Abstract
Free radical-induced oxidation of membrane phospholipids generates complex mixtures of oxidized phospholipids (oxPLs). The combinatorial operation of a few dozen reaction types upon a few dozen phospholipid structures results in the production of a dauntingly vast diversity of oxPL molecular species. Structural identification of the individual oxPL in these mixtures is a redoubtable challenge that is absolutely essential to allow determination of the biological activities of individual species. With an emphasis on cardiovascular consequences, this review focuses on biological activities of oxPLs whose molecular structures are known and highlights two diametric opposite approaches that were used to determine those structures, i.e. (1) the classical approach from bioactivity of a complex mixture to isolation and structural characterization of the active molecule followed by confirmation of the structure by unambiguous chemical synthesis, and (2) hypothesis of products likely to be generated by lipid oxidation, followed by synthesis, and then detection in vivo guided by the availability of authentic standards, and lastly, characterization of biological activities. Especially important for the application of the second paradigm is the capability of LC-MS/MS and derivatizations to selectively detect and quantify specific oxPL in complex mixtures, without the need for their isolation or complete separation. This technology can provide strong evidence for identity by comparisons with pure, well characterized samples available by chemical syntheses. Those pure samples are critical for determining the biological activities attributable to specific molecular species of oxPLs in the complex mixtures generated in vivo as a consequence of oxidative stress.
Keywords: Cardiovascular Disease, Oxidized Phospholipids, Liquid Chromatography-Mass Spectrometry
1.1 Introduction: two approaches to identifying biologically active oxidized phospholipids (oxPLs)
The present review introduces the molecular diversity and biological activities of oxPLs and protein modifications arising from them that occur in vivo and that accumulate with ageing. OxPLs and their protein adducts are involved in cardiovascular phenomena as diverse as angiogenesis, atherogenesis, thrombosis, and inflammation. Progress in understanding the consequences of phospholipid oxidation in human biology is impeded by the logistics of identifying the huge variety of oxPLs generated by free radical-induced lipid oxidation. Determining the biological activities of individual oxPLs requires access to pure samples. Isolation of individual oxPLs from biological extracts that arise from oxidation of the complex mixtures of PLs present in vivo, or even from mixtures of oxPLs generated by in vitro oxidation of pure PLs is a redoubtable task. The present review focuses on two complementary approaches that address this challenge: (1) activity-guided detection and isolation followed by structural characterization confirmed by unambiguous chemical synthesis; and its diametric opposite, (2) structural hypothesis-inspired chemical synthesis followed by detection in vivo and, lastly, determination of biological activities.
1.2 Free radical-induced oxidation generates a large molecular diversity of oxPLs
Nonregioselective hydrogen atom abstraction from doubly allylic methylenes of polyunsaturated fatty acyl groups produces peroxyl radical intermediates (Fig. 1). These are converted into a cascade of products, e.g., through further oxidation, cyclization, fragmentation, and oxygen atom transfer reactions. Hundreds of structurally unique products may be generated from a single fatty acyl precursor. Taking into account stereoisomerism, a variety of polar head groups and alkyl or acyl groups appended at the sn-1 position, the mixtures of oxPLs generated in vivo may include thousands of different species. For example, arachidonyl PLs are converted into various multifunctional cyclic oxPLs such as epoxy isoprostanes (Fig. 1) for which 4 structural isomers are expected as well as 16 stereoisomers of each, or a total of 64 isomers. Structural characterization of the epoxyisoprostane oxPLs of Fig. 1, presented in part I of this review, epitomizes the classical “activity-guided” approach to identification of naturally occurring biologically active oxPLs. Structural identification of the oxidatively truncated γ-hydroxyalkenal oxPLs and the carboxyalkyl pyrrole protein modifications derived from them (Fig. 1), presented in part II of this review, illustrates a powerful alternative “structural hypothesis-guided” approach.
Figure 1. Polyunsaturated phospholipid peroxyl radicals undergo cyclization and fragmentation reactions.
Free radical-initiated oxidation of polyunsaturated phospholipids (PL = 2-lyso phospholipid) by hydrogen atom abstraction from doubly allylic CH2 groups generates pentadienyl radicals that react with molecular oxygen to produce peroxyl radicals. Multifunctional cyclic oxPLs are generated through cyclization of peroxyl radicals and reaction with additional molecular oxygen can produce hydroperoxy endoperoxides that rearrange to hydroperoxy isoprostanes. Dehydrative cyclization delivers epoxy isoprostane phospholipids, and further dehydration produces epoxycyclopentenone PLs. Oxidative fragmentation of peroxyl radicals produces oxidatively truncated oxPLs. γ-Hydroxyalkenal oxPLs adduct with protein lysyl ε-amino groups to produce carboxyalkyl pyrroles after phospholipolysis.
1.3 Oxidation of pure polyunsaturated phospholipids simplifies the isolation of bioactive products
To achieve a fundamental molecular level understanding of the biological consequences of phospholipid oxidation, structural identification of individual oxPL molecular species is critically important. Because the biological activities of PL oxidation product mixtures are a composite of the activities of the component oxPLs, the availability of pure samples of each oxPL is absolutely essential for a deconvoluted, high-resolution analysis of their individual contributions to the biological activities of the complex oxPL mixtures. One tactic to facilitate identification of biologically active oxPLs is to simplify product mixtures by generating them from a single pure precursor phospholipid. Free radical-induced oxidation of 1-palmityl-2-arachidonyl-sn-glycero-3-phosphocholine (PAPC) generates a product mixture that exhibits some of the biological activities of mildly oxidized “minimally modified” low-density lipoprotein (MM-LDL).1 However, even with such a simplified mixture of oxPLs, the biological effects remain complex.2–5 For example, treatment of human aortic endothelial cells (HAECs) with oxPAPC influences the expression of more than a thousand genes6 and results in phosphorylation of 228 proteins.7 Furthermore, these product mixtures contain both phospholipid and non-phospholipid products, and the activity of the oxPL may also be expressed by the corresponding oxidized polyunsaturated free fatty acid. For example, both oxPAPC and oxidized arachidonic acid induce expression of interleukin-6 in osteoblasts.8 Confirmation that a particular activity of a biological extract, MM-LDL or oxPAPC arises from a phospholipid component of the mixture is provided by the observation that the activity is abolished by phospholipolysis. However, only the availability of pure individual oxPLs allows unambiguous determination of their biological activities. The isolation of pure oxPL from biological extracts or even the simpler product mixtures generated from a pure precursor phospholipids is a formidable task. There is a powerful alternative that does not require isolation from such mixtures. Rather, using the “structural hypothesis-guided” approach, the presence of specific oxPL in complex mixtures can be demonstrated by LC-MS/MS comparisons with pure samples of individual oxPLs generated by unambiguous chemical syntheses. In this review, examples of the “activity-guided” approach are presented in Part I while the “structural hypothesis-guided” approach is exemplified in Part II.
Part I. Activity assay, isolation, structural identification, and confirmation by chemical synthesis
2.1 Isolation and identification of oxPLs that promote leucocyte binding to endothelial cells
The observation that MM-LDL promotes adhesion of monocytes to endothelial cells9 suggested that oxidative damage to LDL might foster migration of monocytes into the subendothelial space, an initial step in atherogenesis. To identify the specific oxPL responsible for the biological effect, the activity of MM-LDL was localized in three phospholipid fractions that were isolated by HPLC.1 These fractions exhibited positive mass spectral parent ions at m/z 594.3, 610.3, and 828.6. The same three active oxPL fractions were detected in oxidized 1-palmityl-2-arachidonyl-sn-glycero-3-phosphocholine (oxPAPC) and in fatty streak lesions from cholesterol-fed rabbits. The oxidized phosphatidylcholine (oxPC) with m/z 594.3 was suspected to be 1-palmityl-2-(5-oxovaleryl)-sn-glycero-3-phosphocholine (POVPC). The presence of an aldehyde functional group was indicated by the production of a methoxime upon treatment with methoxyamine and by reduction with sodium borohydride to deliver an alcohol (Fig. 2). The identity of this oxPC was then confirmed by comparisons with a pure sample prepared by an unambiguous chemical synthesis.1
Figure 2. Generation of truncated phospholipids by oxidative fragmentation and chemical synthesis.
Oxidative cleavage of PAPC generates POVPC that is further oxidized to PGPC. Mass spectroscopidc comparison with authentic samples (prepared by unambiguous chemical syntheses) of oxPLs generated from PAPC, as well as derivatives generated by reactions of the oxPLs with methoxyamine, sodium borohydride or pentaflourobenzyl bromide, confirmed their molecular structures. Analogous oxidative cleavage of 1-palmityl-2-linoleyl-sn-glycero-3-phosphocholine (PLPC) generates PONPC that is further oxidized to PAzPC.
Quantities of the oxPC with m/z 610.3 isolated from oxPAPC were initially insufficient for structural identification. However, this same oxPC was serendipitously generated by adventitious autoxidation of the aldehyde group in POVPC during the chemical synthesis of POVPC. This accidental synthesis facilitated identification of this minor oxPC from oxPAPC as 1-palmityl-2-(5-glutaryl)-sn-glycero-3-phosphocholine (PGPC). This molecular structure was confirmed by derivatization with pentafluorobenzyl bromide and by unambiguous synthesis from glutaric anhydride and 1-palmityl-2-lyso-sn-glycero-3-phosphocholine (PC-OH).10 Individual pure oxPL are essential to deconvolute the biological activities of oxPL mixtures. Chemical syntheses provide a convenient supply of pure POVPC11 and PGPC to facilitate confirmation of their biological activities. The association of their abundance with the extent of atherosclerotic lesions in an animal model of atherosclerosis provided presumptive evidence for an atherogenic role in vivo.1
2.2 POVPC and PGPC regulate endothelial binding of monocytes and neutrophils differently
The activities of POVPC and PGPC can be quite different and even diametrically opposed. The effects of these oxPL on leukocyte endothelial interactions are dramatically different. POVPC increases monocyte binding to endothelial cells by inducing the surface expression of the connecting segment (CS)-1 domain of fibronectin, but does not increase neutrophil binding, and also strongly inhibits lipopolysaccharide (LPS)-induced neutrophil binding and expression of E-selectin, a major adhesion molecule that mediates neutrophil-endothelial interactions.12 In contrast, PGPC induces both monocyte and neutrophil binding and expression of E-selectin and vascular cell adhesion molecule (VCAM) 1 (Fig 3B). Eventually it became evident that the activities of POVPC are often also exhibited by the oxPL with m/z 828.6 mentioned above, subsequently identified as one or more epoxyisoprostane phosphatidylcholine (PEIPC) isomers, that are generally more potent than PGPC or POVPC (vide infra). However, there are some activities that are unique to POVPC (Fig. 3A) or PGPC (Fig. 3B). It is noteworthy that the spontaneous nonenzymatic interconversion of POVPC to PGPC is unidirectional, i.e., irreversible. Therefore, under conditions of oxPL accumulation and prolonged oxidation, the levels of POVPC will decrease while those of PGPC increase, and the biological activities of POVPC will be replaced by those of PGPC.
