Abstract
The gonadotropin-releasing hormone (GnRH) neurons represent the final output neurons of a complex neuronal network that controls fertility. It is now appreciated that GABAergic neurons within this network provide an important regulatory influence on GnRH neurons. However, the consequences of direct GABAA receptor activation on adult GnRH neurons have been controversial for nearly a decade now, with both hyperpolarising and depolarising effects reported. This review provides (i) an overview of GABAA receptor function and its investigation using electrophysiological approaches and (ii) re-examines the past and present results relating to GABAergic regulation of the GnRH neuron, with a focus on mouse brain slice data. Although it remains difficult to reconcile the results of the early studies, there is a growing consensus that GABA can act through the GABAA receptor to exert both depolarising and hyperpolarising effects on GnRH neurons. The most recent studies examining the effects of endogenous GABA release on GnRH neurons indicate that the predominant action is that of excitation. However, we are still far from a complete understanding of the effects of GABAA receptor activation upon GnRH neurons. We argue that this will require not only a better understanding of chloride ion homeostasis in individual GnRH neurons, and within subcellular compartments of the GnRH neuron, but also a more integrative view of how multiple neurotransmitters, neuromodulators and intrinsic conductances act together to regulate the activity of these important cells.
The amino acid neurotransmitter γ-aminobutyric acid (GABA) has long been recognized as being of prime importance in the control of GnRH release and thereby gonadotropin secretion in mammals. Indeed, the first report of GABA modulation of luteinizing hormone (LH) secretion was published in Science in 1974 [1] and a PubMed search of “GABA & GnRH” presently generates a list of 413 publications. A large number of in vivo intracerebral and intracerebroventricular studies now support an important role for GABA in the control of many aspects of gonadotropin secretion, and in a variety of species [2]. The prevailing view from these in vivo studies has been that GABA acts through GABAA receptors to suppress both pulsatile and surge modes of LH secretion [2], although there are also examples of stimulatory actions of GABA action on GnRH/LH release [3, 4]. However, the precise mechanisms and loci of GABA action within the GnRH neuronal network (i.e., GnRH neurons, afferent neurons and associated glial cells), remain unclear. Studies examining the effects of GABA on GnRH neurons at a cellular level have progressed from GT1 cell and embryonic nasal placode models through to brain slice work in transgenic mice. Experiments in the latter preparation have enabled GABA actions to be examined on GnRH neurons closer to the in situ situation. This review intends to provide a background to understanding GABAA receptor function and experimental interpretation, and to address the controversy that has arisen regarding the effects of direct GABAA receptor activation on adult GnRH neurons.
GABAA receptor function
Types of GABA receptors
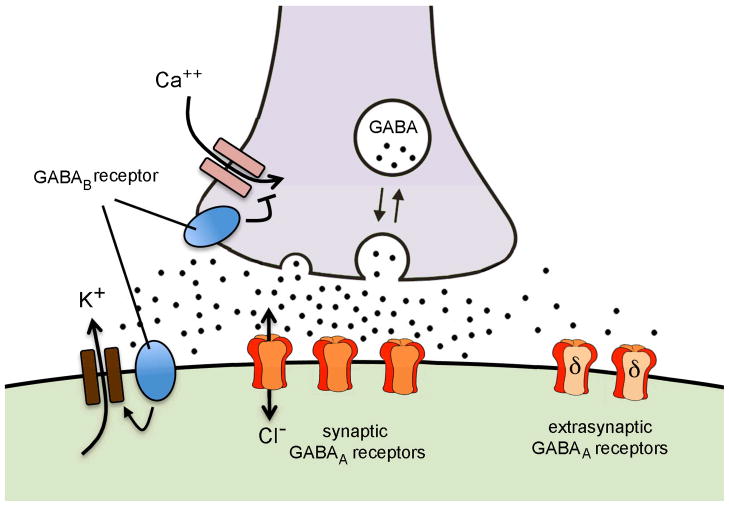
GABA binds to two classes of receptors: GABAA and GABAB with a third class, GABAC, being considered by some to be a subclass of the GABAA group with similar structure and function [3, 4]. GABAA and GABAB receptors can be located on the pre- and post-synaptic membrane, and also outside the synapse at extrasynaptic locations (Fig. 1).
