity in a highly controlled manner.
The original studies of paired associative stimulation dealt not with cortical-cortical connections, but connections between cortex and peripheral nerve (Stefan, Kunesch et al. 2000; Wolters, Sandbrink et al. 2003). If stimuli to the median nerve and motor cortex are paired with an ISI of 25 ms (such that they arrive nearly simultaneously at the motor cortex) a phenomenon similar to long-term potentiation occurs. A subsequent TMS pulse to the motor cortex will result in a larger motor evoked potential in median innervated muscles suggesting that the connection strength has been increased. If the ISI is changed to 10 ms (such that there is an offset of 15 ms at the motor cortex) a phenomenon similar to long-term depression occurs and subsequent MEPs will be decreased. Derivations of this technique have used endogenous motor activity rather than median nerve stimulation (Thabit, Ueki et al. 2010) or timed stimuli to arrive with specific offsets in the spinal cord rather than the motor cortex (Cortes, Thickbroom et al. 2011) with similar effects. However, the most pertinent derivation of this technique for the present discussion is the use of paired associative stimulation to specifically modulate corticocortical connections (Plewnia, Rilk et al. 2008; Rizzo, Siebner et al. 2009; Buch, Johnen et al. 2011).
In the first paper to use this approach, two TMS coils were used to apply simultaneous 10 Hz stimulation to both, the left primary motor cortex and the visual cortex at the occipital pole, with the goal of enhancing polysynaptic connectivity between the two regions (Plewnia, Rilk et al. 2008). An increase in EEG coherence was seen specifically on the stimulated side that was not seen with M1 stimulation alone. While provocative, this study did not vary the timing of the stimuli to show specificity to simultaneous stimulation versus independent effects of rTMS at the two sites. A subsequent study applied single pulses to the left then right motor cortices at a delay of 8 ms and frequency of 1 Hz (Rizzo, Siebner et al. 2009). Following 90 of these paired pulses, but only at this specific delay, there was a marked reduction in inter-hemispheric inhibition. While solidifying the importance of timing, it remains unclear why this study resulted in a decrease rather than an increase in connectivity. Finally, in perhaps the clearest example of this approach, paired associative stimulation was used to modulate connectivity strength between the ventral premotor cortex and M1 (Buch, Johnen et al. 2011). Applying pulses first to ventral premotor cortex followed by M1 at an appropriate delay led to an increase in the connection strength between these two regions. The effect was anatomically specific and reversing the order of the paired stimuli led to a reversal of the effect (i.e. a decrease in connectivity). Particularly promising for improving the duration of therapeutic TMS, residual effects on connectivity could be seen up to 3 hours after the stimulation (Buch, Johnen et al. 2011). Although currently limited to TMS accessible sites on the cortical surface, the technique of corticocortical paired associate stimulation shows great promise for selectively increasing or decreasing connectivity between specific brain regions. Future work is needed to determine if this approach can lead to behavioral manifestations and whether it will be useful for modifying connectivity abnormalities observed with resting state fcMRI in neuropsychiatric disorders in order to promote symptomatic relief.
Conclusions
TMS and resting state fcMRI are complimentary approaches for assessing brain connectivity with individual limitations that might be overcome by combining the two techniques. Areas of particular value include using connectivity to guide TMS target selection and using TMS to modulate abnormal network interactions identified with resting state fcMRI. Together, they may further insight into a variety of interesting neuroscience questions, facilitate the translation of both techniques into clinical care, and move us closer to the goal of a reliable, noninvasive method for controlled, individualized neural network modulation.
Figure 3.
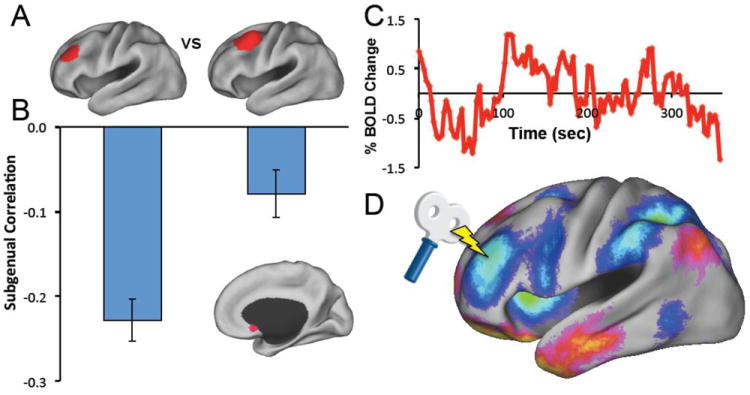
Using resting state fcMRI to target therapeutic TMS. A) TMS targets in the left dorsal lateral prefrontal cortex (DLPFC) known to be more effective (left) versus less effective (right) at producing an antidepressant response. B) Resting state functional connectivity reveals that the more effective target is more negatively correlated (anticorrelated) with the subgenual (inset) compared to the less effective target. C) Resting state BOLD time course extracted from the subgenual. D) Resting state functional connectivity identifies a theoretically optimal stimulation target in the left DLPFC based on anticorrelation with the subgenual. (Modified from (Fox, Buckner et al. 2012))
Highlights.