Figure 3.
Panel A. POVPC activates aortic smooth muscle cell proliferation: POVPC actives UDP-galactose:glucosylceramide(β1→4)galactosyltransferase (GalT-2) to produce lactosylceramide (LacCer) that provokes the production of superoxide, presumably by stimulating NADPH oxidase (Nox).13 This leads to activation of the cytosolic transcription factor p44 MAPK. The phosphorylated form of p44 MAPK translocates to the nucleus where it promotes expression of the protooncogene c-fos and proliferating cell nuclear antigen (PCNA) resulting in cell proliferation. Panel B. PGPC Enhances neutrophil-EC interaction, monocyte maturation and, with PONPC and PAzPC, promotes blood coagulation, and POVPC inhibits LPS-induced expression of E-Selectin: PGPC promotes expression of vascular cell adhesion molecule (VCAM)-1 and E-selectin on the apical EC surface, weakly activates PPARγ (2 fold at 6.6 μg/ml) in the nucleus of monocytes, and triggers a bifurcated cascade initiated by a rise in cytosolic calcium concentration resulting in expression of tissue factor (TF) on ECs.74 POVPC exhibits none of these activities, but rather inhibits LPS-induced E-selectin expression. The procoagulant activity of TF is amplified by the inhibition of tissue factor pathway inhibitor (TFPI) by PONPC and PAzPC, homologues of POVPC and PGPC respectively, neither of which inhibit TFPI. Panel C. POVPC, PGPC & HAzPC initiate apoptosis: OxPC enter cells through TMEM30a. They disrupt the mitochondrial membrane, cause release of cytochrome c and AIF that enter the nucleus and induce expression of caspase resulting in apoptosis.
2.3 Proliferation of vascular smooth muscle cells is promoted by POVPC
The proliferation of aortic smooth muscle cells is a characteristic of the pathogenesis of atherosclerosis. The oxPL POVPC was identified as the biologically active component MM-LDL that initiates this phenomenon (Fig. 3A).11, 13 This aldehydic fragmentation product from PAPC, but not PGPC, the carboxylic acid product of further oxidation, nor the isoprostanoid PEIPC, stimulates the activity of UDP-galactose:glucosylceramide(β1→4)galactosyl-transferase (GalT-2) to produce lactosylceramide (LacCer) that acts as a second messenger leading to the production of superoxide, presumably by stimulating NADPH oxidase (Nox). This results in activation of the cytosolic protein kinase p44 MAPK. The phosphorylated form of p44 MAPK translocates to the nucleus where it promotes expression of the protooncogene c-fos and proliferating cell nuclear antigen (PCNA) resulting in cell proliferation.
2.4 PGPC promotes neutrophil-EC binding, monocyte maturation and expression of tissue factor
PGPC12, but neither POVPC nor PEIPC14, promotes neutrophil-endothelial interaction through the expression of vascular cell adhesion molecule (VCAM)-1 and E-selectin on the apical EC surface (Fig. 3B). PGPC, activates peroxisome proliferator-activated receptor (PPAR)γ in the nucleus of monocytes15 resulting in monocyte maturation, expression of CD36, and uptake of oxLDL, leading ultimately to their transformation into foam cells. PGPC, but not POVPC, also triggers a bifurcated cascade initiated by a rise in cytosolic calcium concentration resulting in expression of tissue factor (TF) on ECs. POVPC exhibits none of these activities, but rather inhibits E-selectin expression triggered by PG-PC, as well as by LPS16 or TNF. It is especially interesting to note that the procoagulant activity of TF is amplified by the inhibition of tissue factor pathway inhibitor (TFPI) by 1-palmityl-2-(9-oxononanoyl)-sn-glycero-3-phosphocholine (PONPC) and 1-palmityl-2-azelayl-sn-glycero-3-phosphocholine (PAzPC), homologues of POVPC and PGPC respectively (Fig. 3B), neither of which inhibit TFPI. This remarkable selectivity further highlights the importance of identifying specific molecular mediators of the biological activities of MM-LDL and of recognizing that PA-PC is only one of many precursors of oxPL contained in LDL. Both PONPC and PAzPC are generated through the oxidative cleavage of 1-palmityl-2-linoleyl-sn-glycero-3-phosphocholine (PLPC) and not PAPC that spawns the homologous oxidative fragmentation products POVPC and PGPC (see Fig. 2). PONPC and PAzPC were also generated in vitro by ozonolysis of 1-palmityl-2-oleyl-sn-glycero-3-phosphocholine.
2.5 PGPC, PAzPC and the ether analog HAzPC promote mitochondrial swelling and apoptosis
Accumulation of apoptotic macrophages and smooth muscle cells parallels the progression of atherosclerosis.17 Treatment of isolated rat liver mitochondria with PGPC caused mitochondria to increase in volume by 50% of that caused by a high concentration of Ca+2 (Fig. 3C) POVPC was less than half as effective whereas the ether phospholipid 1-hexadecyl-2-azelayl-sn-glycero-3-phosphocholine (HAzPC) was only slightly more effective than PGPC (Fig. 3C).18 Treatment of human umbilical vein endothelial cells (HUVECs) with HAzPC caused apoptosis inducing factor (AIF), an integral mitochondrial inner membrane protein, to move to the nucleus and induced cytochrome c leakage from the mitochondrial compartment into the cytoplasm. This non receptor-mediated mitotoxic activity involves internalization of the bioactive phospholipids through transport by a transmembrane 30 Kd protein TMEM30a that provides a mechanism for these oxidatively-truncated PLs found in the extracellular environment during atherogenesis19 to become intracellular effectors.20
2.6 Epoxyisoprostane phospholipids are potent inducers of monocyte binding to endothelial cells
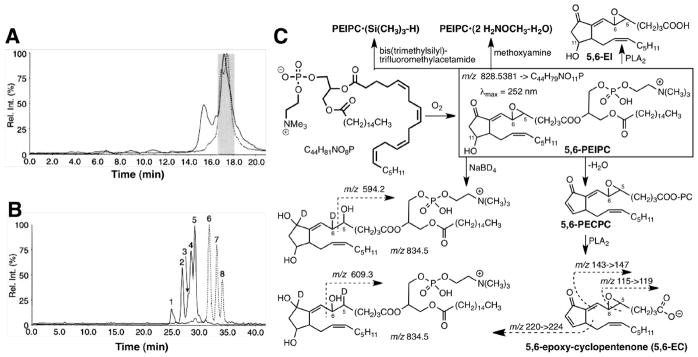
Determining the molecular structure of the biologically active oxPC from oxPAPC with m/z 828 was far more challenging21, and epitomizes characterization of natural products through activity-guided isolation. A biologically active fraction from oxPAPC was eluted from a normal phase HPLC column (Fig. 4A). Reverse phase HPLC separated this fraction into 5 peaks exhibiting m/z 828 that represent at least 5 isomers (Fig. 4B).
Figure 4. LC-MS/MS isolation and structural characterization of epoxyisoprostanes.
Reconstructed selected ion chromatograms from ESI-LC/MS of oxPAPC showing peaks for 1-palmityl-2-(epoxy-isoprostane)-sn-glycero-3-phosphorylcholines (PEIPCs) m/z 828.5 (solid line) and 1-palmityl-2-(epoxycyclopentenone)-sn-glycero-3-phosphorylcholines (PECPCs) m/z 810.5 (dashed line) for (panel A) normal phase HPLC separation of oxPAPC and (panel B) reverse phase separation of the fraction shaded in panel A (adapted from J biol chem. 1999;274:24787–24798). Panel C: the structure of one of the products, 5,6-PEIPC, from oxidation of PAPC was established by analysis of its mass and ultraviolet spectra as well as those of the product of dehydration (5,6-PECPC) and phospholipolysis (5,6-EC), and reduction with sodium borodeuteride.