Figure 1.
Schematic representation of GABA signaling at the GnRH neuron in the mouse. GABA terminals (grey) synapsing on GnRH neurons (green) are likely to have GABAB receptors that act to inhibit calcium entry to the terminal, thereby suppressing presynaptic activity. GABA released from the nerve terminal activates postsynaptic GABAA receptors within the synapse to generate a fast phasic depolarising or hyperpolarising response and also GABAB receptors that activate potassium channels that hyperpolarise the membrane. Spill-over of GABA from the synapse activates extrasynaptic GABAA receptors (expressing the delta receptor subunit) that provide a low level tonic influence on membrane polarisation.
Although this review focuses upon the GABAA receptor, it is important to recognize that GABAB receptors are also likely to play a role in the direct GABAergic modulation of GnRH neuron excitability [5]. GABAB receptors are metabotropic seven-transmembrane domain G-protein- coupled receptors that regulate downstream channels [6]. It is worth emphasizing that the GABAB receptor itself is not an ion channel with changes in membrane current generated in response to GABAB receptor activation being due to receptor-mediated changes in downstream effector channels. Typically, the postsynaptic GABAB receptor is coupled to potassium channels whereas the presynaptic GABAB receptor is linked to calcium channels [6]. Although information remains scarce, this is likely to be the situation for GnRH neurons (Fig. 1). Stimulation of GABAB receptors results in the activation of a specific class of potassium channels that generates membrane hyperpolarisation and inhibition of GnRH neuron firing [5]. Preliminary data also indicate that calcium channel-linked GABAB receptors are present on GABAergic nerve terminals regulating GnRH neuron excitability (Liu & Herbison, unpublished). While there is some evidence that GABAB receptors can act to suppress high-frequency activated GABA inputs to GnRH neurons [7], the roles of endogenous GABA in activating GABAB receptors on GnRH neurons remain largely unknown.
The GABAA receptor is a ligand-gated ion channel composed of five subunits (typically 2α, 2β plus a variable fifth) each of which contributes to a central pore [8]. Binding of two GABA molecules at the α-β interfaces most effectively gates (i.e., opens) the channel [9], although both single ligand and ligand-independent opening are possible [10, 11]. The GABAA receptor pore is permeable in both directions to monovalent anions, the most physiologically relevant of which are Cl− and HCO3− [10, 11]. A variety of GABAA receptor subunits are expressed in GnRH neurons (see below), functional GABAA receptor-mediated currents can be measured in response to both exogenous and endogenous GABA [14–16], and spontaneous GABAergic transmission is altered in different reproductive conditions (see below). Thus an understanding of the consequences of GABAA receptor activation is important in forming a complete picture of GnRH neurobiology.
The opening of the intrinsic ion channel of the GABAA receptor is neither innately excitatory nor inhibitory to a cell [8, 17]. Rather, as with any ion channel, the net current flow through the pore depends primarily on two variables: the concentration gradients of the permeable anions and the membrane potential of the cell at the time the channel opens. These forces define the electrochemical potential that drives ion flow through the open channel. The membrane potential at which these two forces are equal and opposite is referred to as the reversal potential for the current through that class of channels; at this potential there is no net current flow.
As mentioned above, both Cl− and HCO3− can flow through the GABAA receptor pore. The permeability of GABAA receptors to Cl− is typically 2–5 times greater than that to HCO3− [10, 12] in mammalian cells. Under physiological conditions, Cl− is the main charge carrier through the GABAA receptor. However, HCO3− can also contribute to net current via the GABAA receptor depending on both pH and intracellular [Cl−][13, 14]. Hence, although dominated by Cl−, the reversal potential for the GABAA receptor (EGABA) results from the flow of both Cl and HCO3− ions. Intracellular bicarbonate levels tend to accumulate because of the action of carbonic anhydrase to generate HCO3− from CO2 and H2O [15]. As the reversal potential for HCO3− is quite depolarised, EGABA is slightly more depolarised than the reversal potential for Cl− (ECl), alone. Importantly, as detailed below, ECl can vary from cell to cell and with developmental stage, and this results in concomitant variations in EGABA.