Resting state fcMRI and TMS can both be used to measure human brain connectivity
TMS can non-invasively manipulate brain connectivity.
Combining fcMRI and TMS offers several advantages and holds great promise
Resting state fcMRI may guide optimal target selection for TMS
TMS may normalize pathological network interactions identified with fcMRI
Acknowledgments
MDF was supported by NIH Grant R25NS065743. MCE was supported by the Clinical Investigators Training Program of the Beth Israel Deaconess Medical Center and the Harvard-MIT Division of health Sciences and Technology. APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and holds intellectual property on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Work on this study was also supported by grants from the national Institutes of Health and National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758) to Dr. Pascual-Leone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahdab R, Ayache SS, et al. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiologie clinique = Clinical neurophysiology. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, et al. The resting human brain and motor learning. Curr Biol. 2009;19(12):1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, et al. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human brain mapping. 2010;00 doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, et al. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magnetic Resonance Imaging. 2000;18:921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(9):3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions - A new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, et al. Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res. 2008;191(4):383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- Bien N, Roebroeck A, et al. The brain’s intention to imitate: the neurobiology of intentional versus automatic imitation. Cerebral cortex (New York, N Y : 1991) 2009;19:2338–2351. doi: 10.1093/cercor/bhn251. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, et al. Spontaneous Low-Frequency Fluctuations in the BOLD Signal in Schizophrenic Patients: Anomalies in the Default Network. Schizophr Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunholzl C, Claus D. Central motor conduction time to upper and lower limbs in cervical cord lesions. Arch Neurol. 1994;51(3):245–249. doi: 10.1001/archneur.1994.00540150039013. [DOI] [PubMed] [Google Scholar]
- Buch ER, Johnen VM, et al. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(48):17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt T, Lisanby SH, et al. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5(1):73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Korb AS, et al. A review of low-intensity focused ultrasound pulsation. Brain stimulation. 2011;4(3):125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Mima T, et al. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114(9):1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS one. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civardi C, Cantello R, et al. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14(6):1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, et al. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8(11):1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton MV, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cortes M, Thickbroom GW, et al. Spinal associative stimulation: A non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, Holtzheimer PE, 3rd, et al. Disease state prediction from resting state functional connectivity. Magn Reson Med. 2009;62(6):1619–1628. doi: 10.1002/mrm.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Rothwell JC, et al. Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr Biol. 2010;20(2):176–181. doi: 10.1016/j.cub.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf TA, Jacobs C, et al. FMRI effective connectivity and TMS chronometry: complementary accounts of causality in the visuospatial judgment network. PLoS One. 2009;4(12):e8307. doi: 10.1371/journal.pone.0008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, et al. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith SM, et al. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental Brain Research. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, et al. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Peterchev AV, et al. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS) Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5675–5679. doi: 10.1109/IEMBS.2008.4650502. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, et al. Distinct brain networks for adaptive and stable task control in humans. P N A S. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, et al. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, et al. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, et al. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, et al. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, et al. Development of distinct task control networks through segregation and integration. P N A S. 2007 doi: 10.1073/pnas.0705843104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, et al. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Haraldsson HM, et al. A [17F]-fluoromethane PET/TMS study of effective connectivity. Brain Res Bull. 2004;64(2):103–113. doi: 10.1016/j.brainresbull.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy K, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34(5):1255–1262. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- Floyd AG, Yu QP, et al. Transcranial magnetic stimulation in ALS: utility of central motor conduction tests. Neurology. 2009;72(6):498–504. doi: 10.1212/01.wnl.0000341933.97883.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, et al. Intrinsic functional connectivity with the subgenual cingulate predicts clinical efficacy of TMS targets for depression. American Academy of Neurology Annual Meeting; New Orleans. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. P N A S. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. P N A S. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, et al. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56(1):171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, et al. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature Neuroscience. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, et al. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P, Ingham R, et al. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8(12):2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, et al. Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Hum Brain Mapp. 2006;27(6):478–487. doi: 10.1002/hbm.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Freitas C, Fregni F, et al. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophrenia research. 2009;108(1-3):11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, et al. Functional connectivity: the pricipal component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, et al. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: a combined EEG and TMS study. Hum Brain Mapp. 2008;29(1):1–13. doi: 10.1002/hbm.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and light sleep. Magnetic Resonance Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Toro M, Salva J, et al. High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry research. 2006;146(1):53–57. doi: 10.1016/j.pscychresns.2004.08.005. [DOI] [PubMed] [Google Scholar]