Only the oxPC in one of the peaks induced endothelial cells to bind monocytes. Structural identification of the active oxPC in this peak as a 5,6-epoxy-isoprostane E2 ester of 2-lyso-PC (5,6-PEIPC) was achieved by derivatization, mass, ultraviolet, and NMR spectra (Fig. 4C).21 Trimethylsilylation produced a monosilyl ether indicating the presence of a single hydroxyl. Facile dehydration suggested a β-hydroxy-carbonyl array. Generation of an adduct incorporating two molecules of methoxyamine with loss of one molecule of water suggested the presence of one aldehyde or ketone carbonyl and an epoxide. An α,β-unsaturated carbonyl was indicated by a UV absorption at 252 nm. Reduction with borodeuteride added two deuteria and two protons, and the mass spectrum of the products showed fragments with m/z 594.2 and 609.3 confirming the carbonyl group and indicating a 5,6-epoxy group. Phospholipase A2 (PLA2)-catalyzed phospholipolysis delivered free acids 5,6-EI from PEIPC and 5,6-EC (see Fig. 4C) from its dehydration product, 1-palmityl-2-(5,6-epoxycyclopentenone)-sn-glycero-3-phosphorylcholine (5,6-PECPC). The structure of the sn-2 acyl moiety, 5,6-EC, was confirmed by comparison of its mass spectrum with that of the product of 16O→18O exchange of the carboxylic oxygen atoms that adds 4 Da to fragment ions containing the carboxyl group. Ultimately, the putative structure of 5,6-PEIPC was confirmed by an unambiguous chemical synthesis.22, 23
2.7 5,6-PEIPC activates EP2 causing CS-1 fibronectin deposition on ECs and monocyte adhesion
PEIPC enhances monocyte-endothelial cell interaction via multiple signaling pathways. Early studies showed that the MM-LDL component POVPC promotes deposition of CS-1 fibronectin on the apical surface of ECs.12 The availability of 5,6-PEIPC allowed the discoveries that it is a major contributor to this activity of MM-LDL.14 It increases monocyte adhesion to activated endothelial cells by generating intracellular cAMP through activation of a G protein-coupled receptor24, which was shown to be the prostaglandin E2 receptor EP2,25 leading to generation of the second messenger cAMP that then activates a kinase cascade starting with PKA, then R-RAS and PI3K14 leading to α5β1 integrin activation resulting in increased deposition of CS-1 fibronectin that enhances monocyte binding. Notably, the availability of individual oxPL allowed a high resolution analysis of the molecules responsible for the activities of MM-LDL and oxPAPC. Although POVPC promotes fibronectin deposition on ECs, it is not a ligand for EP2 and, therefore, must do so through activation of a different adenylate cyclase-coupled membrane receptor.12
2.8 Oxidation of PAPC generates a large family of epoxyisoprostane phospholipid isomers
Free radical-induced cyclooxygenation of PAPC presumably generates four structurally isomeric families of PEIPC diastereomers from isoprostane endoperoxide precursors (Fig. 5). Of the biologically active oxPCs isolated from the oxidation of PAPC, the mixture of PEIPC isomers is most active, inducing monocyte binding to endothelial cells below 0.5 μM while 5 μM levels of POVPC and PGPC are necessary for bioactivity. The m/z 810.5 dehydration products (i.e. PECPCs) of all the m/z 828.5 isomers were inactive as were the free fatty acids, EIs from PEIPCs and ECs from PECPCs (Fig. 4) liberated from m/z 810.5 and m/z 828.5 by PLA2-catalyzed phospholipolysis. Thus, the hydroxyl group at the 11 position in the oxidized fatty acid is essential for the biological activity of 5,6-PEIPC fostering monocyte binding, and this activity is a property of the phospholipid rather than its fatty acid moiety alone. This counter intuitive finding is remarkable because the prototypical ligand for EP2, prostaglandin E2, is a free acid. This finding underscores the need for pure samples of individual oxPLs that often are best acquired by chemical syntheses.
Figure 5. Dehydrative cyclization of isoprostanoid hydroperoxy endoperoxides generates epoxyisoprostanes.
Oxidation of PAPC generates four structurally isomeric mixtures of stereoisomeric PEIPCs. Mass spectra of the epoxyisoprostane (EI) free acids or the derived EI-diols allowed identification of structural isomers. Dehydration of PEIPCs delivers the corresponding 1-palmityl-2-(epoxycyclopentenone)-sn-glycero-3-phosphorylcholines (PECPCs). The 11,12-PECPC, a cyclopentenone, is 185% more potent than its hydroxy cyclopentanone precursor 11,12-PEIPC for transcriptional activation of PPARα.
Evidence supporting the presence of the three families of structural isomers of 5,6-PEIPC in the product mixture generated upon oxidation of PAPC was secured by detection of characteristic MS fragment ions from the epoxyisoprostane (EI) free acids consistent with the presence of epoxy groups at the 8,9- (m/z 127 and 155), 11,12- (m/z 225) or 14,15- (m/z 265) positions of the sn-2 fatty acyl groups (Fig. 5), in addition to UV spectra, dehydration, perchloric acid-catalyzed epoxide hydrolysis, derivatizations (trimethylsilylation, borohydride reduction, adduction of methoxyamine), and PLA2-promoted phospholipolysis.26 Fractions containing predominately 5,6-, 8,9-, or 11,12-PEIPC were isolated from oxPAPC.
2.9 PEIPCs and PECPCs promote synthesis of the chemokines MCP-1 and IL-8 through activation of endothelial cells
Having established a link between monocyte binding with endothelial cells and the occurrence of POVPC and PEIPCs in atherosclerotic lesions, other potential roles for these oxPCs in atherogenesis were investigated. Since monocyte chemotactic protein (MCP)-1 and interleukin (IL)-8 are important regulators of atherogenesis owing to transmigration of monocytes into the subendothelial space, the possibility was examined that these chemokines are produced by HAECs in response to oxPCs.15 While POVPC and PGPC showed some activity15, PEIPCs and their PECPC dehydration products (Fig. 5) are far more active.26 In contrast with the stringent structural requirement that only 5,6-PEIPC and not other PEIPC structural isomers or their PECPC dehydration products (Fig 5) fosters monocyte-endothelial cell binding, endothelial cell activation resulting in MCP-1 and IL-8 production is promoted by several PEIPC isomers and their PECPC dehydration products. Furthermore, although this activity is substantially reduced, it is not abolished by borohydride reduction, but most activity is lost upon hydrolysis of the epoxy group or PLA2-promoted phospholipolysis.26 Thus, considerable structural isomeric flexibility is tolerated, but the epoxide and intact phospholipid functionality are crucial for induction by these oxPCs of chemokine production in HAECs.
Evidence was secured in follow-up studies that 5,6-PEIPC initially binds to a 37-kDa GPI-anchored protein27 that interacts with Toll-like receptor (TLR)4 to induce IL-8 transcription in HAEC, HeLa, and human microvascular endothelial cells.27 IL-8 induction consequent to TLR4 activation proceeds through activation of PPARα26, which also promotes generation of MCP-1. Notably, the expression of the atherogenic chemotactic factors IL-8 and MCP-1 is promoted by a variety of oxPL. This is understandable because, in contrast with EP2, the TLR has the much broader ligand structural acceptance typical of a pattern recognition receptor. Besides PEIPC and PECPC isomers noted above, both POVPC and PGPC activate ECs to synthesize MCP-1 and IL-8 mRNA and protein by activating the PPARα ligand-binding domain and a consensus PPAR response element (PPRE) in ECs.15 This chemokine production, monocyte attraction and chemotaxis promotes migration of monocytes into the subendothelial space where they can be activated and become macrophages, and eventually, foam cells and components of atherosclerotic plaques.
Other pathways leading to IL-8 induction include a c-Src kinase dependent (1) activation of JAK 228, leading to phosphorylation and nuclear translocation of the signal transducer and activator of transcription (STAT)3 and induction of IL-8 as well as (2) phosphorylation of VEGFR229 (not involving the generation of VEGF) that activates the sterol regulatory element binding protein (SREBP) leading to induction of IL-8. STAT3 may bind to the potential γ-interferon activation sequence (GAS) element in the human IL-8 promoter. PEIPC-induced interleukin (IL)-8 synthesis, in turn, enhances monocyte activation and firm adhesion to endothelial cells. Pathways involved in the oxPAPC-promotion of SREBP-mediated generation of the atherogenic chemotactic factor IL-8 are remarkably diverse. POVPC activates endothelial nitric oxide synthase (eNOS), through an PI3K/Akt-dependent mechanism, that not only generates NO but also superoxide that combine to produce peroxynitrite that, in turn, promotes activation of SREBP. After activation, SREBP translocates to the nucleus where it binds to the sterol regulatory element in the IL-8 promoter and induces IL-8 transcription.30 In addition, a non receptor-mediated PEIPC-induced depletion of cholesterol from the EC plasma membrane through caveolin internalization to the EC endoplasmic reticulum31 also activates SREBP and consequently promotes IL-8 induction as well as transcription of the LDL receptor and hydroxymethylglutaryl CoA synthase. As might be expected for different pathways, the structure-activity requirements for oxPC promotion of monocyte-endothelial cell binding – that is initiated by the EP2 receptor – and transcriptional activation of PPARα – that is initiated by the TLR4 – are entirely different. All of the PEIPC and PECPC isomers promote transcriptional activation of PPARα in transfected HeLa cells.26 11,12-PECPC is 90–280% more active than any of the PEIPCs. Thus, in contrast with the loss of activity, i.e., recognition by EP2, for promoting monocyte binding with endothelial cells upon dehydration of 5,6-PEIPC to 5,6-PECPC, dehydration of 11,12-PEIPC to produce the cyclopentenone 11,12-PECPC, is accompanied by a 185% increase in activity, i.e., recognition by TLR4, for the transcriptional activation of PPARα. Clearly, the hydroxyl group is essential for binding EP2 but not TLR4.
2.10 Epoxyisoprostane phospholipids promote cell and membrane protective responses
Heme oxygenase (HO)-1 protects against oxidative stress owing to potent antioxidant and anti-inflammatory effects. HO-1 levels are strongly increased in HUVECs and HAECs by oxPAPC and PEIPC.32, 33 Thus, PEIPC-induced generation of cAMP by EP2 leads to activation of PKA and consequent phosphorylation of the cAMP-responsive element-binding protein (CREB) that promotes expression of HO-1. OxPAPC also promotes phosphorylation of CREB through activation of the PKC→ERK1/2→EGR-1 cascade and by elevated Ca+2 through activation of p38MAPK.33 Both of these signaling pathways can be initiated by PGPC (Fig. 3B). The oxidative stress response gene OKL38 (bone marrow derived growth factor) is also stimulated by PEIPC but not POVPC or PGPC. OKL38 is expressed in ECs, monocytes, and macrophages and, as for stimulation of HO-1 expression; stimulation of OKL38 expression is mediated via transcription factor nuclear factor E2-related factor 2 (Nrf2). Nrf2 is activated by superoxide produced by activated NADPH oxidase (Nox).