Cation chloride cotransporters
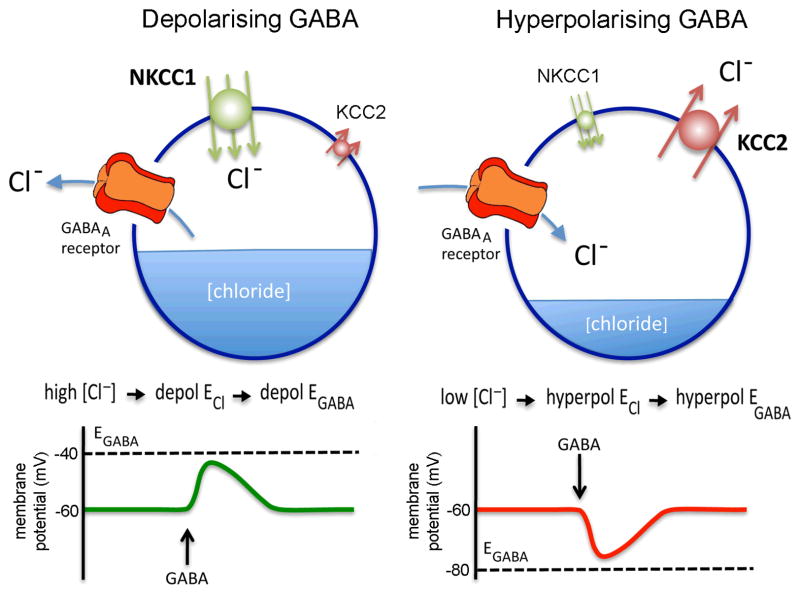
The intracellular chloride concentration of a cell is essentially set by the action of cation chloride cotransporters (Fig. 2). These transporters are secondary active transporters that utilize the driving force in Na+ and K+ gradients established by the Na-K ATPase [16]. The chloride-cation cotransporters are electrically neutral, thus their rate of transport does not contribute in and of itself to membrane potential. There are two primary classes: K-Cl cotransporters (KCCs) and Na-K-Cl cotransporters (NKCCs). Under physiological conditions, KCCs extrude Cl− from the cell, lowering intracellular Cl− levels. In contrast, NKCCs accumulate Cl− and raise intracellular Cl− levels (Fig. 2).
Figure 2.
Schematic diagram showing the typical interrelationships between chloride transporters, chloride levels and GABAA receptor function that underlie depolarising and hyperpolarising actions of GABA in neurons. The balance of chloride ion transport into the neuron determines the intracellular chloride ion concentration. In neurons under physiological conditions this is dependent principally on the activity of the sodium-potassium-chloride co-transporter 1 (NKCC1), bring chloride into the cell, and the potassium-chloride co-transporter 2 (KCC2), removing chloride from the cell. Cells with a relatively high intracellular chloride ion concentration will have a relatively depolarised reversal potential for chloride (depol ECl) that, in turn, sets a relatively depolarised reversal potential for GABA (depol EGABA), in this case imagined to be −40mV. When the cell is exposed to GABA, the GABAA receptor will open and the cell’s membrane potential will move towards EGABA as chloride ions leave the cell, thus generating a depolarising response. The opposite pattern of events occurs for cells with a relatively low intracellular chloride concentration resulting in hyperpolarisation (right figure). Note that a level of intracellular chloride could be attained at which EGABA is similar to the resting membrane potential (imagined here to be −60mV) in which case activation of the GABAA receptor would have minimal effects on membrane potential. Adapted from [80].