- George MS, Stallings LE, et al. Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Human Psychopharmacology: Clinical and Experimental. 1999;14:161–170. [Google Scholar]
- George MS, Wassermann EM, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8(2):172–180. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- Gorsler A, Baumer T, et al. Interhemispheric effects of high and low frequency rTMS in healthy humans. Clin Neurophysiol. 2003;114(10):1800–1807. doi: 10.1016/s1388-2457(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, et al. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50(1):233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings National Academy of Sciences USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, et al. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceeding National Academy of Sciences USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral cortex (New York, N Y : 1991) 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112(10):1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, et al. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Hoffman RE. Transcranial magnetic stimulation and connectivity mapping: tools for studying the neural bases of brain disorders. Frontiers in systems neuroscience. 2010;4:1–8. doi: 10.3389/fnsys.2010.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, et al. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15:1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, et al. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531(Pt 3):849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula H, Neuvonen T, et al. Increasing top-down suppression from prefrontal cortex facilitates tactile working memory. NeuroImage. 2010;49:1091–1098. doi: 10.1016/j.neuroimage.2009.07.049. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Herbsman T, Avery D, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66(5):509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Herwig U, Lampe Y, et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res. 2003;37(4):267–275. doi: 10.1016/s0022-3956(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Herwig U, Padberg F, et al. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001;50(1):58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, et al. Measurement of central motor conduction in multiple sclerosis by magnetic brain stimulation. Lancet. 1986;2(8503):355–358. doi: 10.1016/s0140-6736(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hampson M, et al. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17(11):2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C, Sporns O, et al. Predicting Human Resting-State Functional Connectivity from Structural Connectivity. P N A S. 2009 doi: 10.1073/pnas.0811168106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, et al. Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2007;55(3-4):132–142. doi: 10.1159/000106055. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, et al. Decoupling of the brain’s default mode network during deep sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8(16):3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, et al. Modulation of functional connectivity during the resting state and the motor task. Human Brain Mapping. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(9):1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28(25):6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, et al. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26(28):7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Rothwell JC. TMS investigations into the task-dependent functional interplay between human posterior parietal and motor cortex. Behavioural brain research. 2009;202:147–152. doi: 10.1016/j.bbr.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Koch MA, Norris DG, et al. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage. 2002;16:241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, et al. Resting-state functional connectivity indexes reading competence in children and adults. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(23):8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, et al. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Siebner HR, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23(12):5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, et al. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Li Z, et al. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225(1):253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, et al. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biological psychiatry. 2004;55:882–890. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu C, et al. Whole brain functional connectivity in the early blind. Brain. 2007 doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Beall EB, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Human brain mapping. 2008;29:818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Dzemidzic M, et al. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–587. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, et al. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Marder E, Weimann JM. Modulatory control of multiple task processing in the stomatogastric nervous system. In: Kien J, McCrohan C, Winlow B, editors. Neurobiology of Motor Program Selection: New Approaches to Mechanisms of Behavioral Choice. Manchester, U.K.: Manchester University Press; 1991. pp. 3–19. [Google Scholar]
- Massimini M, Ferrarelli F, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309(5744):2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119(4):717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, et al. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(Pt 2):429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Price RR. The effect of sensorimotor activation on functional connectivity mapping with fMRI. Magnetic Resonance Medicine. 2004;22:1069–1075. doi: 10.1016/j.mri.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, et al. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, et al. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, et al. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30(4):1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, et al. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54(3):479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Strens LH, et al. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res. 2003;149(1):107–113. doi: 10.1007/s00221-002-1344-x. [DOI] [PubMed] [Google Scholar]
- Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219(1):2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Paillère Martinot M-L, Galinowski A, et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010;13:45–59. doi: 10.1017/S146114570900008X. [DOI] [PubMed] [Google Scholar]
- Pal PK, Hanajima R, et al. Effect of low-frequency repetitive transcranial magnetic stimulation on interhemispheric inhibition. J Neurophysiol. 2005;94(3):1668–1675. doi: 10.1152/jn.01306.2004. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292(5516):510–512. [Google Scholar]