The oxPAPC-induced generation of cAMP also can inhibit the acute inflammatory response induced by tissue necrosis factor (TNF)α or LPS that results in NFκB-mediated expression of E-selectin and consequent neutrophil adhesion to ECs. Although POVPC, but not PGPC, was first recognized to exert this inhibition12, 34, PEIPC accounts for the majority of the inhibitory activity in oxPAPC. In MM-LDL the inhibitory effects of POVPC and PEIPC overpower12, 34 the promotion of E-selectin expression by PGPC (Fig. 3B). Nevertheless, since POVPC is readily oxidized to PGPC, it is tempting to speculate that PGPC may accumulate in foam cells or advanced atherosclerotic plaques, and that inflammatory activities of PGPC could overpower the cell protective activities of POVPC and PEIPC. PEIPC (or perhaps the free acid EI) but not POVPC, down regulates the expression of the inflammatory protein TNFα and upregulates expression of the antiapoptotic interleukin 10 (IL10) in human macrophages and monocytes owing to the EP2-mediated generation of cAMP.25 The inhibition of TNFα expression could provide damage control by protecting cells from the overproduction of toxic reactive oxygen species produced by activated neutrophils, and thus could promote resolution of an acute inflammatory response. Counter intuitively, the antiapoptotic activity of IL10 can contribute to the pathogenesis of atherosclerosis. This is because it can inhibit the apoptosis of foam cells, smooth muscle cells, and macrophages in the growing atherosclerotic plaque.
In vitro studies on the effects of individual oxPC found in oxPAPC on the barrier function of human pulmonary artery endothelial cells (HPAECs) revealed structurally specific activities. In contrast to POVPC and PGPC that promote decreased endothelial barrier function, PEIPC promotes the repair of vascular leakage such as that associated with the chronic inflammation of atherosclerosis.35 This activity is only exhibited by one or a small number of PEIPC isomers. This might imply EP2 or DP3 receptor involvement. However, although the EP2-mediated activation of endothelial α5β1 integrin only responds to PEIPC, one or a few PECPC isomers are also active in promoting endothelial barrier function. Therefore, this structure-activity relationship is more reminiscent of the TLR4-mediated induction of IL-8. OxPAPC induces translocation of the small GTPases Rac and Cdc42 from the cytosol to the EC membrane where they mediate cytoskeletal remodeling, inducing the formation of lamellipodia and filopodia respectively and promoting new actin polymerization at the cell-cell interface.35 Thus, Rac activation stimulates cell spreading and enhanced cortical actin rim formation. These cell barrier repair activities could be important for the resolution of vascular leakage associated with acute inflammation.
2.11 Epoxyisoprostane phospholipids can dampen the innate immune response to pathogens
The high resolution identification of specific oxPL that mediate the pathological sequelae of phospholipid oxidation can reveal remarkable structural specificity. Among the host derived oxPL generated upon microbial infection with Mycobacterium leprae that accumulate in macrophages, only a mixture of PEIPC isomers, but not PECPC, POVPC, or PGPC inhibited the expression on differentiating dendritic cells of CD1b.36 Consequently, PEIPC inhibited antigen presentation to T cells. It also altered the TLR2/1 response to microbial ligands that occurs consequent to activation of the innate immune system. Thus, PEIPC selectively inhibited production of the proinflammatory cytokine IL-12 while enhancing production of the anti-inflammatory cytokine IL-10 in primary human monocytes.36 It also inhibited the TLR2/1-mediated upregulation of CYP27b1 and, consequently activation of its target, vitamin D, and production of the antimicrobial peptide cathelicidin that protects human monocytes and macrophages against mycobacteria.36 Consequently, PEIPC promotes the survival of the mycobacterially infected cells. A provocative hypothesis, based on the strikingly similar accumulation of host-derived oxPL in atherosclerosis, is that “the link between host lipid metabolism and innate immunity contributes to the pathogenesis of both microbial infection and metabolic disease.”36
Part II. Structural hypothesis, chemical synthesis, then in vivo detection and bioactivity assay
An alternative approach to the identification of naturally occurring oxPL is to postulate the structures of oxPL based on mechanistic considerations or analogy with structures of known products of lipid oxidation. Coupled with the chemical synthesis of candidate molecules, LC-MS/MS comparisons and derivatizations can obviate the necessity for isolation of pure samples of individual oxPLs from biological extracts and can provide a convenient source of well-characterized, pure individual oxPLs.37
Pioneering studies on bioactive phospholipids explored a structural hypothesis that short chain oxidatively-truncated oxPCs, which bear some structural analogy with platelet activating factor and might be generated through oxidative cleavage of polyunsaturated PCs, are endogenous ligands for the platelet activating factor receptor (PAFR).38 POVPC, prepared by ozonolysis of PAPC, exhibited biological activity attributable to activation of the PAFR. It induces smooth muscle cell proliferation and neutrophil activation in vitro.11, 38 However, these early studies did not identify POVPC as a component of oxidatively modified LDL or obtain evidence for its occurrence in vivo.
3.1 γ-Hydroxyalkenal phospholipids: from hypothesis to detection in vivo
Rather than attempting to isolate and characterize oxPL components of oxLDL, we opted to predict likely candidates. By analogy with the production of the 4-hydroxy-2-nonenal (HNE) through free radical-induced oxidative cleavage of bond “a” of PAPC (Fig. 6A), we postulated that cleavage of bond “b” would generate an analogous γ-hydroxyalkenal phospholipid, 1-palmityl-2-(5-hydroxy-8-oxooct-6-enoyl)-sn-glycero-3-phosphocholine (HOOA-PC). The first evidence supporting this hypothesis was provided by examination of the direct infusion MS of the PAPC oxidation product mixture in which PGPC and POVPC had previously been identified. A parent ion with the correct m/z 650 expected for HOOA-PC was evident.1 To obtain further evidence, a sample of HOOA-PC was secured through unambiguous chemical synthesis. The presence of HOOA-PC in oxPAPC was then confirmed by LC-MS/MS tandem mass spectrometric comparisons with the pure synthetic HOOA-PC, its NaBH4 reduction product and methoxime derivative.39 Subsequently, HOOA-PC was also found in lipid extracts from oxLDL and human atheroma (vide infra).19, 40
Figure 6. Panel A. Alternative oxidative fragmentations generate “mirror image” γ-hydroxyalkenals.
Oxidative cleavage of PAPC generates HOOA-PC, a γ-hydroxyalkenal oxPL analogue of HNE. Panel B. Aduction of γ-hydroxyalkenals with primary amines generates carboxyalkylpyrroles: Adduction of γ-hydroxyalkenal phospholipids with primary amino groups, e.g., of proteins, generates ω–carboxyalkyl pyrroles. Panel C. CEP and VEGF Mediated Angiogenesis: ω–Carboxyethylpyrrole (CEP) promotes MyD88-dependant GTP loading (activation) of Rac1 through binding with TLR1/2 leading to NFkB stimulation, integrin expression and proangiogenic activities. The VEGF/VEGFR pathway is an independent parallel integrin-mediated proangiogenic pathway. The tripeptide RGD blocks both pathways.
3.2 HOOA-PC promotes monocyte entry into chronic lesions
As found previously for POVPC and PGPC1, HOOA-PC dose-dependently activates HAECs to bind monocytes and increases levels of MCP-1 and IL-8 – chemokines that are important in monocyte entry into chronic lesions. These proinflammatory activities39 cause leukocyte–endothelial interaction resulting in atherogenic extravasation of monocytes into the subendothelial space. Thus, HOOA-PC may play a role in chronic inflammation. Since elevated levels of myeloperoxidase (MPO) are associated with the presence of coronary artery disease,41 one likely scenario for atherogenesis involves MPO-initiated free radical-induced lipid oxidation. The resulting oxPC promote extravasation of monocytes into the subendothelial space and endocytosis of oxLDL by the derived macrophages leading to their conversion into foam cells and, ultimately, atheroma formation. As observed previously for POVPC, HOOA-PC also promotes the antiinflammatory inhibition of LPS-induced expression of E-Selectin in a model of bacterial infection, and it therefore counteracts the activity of PGPC that promotes the E-Selectin-mediated neutrophil-endothelial interactions of acute inflammation.39
3.3 Adduction of γ-hydroxyalkenal phospholipids to protein lysyl ε-amino groups generates bioactive carboxyalkyl pyrroles
Inspired by the reaction of γ-hydroxyalkenal HNE with proteins to form adducts that incorporate the ε-amino group of lysyl residues in pentylpyrrole modifications,42 analogous reactions were postulated for γ-hydroxyalkenal PLs. Lipolysis of intermediate oxPL adducts was expected to produce ω-carboxyalkylpyrrole modifications of proteins. Enzyme-linked immunosorbent assays, using antibodies raised against carboxyheptyl pyrrole (CHP), carboxypropyl pyrrole (CPP), and carboxyethyl pyrrole (CEP)43 protein modifications (Fig. 6B), prepared by chemical syntheses, revealed the presence of CHP and CPP in oxLDL. Elevated levels of CHP are found in plasma from patients with end-stage renal disease or atherosclerosis compared to healthy volunteers.44 Elevated levels of CEP are found in plasma from patients with age-related macular degeneration (AMD).45 CEP accumulates in retinas of these patients where it promotes the atrophy of “dry” AMD46 and the angiogenesis of “wet” AMD.47 CEP accumulates and promotes angiogenesis in melanoma and in healing wounds where it is transiently present.48
Neither of the pattern recognition receptors, CD36 and SR-B1, which recognize the phospoholipid precursors of CEPs and CPPs (vide infra), recognize these carboxyalkyl pyrroles.48 The proangiogenic effect of CEP on HUVECs is comparable to that of vascular endothelial growth factor (VEGF).47 However, CEP does not promote angiogenesis through the VEGF receptor (VEGFR), but rather acts as a ligand for TLR2, apparently as a heterodimer with TLR1 (Fig. 6C).48 CEP triggers MyD88-dependent GTP loading of Rac1 leading to stimulation of NFκB, but does not result in phosphorylation (activation) of VEGFR2. That both the VEGF/VEGFR and CEP/TLR2 induced angiogenic activities are mediated by integrins was demonstrated by inhibition of HUVEC migration by the RGD peptide an integrin receptor binding ligand.48 On the other hand, aortic rings from TLR2 knockout mice exhibit angiogenesis in response to VEGF but not CEP. The homologous carboxyalkyl pyrrole CPP is also proangiogenic. It remains to be determined whether this indicates a tolerance for ligand structural variability of the same Toll-like pattern recognition receptor that is activated by CEP, or if CPP signals through a different receptor or through a different heterodimer of TLR2. It seems likely that CEP-induced angiogenesis will compromise antiVEGF therapeutic measures by compensating for inhibition of the VEGF pathway.