The intracellular Cl− concentration in most neurons is reduced from elevated to low levels during early postnatal life. This shift has been associated with an increase in KCC expression and function, in particular KCC2B, concomitant with a reduction in NKCC1 [17] (Fig. 2). Although this neonatal developmental shift has become somewhat dogma, cell types are still being identified that do not follow this developmental pattern, with several cell types continuing to exhibit elevated intracellular Cl− concentrations either locally or globally into adult life [23–25]. For example, primary olfactory neurons maintain elevated intracellular Cl− throughout adult life due to continued function of NKCC1 and low expression of KCC2 [18, 19]. In addition to global expression patterns, posttranslational modification and subcellular location of cation chloride transporters appear to be critical to sculpting response to GABA. For example, NKCCs are activated by phosphorylation, whereas KCCs can be inhibited or activated by phosphorylation [28–31], and NKCC1 may be targeted to the axon initial segment where axon potentials are initiated [32, 33]. Thus, KCCs and NKCCs set intracellular Cl− concentrations in a cell-type- and subcellular location- specific manner and this, in turn, provides the dominant force setting EGABA of the cell/region (Fig. 2).
Consequence of GABAA receptor activation
Whether activation of GABAA receptors results in depolarisation or hyperpolarisation of a neuron’s membrane potential depends on the relationship of EGABA to the membrane potential of the cell at the time the receptor is gated by GABA (Fig. 2). When the membrane potential of a cell is depolarised relative to EGABA, opening the intrinsic channel of the GABAA receptor will hyperpolarise the membrane; likewise when the membrane potential is hyperpolarised relative to EGABA, opening this channel will depolarise the membrane (Fig. 2). In other words, if the membrane potential of a cell was −60mV and EGABA was −40mV then opening the GABAA receptor would result in an outward flow of Cl− in an attempt to depolarise the cell to a membrane potential of −40mV. If EGABA was −80mV, then GABAA receptor activation would result in Cl− entering the cell to hyperpolarise it. Hence, it can be seen that EGABA is critical to determining whether GABA will depolarise or hyperpolarise a cell. The relationship of EGABA to action potential threshold is also important; if EGABA is more depolarized than threshold, activation of GABAA receptors can depolarize a cell sufficiently to initiate action potential firing. As noted above, EGABA is determined primarily by ECl, which is established by the activity of the chloride ion cotransporters (Fig. 2). This is why the neonatal developmental changes in NKCCs and KCCs noted above are so fundamental to the shift from depolarising to hyperpolarising responses to GABAA receptor activation in many forebrain neurons in altricial rodents [20, 21]. It also explains why it is not easy to predict whether GABAA receptor activation will be hyperpolarising or depolarising as Cl− homeostasis is not static in neurons, even in the adult. Furthermore, EGABA can be near the resting potential membrane potential of a cell thereby making its effects on membrane potential small or even negligible.
A further complication in defining GABAA receptor activation as being excitatory or inhibitory is that hyperpolarisation and depolarisation may not necessarily be equated with inhibition and excitation, respectively. For excitation to occur, that is initiation of action potential firing, a depolarising response must be sufficient to activate the voltage-gated sodium channels that generate the sharp spike of the action potential [22, 23]. That is, the membrane must depolarise to the threshold for action potential initiation. A depolarisation in membrane potential that is insufficient to reach threshold for action potential initiation can generate two main responses. First, it can activate other mechanisms such as voltage-dependent channels, that further depolarise the membrane to the threshold for action potential generation [19, 32, 38, 39]. Second, it can induce what appears to be a paradoxical inhibition of action potentials through what is called “depolarising” or shunting inhibition. Whenever the GABAA receptor channel is open, there is a transient increase in membrane conductance through the pores and thus a decrease in membrane resistance (also called input resistance, Rin). During this transient reduction in input resistance, a greater amount of current is required to produce a unit change in membrane potential (from Ohm’s Law ΔVmembrane=IRin). As a result, a depolarisation in membrane potential subsequent to GABAA receptor activation may render a cell less responsive to other inputs, leading to shunting inhibition [24]. In cells with slow membrane time constants (typically big cells with extensive dendritic trees) the resultant slow depolarisation can also inactivate sodium channels needed for action potential generation, causing transient inhibition through this mechanism [25].