- Paus T, Castro-Alamancos Ma, et al. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. European Journal of Neuroscience. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, et al. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17(9):3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, et al. Functional connectivity changes with concentration of sevoflurane anaesthesia. Neuroreport. 2005;16(3):285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European journal of neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, et al. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J Neurosci. 2006;26(7):1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Rilk AJ, et al. Enhancement of long-range EEG coherence by synchronous bifocal transcranial magnetic stimulation. The European journal of neuroscience. 2008;27(6):1577–1583. doi: 10.1111/j.1460-9568.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- Reithler J, Peters JC, et al. Multimodal transcranial magnetic stimulation: Using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Progress in Neurobiology. 2011:1–17. doi: 10.1016/j.pneurobio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, et al. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cerebral cortex. 2009;19(4):907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, et al. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25(1):230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, et al. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007 doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Y, Amir A, et al. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol. 2007;24(1):31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J, et al. Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex. 2009;45(9):1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Cohen Kadosh R, et al. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21(2):207–221. doi: 10.1162/jocn.2009.21126. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, et al. The relation of ongoing brain activity, evoked neural responses, and cognition. Frontiers in systems neuroscience. 2010;4:20. doi: 10.3389/fnsys.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2(2):58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54(4):956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cowey A, et al. Striate cortex (V1) activity gates awareness of motion. Nat Neurosci. 2005;8(2):143–144. doi: 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, et al. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96(2):941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, et al. The perceptual and functional consequences of parietal top-down modulation on the visual cortex. Cereb Cortex. 2009;19(2):327–330. doi: 10.1093/cercor/bhn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, et al. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43(3):554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, et al. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ. Analyzing effective connectivity with fMRI. Wiley Interdiscip Rev Cogn Sci. 2010;1(3):446–459. doi: 10.1002/wcs.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, et al. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113(8):1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- Sun FT, Miller LM, et al. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cerebral Cortex. 2006;17(5):1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, et al. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabit MN, Ueki Y, et al. Movement-related cortical stimulation can induce human motor plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(34):11529–11536. doi: 10.1523/JNEUROSCI.1829-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain topography. 2010;22(4):219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, et al. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, et al. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176(4):603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, et al. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163(1):1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, et al. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28(43):10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, et al. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, et al. Efficiency of functional brain networks and intellectual performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf YD, Sanz-Arigita EJ, et al. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC neuroscience. 2010;11:145. doi: 10.1186/1471-2202-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varella F, Lachaux J-P, et al. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, et al. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biological psychiatry. 2010;67(10):912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, et al. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. Journal of psychiatric research. 2010;44(11):725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, et al. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, et al. Coherent spontaneous activity identifies a hippocampal-parietal mnemonic network. Journal of Neurophysiology. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of comparative neurology. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Farzan F, et al. The Role of the Corpus Callosum in Transcranial Magnetic Stimulation Induced Interhemispheric Signal Propagation. Biological psychiatry. 2010;68:825–831. doi: 10.1016/j.biopsych.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, et al. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wahl M, Hubers A, et al. Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Hum Brain Mapp. 2011;32(6):846–855. doi: 10.1002/hbm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27(45):12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Jiang T, et al. Discriminative analysis of early Alzheimer’s disease based on two intrinsically anti-correlated networks with resting-state fMRI. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2006;9(Pt 2):340–347. doi: 10.1007/11866763_42. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GF, Werhahn KJ, et al. Functional connectivity between somatosensory and visual cortex in early blind humans. Eur J Neurosci. 2004;20(7):1923–1927. doi: 10.1111/j.1460-9568.2004.03630.x. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89(5):2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Yu C, Liu Y, et al. Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp. 2008;29(5):533–543. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, et al. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature neuroscience. 2011;14(5):656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nature Reviews Neurology. 2010;6(Jan) doi: 10.1038/nrneurol.2009.198. In press. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, et al. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20(5):1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, et al. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(28):10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