3.4 Identification of oxPL ligands (oxPCCD36) for the scavenger receptor CD36
Previously we had shown that lipid-derived protein modifications that are present in oxLDL promote uptake and degradation of the particles by macrophages owing to recognition and binding by the scavenger receptor CD36.49 However, the discovery that binding and endocytosis of oxidized low-density lipoprotein (oxLDL) by macrophages is primarily a consequence of recognition of oxPL components of the LDL particle prompted efforts to identify the oxPL molecular species that confer recognition of oxLDL by CD36. The complex mixture of oxidation products in oxPAPC was analyzed and fractionated by preparative reverse phase HPLC to deliver three fractions that exhibited the ability to compete for the binding of 125I-NO2-LDL to CD36-transfected 293 cells (Fig 7A).37
Figure 7. Chemical synthesis enabled structural and biological characterization of OxPCCD36.
HPLC analysis and fractionation of CD36 ligands generated during oxidation of PAPC. OxPAPC was fractionated by preparative reverse phase HPLC and monitored by evaporative light scattering (panel a bottom graph). Three fractions (I-III in panel A upper graph) were found to contain CD36 ligands. Panel B: (top) CD36 binding, LC-MS/MS detection of (middle) HOOA-PC and HOOA-PC methoxime (bottom). Panel C: structures of CD36 binding oxPL shown to be present in fractions isolated from (left) oxPAPC or (right) oxPLPC. Adapted from J biol chem (2002) 38503–16.
To test the speculation that HOOA-PC, which we had previously identified as a component of oxPAPC39, might be one of the CD36 binding oxPLs, the retention times and MS fragmentations were compared by LC-MS/MS with pure synthetic HOOA-PC, as well as those of derivatives generated by treatment of the CD36 active fraction with multiple agents (NaBH4, NaBD4, and NaCNBH3) for reducing the aldehyde carbonyl; methoxamine and dinitrophenyhydrazine for generating a methoxime and hydrazone derivative and subsequent tandem MS analysis in both positive ion and negative ion modes.37 For example, methoxime isomers coeluted with the methoxime from synthetic HOOA-PC (Fig. 7B, bottom). This technique conveniently eliminates background signals from other components in the fractions. The LC-MS/MS signal for a mother-daughter transition m/z = 650→184 characteristic of HOOA-PC from fraction II coeluted with that of authentic HOOA-PC (Fig. 7B, middle). It should be noted that this approach obviated the need for isolating pure HOOA-PC from oxPAPC. Indeed fraction II was contaminated with another CD36 binding oxPL that was later shown to be HOdiA-PC (Fig. 7B, top).
Based on the polarities of fractions I – III, and the speculation that the other CD36 binding oxPLs were structurally related to HOOA-PC and derived from it by further oxidative transformations, we postulated that the less polar fraction III contained a γ-ketoaldehyde KOOA-PC while the more polar fraction I contained the corresponding acids HOdiA-PC and KOdiA-PC (for structures see Fig. 7C). To test these predictions, pure samples of these putative oxPLs were generated by unambiguous chemical syntheses50 and used to confirm their presence in the CD36-binding fractions from oxPAPC by LC-MS/MS comparisons and derivatizations (including esterification with pentafluorobenzyl bromide). Identification of each species was based upon the detection of ions with mass to-charge (m/z) ratio identical to that of the parent lipid, which following collision-induced dissociation, subsequently also gave rise to a characteristic daughter ion and retention time determined by analysis of authentic synthetic oxPC species.
Similarly, a series of CD36-binding oxPC was postulated to be generated from PLPC. These were prepared by chemical synthesis and then used to confirm their presence in oxPLPC. Although none of these CD36-binding oxPLs, referred to collectively as oxPCCD36, have ever been isolated from oxLDL, oxPAPC, or oxPLPC, it was possible to establish their presence in these oxPL mixtures and in biological extracts by multiple analytical comparisons with pure synthetic samples. These pure samples also allowed the demonstration that each of these molecules recapitulate the biological activities, including the promotion of macrophage foam cell formation, of oxPL detected in oxLDL, oxPAPC, or oxPLPC as well as in atherosclerotic plaques.19 It is important to recognize that chemical syntheses preceded the identification of each naturally occurring oxPL exhibiting CD36 binding activity. These syntheses were absolutely essential for studies on the biological activities of individual molecular species of oxPCCD36. This was especially important for the mixtures of HOdiA-PC and KOdiA-PC and of HDdiA-PC and KDdiA-PC from oxidation of PAPC and PLPC, respectively, that were inseparable under the LC conditions employed. All of these oxPCs, which contain γ-oxygenated-α,β-unsaturated aldehyde or carboxylic acid functionality were confirmed to be especially potent inducers of CD36-mediated endocytosis of oxLDL by macrophage cells. LC-ESI/MS/MS analysis of lipid extracts from rabbit aortas confirmed the presence of each oxPCCD36 species in vivo and demonstrated 5–7 fold elevated levels of PAPC-derived oxPCCD36 in atherosclerotic versus normal aortas.19
Addition of pure synthetic oxPCCD36 to cholesterol-containing particles promotes CD36-dependent macrophage binding (but not to macrophage cells from CD36 null mice), uptake, and metabolism of cholesterol esters resulting in accumulation of cholesterol and foam cell formation.19 Notably, binding of oxPCCD36-containing particles to CD36 increased with increasing mol % of ligand within the surface of a particle, consistent with enhancement of binding through mutivalent, i.e., multiple receptor-ligand interactions. That only a few molecules per LDL particle are needed to confer recognition of oxPCCD36, supports their physiological relevance. Analogously, oxPCCD36 promote endocytosis of oxidatively damaged photoreceptor rod cell outer segments (PhROS) by retinal pigmented epithelial (RPE) cells51, a process that replaces the entire stack of photoreceptor disks within these cells every 10 days.
3.5 OxPCCD36-induced endocytosis of oxLDL delivers toxic oxPL into macrophage cells
Like a Trojan horse, oxPCCD36-foster endocytosis of toxic cargo within an oxLDL particle into CD36-expressing macrophages or RPE cells. This might lead to (1) lipolysis of oxysterol-containing esters that releases toxic oxysterols or (2) covalent adduction of electrophilic oxidized phospholipids or oxysterols to proteins that impairs protein function. Thus, oxPCCD36 can indirectly promote biological activities such as oxysterol-induced cytotoxicity.52 Covalent adduction of γ-hydroxyalkenal-phospholipids, e.g., HOOA-PC and HODA-PC, with a cysteine thiol in the lysosomal protease cathepsin B, reduces the proteolytic degradation by mouse peritoneal macrophages of macromolecules previously internalized by receptor mediated endocytosis or phagocytosis.40 This can impair processing of the lipid-modified proteins by interfering with the fusion with lysosomes of endosomes that is crucial for degradation of lipid-modified proteins, e.g., in endocytosed oxLDL, by macrophage cells. The analogous processing of PhROS by RPE cells is also perturbed by oxLDL.53–55 Rab5a is a fusion protein believed to be critical for phagosome and possibly endosome maturation through fusion with lysosomes.56 HODA-PC blocked the posttranslational modification, i.e., isoprenylation and proteolytic cleavage, of inactive 25 kDa Rab5a within RPE cells into the active 23 kDa form required for phagosome-lysosome fusion in these phagocytes.56
3.6 OxPCCD36 inhibits HDL binding with hepatocyte SR-B1 impeding delivery of cholesterol to the liver for excretion
There is appreciable sequence homology between the scavenger receptors CD36 and SR-B1, and they bind common ligands, e.g., HDL, oxLDL, and anionic phospholipids.57–59 Therefore, it was postulated that oxPCCD36 might serve as ligands for SR-B1, and that such binding could inhibit reverse cholesterol transport from macrophages in atherosclerotic plaques to HDL and then to liver for excretion, the main mechanism whereby HDL protects against the development of atherosclerosis. Thus, cholesterol is transferred from macrophages to the surface of HDL particles where it is convered to cholesteryl esters that migrate into the hydrophobic HDL core. HDL delivers cholesteryl esters to the liver, via SR-B1 on hepatocytes, where it can be converted into bile acids and excreted. OxLDL, oxPAPC, and individual pure oxPCCD36 exhibited saturable binding with human SR-B1.60 Importantly, the Kd value is very similar for binding of HDL or small unilammelar vesicles containing oxPCCD36 to a GST-SR-B1 fusion protein, containing the amino acid 144-205 fragment of the extracellular amino-terminal domain of human SR-B1. Furthermore, oxLDL and individual pure oxPCCD36 inhibited binding of HDL with hepatocytes and almost completely inhibited the selective uptake of cholesteryl esters from HDL by hepatocytes via SR-B1.60 Thus, oxidative stress and accumulation of specific oxidized phospholipids in plasma may promote the development of hypercholesterolemia and progression of atherosclerosis, not only by inducing uptake of oxLDL by magrophages via CD36 and interfering with lysosomal processing, e.g., by preventing maturation of the fusion protein Rab5a (vide supra), but by inhibiting SR-B1-mediated transfer of cholesteryl from macrophages to hepatocytes.
3.7 OxPCCD36 induce a prothrombotic state through activation of platelet CD36 and αIIβ3, and expression of P-selectin
Through binding with platelet scavenger receptor CD36, oxPCCD36 activate platelets, as assessed by an increase in P-selectin surface expression and activation of platelet fibrinogen receptor integrin αIIβ3 that primes or sensitizes the platelets for subsequent activation by the classical platelet agonist ADP (Fig. 8A).61 In vivo, a mesenteric thrombosis model revealed that occlusion times are significantly shorter in hyperlipidemic mice than in wild-type mice on a Western diet, and functional deficiency of CD36 protects mice from the hyperlipidemia-related prothrombotic phenotype.61 These observations suggest a role for oxPCCD36 in the pathophysiology of occlusive arterial thrombi associated with myocardial infarction and stroke. They provide a molecular mechanistic link between hyperlipidemia, oxidant stress and a prothrombotic phenotype because oxPCCD36 accumulate in plasma of hyperlipidemic mice at concentrations up to 40-fold higher than those found in normolipidemic mice, and they are present in substantial amounts in human plasma at elevated levels in individuals with low HDL.61 In normolipidemic human plasma, oxPCCD36 levels are inversely correlated with levels of HDL, but not LDL. They are 2.5 times higher in plasma from subjects in the lowest HDL tertile. This is consistent with the anti-inflammatory and antioxidant effects of HDL62, and suggests that it is an interaction between CD36 and oxidative stress, not merely dyslipidemia (or elevated LDL), that is responsible for enhanced platelet reactivity in vivo.