The classic response to GABAA receptor activation in adults is a hyperpolarisation in membrane potential that is typically inhibitory. It is possible, however, for hyperpolarisation to remove inactivation from sodium channels. This can result in rebound action potentials after decay of the inhibitory postsynaptic potential [42–44]. There are thus many factors that must be taken into account before classifying a response to GABAA receptor activation as excitatory or inhibitory.
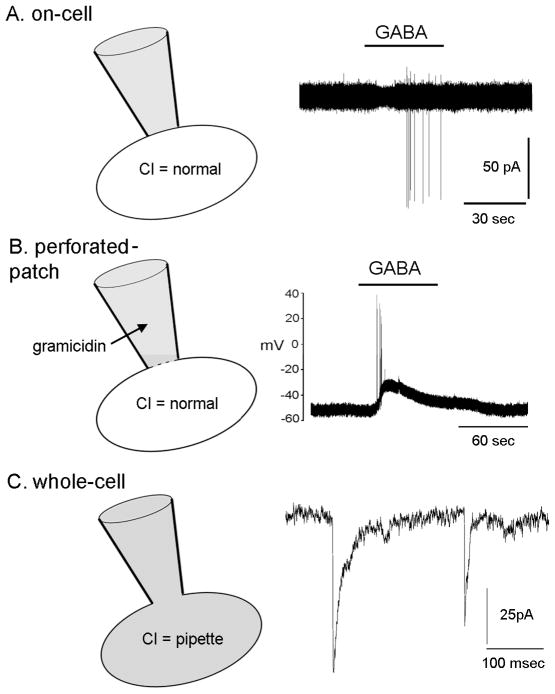
An important final consideration when examining the effects of GABAA receptor activation on membrane potential is that of experimental approach (Fig. 3). As noted above, intracellular Cl− levels are absolutely critical in determining EGABA and so it is essential that the recording configuration does not alter the internal chloride milieu of the recorded cell. There two practical ways to achieve this; the first is to use an “on-cell” recording approach in which an electrode is placed on the cell without any attempt to break the membrane of the cell (Fig. 3A). This allows the investigator to record the firing pattern of the cell [26] without any perturbation of the intracellular environment, or to estimate membrane potential [27]. The second approach is to use the “perforated-patch” approach (Fig. 3B). In this case, an electrode with the antibiotic gramacidin in its tip is placed on the cell membrane. With time, gramicidin forms small pores in the membrane beneath the electrode through which monovalent ions such as sodium and potassium can pass, but these pores are not permeable to chloride ions. Hence, in this configuration, the investigator has a relatively good electrical connection with the cell and can measure firing rate and membrane potential changes without altering the internal chloride ion balance [28]. It is important to note here that the most common electrophysiological technique called “whole-cell” recording (Fig. 3C) is not appropriate for determining the effect of GABAA receptor activation on membrane potential or firing rate as this method dialyzes the intracellular milieu of the cell with the solution in the patch electrode buffer, thus alters the native intracellular [Cl−]. The whole-cell mode can, however, be used to good effect to examine the dynamics of the individual GABAA receptors as, by filling the cell with a high Cl− concentration through the pipette, one can amplify the Cl− ion movements and observe GABAA receptor ion channel openings and closings with ease.
Figure 3.