Figure 8. Prothrombotic activities of oxidatively-truncated phospholipids.
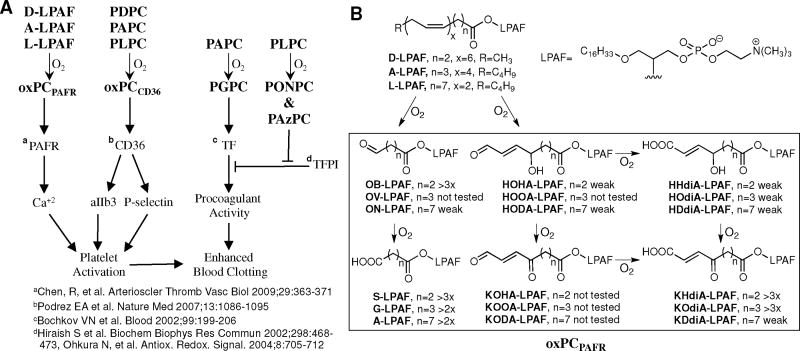
Panel A: Oxidatively truncated phospholipids promote thrombosis by activating platelets through the scavenger receptor CD36 and PAFR, inducing expression of procoagulant tissue factor (TF) on vascular endothelium, and blocking inhibition of that activity by tissue factor pathway inhibitor (TFPI). Panel B: Oxidatively truncated ether phospholipids, referred to collectively as oxPAFPAFR are generated by oxidative cleavage of PUFA esters of lyso-PAF (LPAF) and cause transient (peak in ~100 sec) elevations in Ca+2 levels ranging from 1.5x to more than 3x.
Oxidative stress-related generation of oxPL might contribute in numerous ways to the production of occlusive arterial thrombi associated with myocardial infarction and stroke (Fig. 8A). As noted above, PGPC triggers a bifurcated cascade resulting in expression of tissue factor (TF), a procoagulant, on ECs, and the activity of TF is amplified by the inhibition of tissue factor pathway inhibitor (TFPI) by PONPC and PAzPC. It seems reasonable to anticipate that platelet activation by oxPCCD36 working in concert with TF expression on the vascular endothelium promotes thrombosis consequent to the rupture of an atherosclerotic plaque owing to the release of a bolus of oxidatively-truncated phospholipids into the circulation. In addition to those mentioned above, some oxidatively-truncated ether phospholipids are potent platelet activators that masquerade as platelet activating factor (PAF) (vide infra).
3.8 A family of ether oxPCs, oxPCPAFR, activate platelets through the PAF receptor (PAFR)
Platelet-activating factor (PAF), 1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine, is an ether phospholipid with potent, diverse physiological actions, particularly as a mediator of inflammation.63 It exerts its effects through a single, highly specific G-protein-coupled receptor. As the most potent phospholipid agonist yet identified, PAF’s biosynthesis is closely controlled. In contrast, PAF-like ether phospholipids with oxidatively truncated sn-2 acyl chains (oxPAFs) can be generated nonenzymatically under oxidative stress. We predicted that oxPAFs shown in Fig. 8B would be generated through the autoxidation of PUFA esters of lyso-PAF (LPAF). To facilitate the identification, quantification, and biological testing of these putative oxPAFs, we prepared pure samples by unambiguous chemical syntheses.64 The electrospray mass spectrum of lipids extracted from oxLDL exhibited molecular ions corresponding to G-PAF, KOdiA-LPAF, KDdiA-LPAF, KODA-LPAF, and KOOA-LPAF.64 The entire family of oxidatively truncated ether phospholipids depicted in Fig 8B was generated through oxidative cleavage of 2-lyso-PAF esters of LA, AA, and DHA in small unilamellar vesicles (SUVs) exposed to the biologically relevant myeloperoxide (MPO)/H2O2/NO2 system to initiate autoxidation.
In contrast with the sensitization of platelets toward activation through binding of oxPC with CD36, oxPAFs act through the platelet PAF receptor (PAFR). OxPAFs account for the activation of platelets upon treatment with low concentrations of oxLDL as indicated by surface expression of P-selectin and the production of highly spread aggregates. Many of these oxPAFs at submicromolar concentrations induce a rapid increase in platelet cytoplasmic Ca+2 level (elevations are indicated in Fig. 8B). The short chain aldehyde OB-PAF and acid S-LPAF, which most closely resemble PAF, were among the most active while longer chain aldehydes were all weak agonists. The longer chain γ-hydroxyalkenoic acids HHdiA-, HOdiA, and HDdiA-LPAF were all weak agonists. In contrast, the shorter chain γ-ketoalkenoic acids KHdiA-LPAF and KOdiA-LPAF are highly active while the longer chain analogue was only weakly active. The activity of all oxPAFs and oxLDL is completely suppressed by the PAFR antagonist WEB 2086.64 The action profile is bell shaped, with concentrations of oxLDL from just under 1.5 μg/ml up to 5μg/mL stimulating intracellular Ca2+ flux, but then activation is significantly reduced as the concentration of oxLDL increases above 7 μg/mL, perhaps owing to the presence of antiinflammatory agents in the complex mixture of oxidized lipids and lipid-derived oxidative protein modifications in oxLDL.
3.9 α,β-Unsaturated carboxylic oxPEs and oxPCs inhibit LPS-induced expression of IL-8
OxLDL inhibits the acute inflammatory response elicited by LPS, a model of bacterial infection, as evidenced by the expression of IL-8 by endothelial cells. Since oxPAPC and oxidized 1-palmityl-2-arachidonyl-sn-glycero-3-phosphoethanolamine (oxPAPE) both mimic this effect, the head group is apparently not important for activity. Furthermore, the polar phospholipid head group is not expected to have a major influence on the free radical-induced oxidative modification of their polyunsaturated acyl groups. To test the hypothesis that oxidatively truncated ethanolamine PLs, analogous to the oxidatively-truncated choline PLs described above, are generated in vivo, a family of oxPEs was prepared by chemical syntheses.65 Their natural occurrence was then demonstrated by LC-MS/MS comparisons with lipids extracted from retina.66
The inhibition of LPS induction of IL-8 was measured for a variety of pure oxPCs and oxPEs available through unambiguous chemical syntheses. While all the oxPEs have some activity, those containing α,β-unsaturated carboxylic acids, i.e., KHdiA-PE, KOdiA-PE, KDdiA-PE, and HDdiA-PE (analogues of the oxPAFs depicted in Fig. 8B), are much more potent inhibitors of the LPS induction of IL-8 than are those containing short-chain aldehydes or acids, i.e., POVPE, PON, PGPE and PAzPE (analogues of the oxPC depicted in Fig. 2).67 In contrast, the latter are among the most active proinflammatory oxPEs, i.e., for fostering monocyte-endothelial interactions. A similar dichotomy is found for oxPCs. Thus oxidation products with different oxidatively truncated sn-2 acyl chains – γ-oxygenated-α,β-unsaturated carboxylic acids versus short-chain aldehydes or acids – are most potent in regulating the pro- and anti-inflammatory effects of oxidized phospholipids. Thus, the sn-2 acyl chain, and not the polar head group, is the primary determinant of the biological activities tested.
3.10 OxPL alter membrane structure and function
The inhibition of LPS induction of IL-8 by oxPAPC or KOdiA-PC is abrogated by a neutral sphingomyelinase inhibitor, and cell-permeant C6 ceramide. OxPAPC or KOdiA-PC activate neutral sphingomyelinase and increases levels of the product of its activity, ceramide. This suggests that the anti-inflammatory inhibition of LPS induction of IL-8 by KOdiA-PC is mediated, at least in part, by the neutral sphingomyelinase. These effects may reflect ceramide-induced alterations to lipid rafts and caveolae resulting in deficient assembly of the LPS receptor complex. These observations also suggest that certain oxPCs can foster modification of cell membrane structure, and thereby alter membrane protein function, e.g., the LPS receptor complex.
The close proximity of oxidatively truncated sn-2 acyl chains with the choline head group in phospholipid membranes was demonstrated by nuclear Overhauser effect experiments.68 Oxidation resulting in the sn-2 acyl chain of phospholipids becoming shorter and richer in polar functional groups increases their hydrophilicity leading to their expulsion from the hydrophobic core of the membrane lipid bilayer. Thus, when cellular membranes are oxidatively damaged, truncated acyl chains sprout from the membrane like whiskers.69 Molecular dynamics simulations support this “lipid whisker model” for oxidatively damaged membranes.70 The conformational change that results in sprouting of lipid whiskers may have several consequences: (a) facilitating specific binding with receptors, e. g., endothelial cell EP2 or TLR4, macrophage or platelet CD36, platelet PAFR and hepatocyte SR-B1; (b) inducing higher local positive membrane curvature owing to the presence of one rather than two lipophilic tails inserted into the membrane; (c) allowing interaction and transfer of oxPC, e.g., from oxLDL, to other lipoprotein particles through specific binding interactions, e.g., with small high-density lipoprotein (HDL) particles or peptide fragment analogues of the HDL protein Apo-A1;71 and (d) allowing interaction with lipases such as PAF acetylhydrolase that preferentially cleaves oxidatively truncated acyl groups, abrogating the biological activities of oxPL and possibly facilitating the repair of oxidatively damaged phospholipids. Recognition of the polar choline head group of oxPC may also contribute to binding with pattern recognition receptors. Thus POVPC adducted to BSA or a peptide binds with CD36 competing with oxLDL. However, the hydrolysis products of the POVPC-peptide adduct after treatment with PLD or PLC to remove choline or PC, respectively, were no longer competitors for the OxLDL ligand. Similarly, the substitution of the PC headgroup by phosphoethanolamine abrogated the inhibitory effect of POVPC-peptide.72
4.1 Conclusions
Even applying the most sophisticated chromatographic techniques available, isolation of pure oxPL molecular species from biological extracts, or even from the less complex mixtures generated through oxidation of individual pure polyunsaturated phospholipids, is a redoubtable task that has met with very limited success, and is an impractical source of pure molecular species for biological evaluation. In contrast, the nonclassical approach, which proceeds from structural hypothesis, to chemical synthesis, then detection in vivo and finally bioactivity assay, has been a cornucopia for the discovery of naturally occurring, biologically active phospholipids and the derived protein adducts. It has provided ready access to pure samples that enabled rapid progress in understanding their involvements, inter alia, in artherogenesis, thrombosis, inflammation and angiogenesis. Without a focus on understanding a particular disease, research adopting this unusual paradigm is proving outstandingly productive for uncovering unexpected involvements of oxidatively-truncated phospholipids and their protein adducts in diseases such as AMD, atherosclerosis, and cancer, as well as in wound healing. Besides their utility as standards for identification and quantification of oxPC in biological samples, the availability of pure individual oxPCs through chemical syntheses also enabled a breakthrough in our understanding of their conformations in membranes. Thus, the “lipid whisker model”, which is a refinement of the fluid mosaic and lipid raft models,73 provides a basis for understanding the receptor recognition of oxPLs displayed on the surface of cellular membranes or lipoprotein particles.
OxPL ligands probably appeared before receptors that recognize them. Primordial lipid oxidation became a problem as a consequence of oxygen generated after photosynthetic organisms evolved. In response, ancient receptors, e.g. pattern recognition receptors (CD36, SR-B1, TLRs), evolved that are abundantly represented among those recognizing oxPLs. Those receptors conferred a competitive advantage allowing organisms to respond to oxidative injury. Subsequently, the ability to generate messenger molecules as receptor ligands that could be used to control biological processes evolved. Refinement of ligand and receptor structures allowed fine tuning of receptor-mediated signaling and control. Some more highly evolved receptors, e.g., EP2 and PAFR, probably respond to products of nonenzymatic chemistry, e.g., free radical-induced lipid oxidation, because those products resemble the enzymatically generated ligands for those receptors.
The activities of oxPL mixtures generated in vivo are a composite of the activities of the individual components, and lipid oxidation can generate molecules that have opposing biological activities. Therefore, understanding the biological consequences of lipid oxidation depends not only on knowledge of the activities of individual oxPL, but also on their relative amounts. Since some oxPL are readily converted to others by further oxidation, the relative amounts, and hence, the composite biological activities can vary with duration and severity of oxidative injury. As knowledge of the identities and activities of individual oxPL matures, temporal studies of their levels, distribution and clearance in vivo are needed to fully understand their involvements in health and disease.
Acknowledgments
Sources of Funding
The participation of my laboratory in studies mentioned above was generously support by National Institutes of Health Grants GM021249 and EY016813.
Non-standard Abbreviations and Acronyms
- 5,6-EC
5,6-epoxyisoprostane A2 (5,6-epoxycyclopentenone)
- αIIβ3
platelet fibrinogen receptor integrin
- AA
arachidonic acid
- ADP
adenosine diphosphate
- AIF
apoptosis inducing factor
- AMD
age-related macular degeneration
- Apo-AI
apolipoprotein A I
- cAMP
cyclic adenosine mono-phosphate
- CD36
cluster of differentiation 36, a scavenger receptor
- CEP
2-(ω-carboxyethyl)pyrrole
- CHP
2-(ω-carboxyheptyl)pyrrole
- CPP
2-(ω-carboxypropyl)pyrrole
- CREB
cAMP-responsive element-binding protein
- CS-1
connecting segment 1
- CYP
cytochrome p450
- DHA
docosahexaenoic acid
- EC
endothelial cell
- EGR1
early grown factor response protein 1
- EI
epoxyisoprostane
- eNOS
endothelial nitric oxide synthase
- EP2
E-type prostaglandin receptor
- Erk
extracellular-signal-regulated kinase
- GalT-2
UDP-galactose:glucosylceramide(β1→4)galactosyltransferase
- GAS
γ-interferon activation sequence
- GPI
glycosylphosphatidylinositol
- HAEC
human aortic endothelial cell
- HAzPC
1-hexadecyl-2-azelayl-sn-glycero-3-phosphocholine
- HDdiA-PC
9-hydroxy-10-dodecenedioic acid ester of 2-lysoPC
- HDL
high-density lipoprotein
- HeLa
Henrietta Lacks (cervical cancer cell line)
- HNE
4-hydroxy-2-nonenal
- HO-1
heme oxygenase 1
- HOdiA-PC
5-hydroxy-8-oxo-6-octenedioic acid ester of 2-lysoPC
- HODA-PC
1-palmityl-2-(9-hydroxy-12-oxododec-10-enoyl)-sn-glycero-3-phosphocholine
- HOHA-PC
1-palmityl-2-(4-hydroxy-7-oxohept-5-enoyl)-sn-glycero-3-phosphocholine
- HOOA-PC
1-palmityl-2-(5-hydroxy-8-oxooct-6-enoyl)-sn-glycero-3-phosphocholine
- HUVEC
human umbilical vein endothelial cell
- IL-8 or 10
interleukin 8 or 10
- JAK2
Janus kinase 2
- KDdiA-PC
1-palmityl-2-(9-keto-10-dodecendioyl)-sn-glycero-3-phosphocholine
- KOdiA-PC
1-palmityl-2-(5-keto-6-octendioyl)-sn-glycero-3-phosphocholine
- KODA-PC
1-palmityl-2-(9-keto-12-oxo-10-dodecenoyl)-sn-glycero-3-phosphocholine
- KOOA-PC
1-palmityl-2-(5-keto-8-oxo-6-octenoyl)-sn-glycero-3-phosphocholine
- LA
linoleic acid
- LacCer
lactosylceramide
- LDL
low-density lipoprotein
- LPAF
2-lysoPAF
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein 1
- MM-LDL
minimally modified low-density lipoprotein
- MPO
myeloperoxidase
- MyD88
myeloid differentiation primary response gene (88)
- NfκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nox
NADPH oxidase
- Nrf2
nuclear factor E2-related (erythroid derived 2)-like 2
- OxLDL
oxidized low-density lipoprotein
- OxPAPC
oxidized 1-palmityl-2-arachidonyl-sn-glycero-3-phosphocholine
- OxPAPE
oxidized1-palmityl-2-arachidonyl-sn-glycero-3-phosphoethanolamine
- OxPC
oxidized phosphatidylcholine
- OxPCCD36
OxPC ligands for CD36
- OxPCPAFR
OxPC ligands for PAFR
- OxPE
oxidized phosphatidylethanolamine
- OxPL
oxidized phospholipid
- OxPLPC
oxidized 1-palmityl-2-linoleyl-sn-glycero-3-phosphocholine
- PAF
platelet activating factor
- PAFR
PAF receptor
- PAPC
1- palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine
- PAzPC
1-palmityl-2-azelyl-sn-glycero-3-phosphocholine
- PC
phosphatidylcholine
- PCNA
proliferating cell nuclear antigen
- PC-OH
1-palmityl-2-lyso-sn-glycero-3-phosphocholine
- PE
phosphatidylethanolamine
- PGPC
1-palmityl-2-glutaryl-sn-glycero-3-phosphocholine
- PECPC
1-palmityl-2-(epoxycyclopentenone)-sn-glycero-3-phosphocholine
- PEIPC
1-palmityl-2-(epoxyisoprostane)-sn-glycero-3-phosphocholine
- PhROS
photoreceptor rod outer segments
- PI3K
phosphoinositide 3 kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PL
phospholipid
- PLA2
phospholipase A2
- PLPC
1-palmityl-2-linoleyl-sn-glycero-3-phosphocholine
- PONPC
1-palmityl-2-(9-oxononanoyl)-sn-glycero-3-phosphocholine
- POVPC
1-palmityl-2-(5-oxovaleryl)-sn-glycero-3-phosphocholine
- PPAR
peroxisome proliferator-activated receptor
- RPE
retinal pigmented epithelial
- R-RAS
RAS-related protein
- SR-B1
scavenger receptor B1
- Src
sarcoma
- SREBP
sterol regulatory element binding protein
- STAT3
signal transducer and activator of transcription 3
- TF
tissue factor
- TFPI
tissue factor protein inhibitor
- TLR2 or 4
Toll-like receptor 2 or 4
- TMEM30a
transmembrane protein 30A
- TNF
tumor necrosis factor
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Disclosures
None
References
- 1.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. The Journal of biological chemistry. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Gharavi NM. Endothelial cell regulation by phospholipid oxidation products. Free radical biology & medicine. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. Journal of lipid research. 2009;50 (Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochkov VN. Inflammatory profile of oxidized phospholipids. Thrombosis and haemostasis. 2007;97:348–354. [PubMed] [Google Scholar]
- 5.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxidants & redox signaling. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, Nelson SF, Horvath S, Berliner JA, Kirchgessner TG, Lusis AJ. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimman A, Chen SS, Komisopoulou E, Titz B, Martinez-Pinna R, Kafi A, Berliner JA, Graeber TG. Activation of aortic endothelial cells by oxidized phospholipids: A phosphoproteomic analysis. Journal of proteome research. 2010;9:2812–2824. doi: 10.1021/pr901194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng W, Lu J, Bishop GA, Watson AD, Sage AP, Demer L, Tintut Y. Regulation of interleukin-6 expression in osteoblasts by oxidized phospholipids. Journal of lipid research. 2010;51:1010–1016. doi: 10.1194/jlr.M001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. The Journal of clinical investigation. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokumura A, Asai T, Takauchi K, Kamiyasu K, Ogawa T, Tsukatani H. Novel phospholipids with aliphatic dicarboxylic acid residues in a lipid extract from bovine brain. Biochemical and biophysical research communications. 1988;155:863–869. doi: 10.1016/s0006-291x(88)80575-8. [DOI] [PubMed] [Google Scholar]
- 11.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified ldl contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. The Journal of clinical investigation. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Berliner JA, Subbanagounder GG, Bhunia AK, Koh S. Identification of a biologically active component in minimally oxidized low density lipoprotein (mm-ldl) responsible for aortic smooth muscle cell proliferation. Glycoconjugate journal. 2004;20:331–338. doi: 10.1023/B:GLYC.0000033629.54962.68. [DOI] [PubMed] [Google Scholar]
- 14.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a camp-dependent r-ras/pi3-kinase pathway. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circulation research. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 16.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 17.Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 18.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. The Journal of biological chemistry. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor cd36 and is enriched in atherosclerotic lesions. The Journal of biological chemistry. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Brady E, McIntyre TM. Human tmem30a promotes uptake of antitumor and bioactive choline phospholipids into mammalian cells. J Immunol. 2011;186:3215–3225. doi: 10.4049/jimmunol.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, Fogelman AM, Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. The Journal of biological chemistry. 1999;274:24787–24798. doi: 10.1074/jbc.274.35.24787. [DOI] [PubMed] [Google Scholar]
- 22.Jung ME, Berliner JA, Angst D, Yue D, Koroniak L, Watson AD, Li R. Total synthesis of the epoxy isoprostane phospholipids peipc and pecpc. Organic letters. 2005;7:3933–3935. doi: 10.1021/ol051415y. [DOI] [PubMed] [Google Scholar]