Schematic diagram showing the different electrophysiological recording modes used to examine GABAA receptor functioning in GnRH neurons. A. On-cell configuration in which the recording electrode (grey) is placed on the cell membrane, a tight seal is formed and currents recorded from the GnRH neuron. The current underlying an action potential is recorded as an action current (in pA); this enables the firing pattern of a GnRH neuron to be monitored without altering the intracellular constituents (e.g., the chloride ion concentration) of the GnRH neuron. The example trace shows a GnRH neuron activated to fire action currents by GABA added to the bathing medium. B. Perforated patch recording mode in which the presence of an antibiotic (in this case gramicidin) in the patch electrode results in ion-selective pores being made in the membrane beneath the electrode. Gramicidin allows some ions (potassium and sodium) to pass through the pores but not chloride. This enables the membrane potential to be monitored while maintaining the normal chloride ion concentration of the cell. The example shows a GnRH neuron with a resting membrane potential of approximately −55mV that responds to GABA application with a short burst of action potentials and more prolonged depolarization. C. Whole-cell recording mode in which the membrane within the electrode is ruptured so that the intracellular contents of the cell become dialysed with that of the pipette. In this way, the intracellular ion environment of the GnRH neuron can be manipulated by the contents of the pipette. This mode is commonly used to help isolate or augment a particular current for measurement. In this example, the recording was optimized to monitor Cl- currents to measure the frequency of activation of GABAA receptors. The recording on the right shows postsynaptic currents due to chloride flow through GABAA receptors of the recorded cell that were opened by synaptic release of GABA. Note the different timescale of this recording compared to that in A and B. It is important to note that one cannot use this approach to study the physiological response of the recorded cell to activation of those receptors as the chloride ion concentration in the cell is artificial. Kiho Lee and Stephanie Constantin are thanked for help in generating this figure.
GABA inputs, GABAA receptors and GnRH neurons in the mouse
GnRH neurons receive GABAergic input
One of the most consistent observations revealed during the initial electrophysiological investigations of GnRH neurons in the brain slice preparation was that these cells receive GABAergic input [29, 30]. Virtually all GnRH neurons exhibit GABAA receptor-mediated postsynaptic currents (p.s.c.s.) and respond to exogenous GABA [14, 16, 48, 49]. In both voltage- and current-clamp recording configurations, the great majority of fast post-synaptic activity at the GnRH neuron soma is attributable to GABAA receptor activation [29, 30]. The remaining fast p.s.c.s. result from the activation of glutamate receptors, principally the AMPA receptor [14, 50–52], however it is important to note that the glutamatergic component is small compared to the GABAergic component. In experiments in which GABA and glutamate p.s.c.s. have been recorded from GnRH neurons, the rate of GABAA-mediated events is typically 5–10 fold greater than glutamate events [7, 50, 53–55]. The potential role of glutamatergic and GABAergic transmission to distal dendrites remains to be determined [31, 32]
GABAA receptor signalling occurs in two forms; “phasic” and “tonic”. Phasic refers to the normal fast activation of GABAA receptors within the synapse, whereas tonic represents the activation of extrasynaptic GABAA receptors by GABA in the extracellular space [33] (Fig. 1). Phasic activation is observed electrophysiologically as the brief opening and closing of GABAA receptors, resulting in p.s.c.s. Tonic currents are evident as a persistent current that has a sustained influence on membrane potential. Although phasic GABAA receptor signalling has long been recognized in GnRH neurons, it is only recently that tonic GABAA signaling has been identified in these neurons [34, 35]. Mediated by δ subunit-containing GABAA receptors and partly dependent upon the activity of glial and neuronal GABA transporters, which clear GABA from the extracellular space, tonic GABAA receptor signaling was found to hyperpolarise the membrane by ~5mV in mouse GnRH neurons [35]. As such, modulation of this tonic GABA current could have important roles in determining the excitability of GnRH neurons.
A feature of phasic GABAA receptor activity in GnRH neurons in the brain slice preparation is that it often does not appear dependent to any large extent upon electrical activity in the presynaptic GABA terminal. The frequency of GABAA-mediated miniature (action potential independent) p.s.c.s. is often similar to that of spontaneous (action potential dependent and independent) p.s.c.s. [55, 61, 62]. This type of activity-independent GABA release is observed in other neurons [63–65], however, its regulation and function remain poorly understood [36, 37]. Indeed, a variety of chronic and acute gonadal steroid treatments known to modulate the frequency of GABAA p.s.c.s. in GnRH neurons (see below) do so by changing the rate of action potential-independent GABA release with no or little measured effect on activity-dependent GABA release [55, 59, 61, 62, 68]. As evidence suggests that activity-independent GABA release is related to the degree of GABAergic input existing at a cell [38], it is possible that the changes in GABAA m.p.s.c. frequency represent alterations in the number or extent of GABAergic inputs to GnRH neurons. Unfortunately, little is presently known about GABA-GnRH neuron ultrastructural rearrangements with steroid treatment. On the basis of in vivo microdialysis and neurochemical studies it has, however, been suggested that the estrogen regulation of GABA release in the preoptic area results from a reorganization of GABA presynaptic terminal dynamics [39]. Recent work also suggests retrograde endocannbinoid signalling from GnRH neurons can alter m.p.s.c. frequency, suggesting signalling at the presynaptic terminal can bring about functional changes not dependent on structural rearrangements [40].