- 23.Jung ME, Berliner JA, Koroniak L, Gugiu BG, Watson AD. Improved synthesis of the epoxy isoprostane phospholipid peipc and its reactivity with amines. Organic letters. 2008;10:4207–4209. doi: 10.1021/ol8014804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA. Oxidized phospholipids increase interleukin 8 (il-8) synthesis by activation of the c-src/signal transducers and activators of transcription (stat)3 pathway. The Journal of biological chemistry. 2004;279:30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- 25.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin e2 receptor subtype 2 as a receptor activated by oxpapc. Circulation research. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 26.Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. The Journal of biological chemistry. 2002;277:7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 27.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. The Journal of biological chemistry. 2003;278:29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 28.Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, Wei L, Pfeffer LM, Berliner JA. Role of the jak/stat pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. The Journal of biological chemistry. 2007;282:31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 29.Zimman A, Mouillesseaux KP, Le T, Gharavi NM, Ryvkin A, Graeber TG, Chen TT, Watson AD, Berliner JA. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:332–338. doi: 10.1161/01.ATV.0000252842.57585.df. [DOI] [PubMed] [Google Scholar]
- 30.Gharavi NM, Baker NA, Mouillesseaux KP, Yeung W, Honda HM, Hsieh X, Yeh M, Smart EJ, Berliner JA. Role of endothelial nitric oxide synthase in the regulation of srebp activation by oxidized phospholipids. Circulation research. 2006;98:768–776. doi: 10.1161/01.RES.0000215343.89308.93. [DOI] [PubMed] [Google Scholar]
- 31.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circulation research. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 32.Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, Szeto WL, Watson AD, Lusis AJ, Berliner JA. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]
- 33.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of camp-responsive element-binding protein. The Journal of biological chemistry. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 34.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 35.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via cdc42 and rac. Circulation research. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 36.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and hdl regulate innate immunity in human leprosy. The Journal of clinical investigation. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor cd36. The Journal of biological chemistry. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 38.Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIntyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. The Journal of biological chemistry. 1991;266:11104–11110. [PubMed] [Google Scholar]
- 39.Subbanagounder G, Deng Y, Borromeo C, Dooley AN, Berliner JA, Salomon RG. Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vascular pharmacology. 2002;38:201–209. doi: 10.1016/s1537-1891(02)00170-2. [DOI] [PubMed] [Google Scholar]
- 40.Hoff HF, O’Neil J, Wu Z, Hoppe G, Salomon RL. Phospholipid hydroxyalkenals: Biological and chemical properties of specific oxidized lipids present in atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:275–282. doi: 10.1161/01.atv.0000051407.42536.73. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. Jama. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 42.Sayre LM, Sha W, Xu G, Kaur K, Nadkarni D, Subbanagounder G, Salomon RG. Immunochemical evidence supporting 2-pentylpyrrole formation on proteins exposed to 4-hydroxy-2-nonenal. Chemical research in toxicology. 1996;9:1194–1201. doi: 10.1021/tx960094j. [DOI] [PubMed] [Google Scholar]
- 43.Salomon RG, Hong L, Hollyfield JG. Discovery of carboxyethylpyrroles (ceps): Critical insights into amd, autism, cancer, and wound healing from basic research on the chemistry of oxidized phospholipids. Chemical research in toxicology. 2011;24:1803–1816. doi: 10.1021/tx200206v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur K, Salomon RG, O’Neil J, Hoff HF. (carboxyalkyl)pyrroles in human plasma and oxidized low-density lipoproteins. Chemical research in toxicology. 1997;10:1387–1396. doi: 10.1021/tx970112c. [DOI] [PubMed] [Google Scholar]
- 45.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. The Journal of biological chemistry. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 46.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating tlr2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoppe G, Subbanagounder G, O’Neil J, Salomon RG, Hoff HF. Macrophage recognition of ldl modified by levuglandin e2, an oxidation product of arachidonic acid. Biochimica et biophysica acta. 1997;1344:1–5. doi: 10.1016/s0005-2760(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Deng Y, Batyreva E, Sha W, Salomon RG. Novel bioactive phospholipids: Practical total syntheses of products from the oxidation of arachidonic and linoleic esters of 2-lysophosphatidylcholine. The Journal of organic chemistry. 2002;67:3575–3584. doi: 10.1021/jo0105383. [DOI] [PubMed] [Google Scholar]
- 51.Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, Silverstein RL, Hazen SL. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for cd36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. The Journal of biological chemistry. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colles SM, Irwin KC, Chisolm GM. Roles of multiple oxidized ldl lipids in cellular injury: Dominance of 7 beta-hydroperoxycholesterol. Journal of lipid research. 1996;37:2018–2028. [PubMed] [Google Scholar]
- 53.Hoppe G, Marmorstein AD, Pennock EA, Hoff HF. Oxidized low density lipoprotein-induced inhibition of processing of photoreceptor outer segments by rpe. Investigative ophthalmology & visual science. 2001;42:2714–2720. [PubMed] [Google Scholar]
- 54.Hoppe G, O’Neil J, Hoff HF, Sears J. Accumulation of oxidized lipid-protein complexes alters phagosome maturation in retinal pigment epithelium. Cell Mol Life Sci. 2004;61:1664–1674. doi: 10.1007/s00018-004-4080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoppe G, O’Neil J, Hoff HF, Sears J. Products of lipid peroxidation induce missorting of the principal lysosomal protease in retinal pigment epithelium. Biochimica et biophysica acta. 2004;1689:33–41. doi: 10.1016/j.bbadis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Dominguez C, Stahl PD. Interferon-gamma selectively induces rab5a synthesis and processing in mononuclear cells. The Journal of biological chemistry. 1998;273:33901–33904. doi: 10.1074/jbc.273.51.33901. [DOI] [PubMed] [Google Scholar]
- 57.Connelly MA, Williams DL. Scavenger receptor bi: A scavenger receptor with a mission to transport high density lipoprotein lipids. Current opinion in lipidology. 2004;15:287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Jessup W, Gelissen IC, Gaus K, Kritharides L. Roles of atp binding cassette transporters a1 and g1, scavenger receptor bi and membrane lipid domains in cholesterol export from macrophages. Current opinion in lipidology. 2006;17:247–257. doi: 10.1097/01.mol.0000226116.35555.eb. [DOI] [PubMed] [Google Scholar]
- 59.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor sr-bi in the lipid metabolism of endocrine and other tissues. Endocrine reviews. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 60.Ashraf MZ, Kar NS, Chen X, Choi J, Salomon RG, Febbraio M, Podrez EA. Specific oxidized phospholipids inhibit scavenger receptor bi-mediated selective uptake of cholesteryl esters. The Journal of biological chemistry. 2008;283:10408–10414. doi: 10.1074/jbc.M710474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet cd36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature medicine. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of hdl. Circulation research. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 63.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annual review of biochemistry. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 64.Chen R, Chen X, Salomon RG, McIntyre TM. Platelet activation by low concentrations of intact oxidized ldl particles involves the paf receptor. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:363–371. doi: 10.1161/ATVBAHA.108.178731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gugiu BG, Salomon RG. Total syntheses of bioactive oxidized ethanolamine phospholipids. Organic letters. 2003;5:2797–2799. doi: 10.1021/ol034729z. [DOI] [PubMed] [Google Scholar]
- 66.Gugiu BG, Mesaros CA, Sun M, Gu X, Crabb JW, Salomon RG. Identification of oxidatively truncated ethanolamine phospholipids in retina and their generation from polyunsaturated phosphatidylethanolamines. Chemical research in toxicology. 2006;19:262–271. doi: 10.1021/tx050247f. [DOI] [PubMed] [Google Scholar]
- 67.Walton KA, Gugiu BG, Thomas M, Basseri RJ, Eliav DR, Salomon RG, Berliner JA. A role for neutral sphingomyelinase activation in the inhibition of lps action by phospholipid oxidation products. Journal of lipid research. 2006;47:1967–1974. doi: 10.1194/jlr.M600060-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Li XM, Salomon RG, Qin J, Hazen SL. Conformation of an endogenous ligand in a membrane bilayer for the macrophage scavenger receptor cd36. Biochemistry. 2007;46:5009–5017. doi: 10.1021/bi700163y. [DOI] [PubMed] [Google Scholar]
- 69.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. The Journal of biological chemistry. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 70.Khandelia H, Mouritsen OG. Lipid gymnastics: Evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations. Biophysical journal. 2009;96:2734–2743. doi: 10.1016/j.bpj.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epand RF, Mishra VK, Palgunachari MN, Anantharamaiah GM, Epand RM. Anti-inflammatory peptides grab on to the whiskers of atherogenic oxidized lipids. Biochimica et biophysica acta. 2009;1788:1967–1975. doi: 10.1016/j.bbamem.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for cd36. Journal of lipid research. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Catala A. Lipid peroxidation modifies the picture of membranes from the “fluid mosaic model” to the “lipid whisker model”. Biochimie. 2012;94:101–109. doi: 10.1016/j.biochi.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 74.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of erk/egr-1 and ca(++)/nfat. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]