It is important to note that all of the GABAA p.s.c.recordings have been undertaken in the acute brain slice preparation in which many, or possibly most, of the GABAergic (and other) afferents to GnRH neurons have been severed. As such, it may be that much of the activity-dependent GABA input is absent in the slice preparation. Indeed, recordings from other neuronal cell types in vivo have revealed that levels of synaptic activity and firing rates are up to 50-times higher in vivo compared with the acute brain slice preparation [41, 42]. Establishing the actual rate and pattern of GABAA p.s.c.s in GnRH neurons in a more intact preparation is an important future goal for this field.
At present, the locations of the GABAergic cell bodies innervating GnRH neurons are not well established. The anteroventral periventricular nucleus (AVPV), a region that has been suspected as providing GABAergic inputs for many years [74–77], has just recently been confirmed using an electrophysiological approach [7]. By cutting horizontal brain slices it was found possible to maintain the AVPV input to GnRH neurons in GnRH-GFP transgenic mice and this was shown to provide a very substantial GABAergic input to GnRH neurons. Another study has examined the potential locations of GABA neurons contributing to p.s.c.s. in GnRH neurons at the time of estradiol positive feedback by looking for differences between lateral and medial (containing AVPV) sagittal and coronal brain slices [43]. However, with one exception, no differences were detected in GABAA p.s.c. frequency between any of the slice configurations, or any TTX-sensitivity found. The exception was that the p.s.c. frequency of GnRH neurons recorded in medial sagittal slices and exhibiting high p.s.c. frequency at the time of the GnRH surge, was reduced by ~50% by TTX or by a cut through the slice made just caudal to the AVPV. One interpretation of this finding is that an activity-dependent GABA input originating caudal to the AVPV, perhaps the SCN, is activated at the time of the surge [43]. Further work is required to establish the origins of GABAergic inputs to GnRH neurons.
GnRH neurons express a range of GABAA receptor subtypes
As mentioned above, the GABAA receptor is a pentamer made up of different combinations of subunits that define its binding affinity to a variety of allosteric modulators [44]. Several methodologies including dual-label in situ hybridisation and single cell RT-PCR have been used to define the GABAA receptor subunits expressed by GnRH neurons in the rodent [48, 59, 60, 79–85]. These studies suggest that there are sex differences as well as postnatal developmental alterations in the α, β and γ GABAA receptor subunits expressed by GnRH neurons. For example, in the adult female mouse there is a predominance of α1, α3, α5, β1 and γ2 mRNA in contrast to the male in which transcripts for nearly all of the α, β and γ GABAA receptor subunits can be detected [45]. Also, whereas many different subunits are detected in prepubertal GnRH neurons, this appears to become more restricted in adults [29, 46]. Much less attention has been paid to the uncommon GABAA receptor subunits, although the δ and ε subunits have now been identified in these cells [59, 60, 65, 81]. While studies have shown that both androgen and progesterone derivatives are potent allosteric modulators of GABAA receptors expressed by GnRH neurons [47, 48], the full functional relevance of the many different GABAA receptors likely to be expressed by GnRH neurons is not known.
The precise locations of GABAA receptors on GnRH neurons also remain unexplored. Recent studies have shown that GnRH neurons in the mouse extend long dendrites that are often well over 1000 μm in length [49] and often intertwine [50]. This, along with evidence that action potentials can be initiated in the dendrites of GnRH neurons [51], has required a re-evaluation of how GnRH neurons receive and integrate afferent input. Although not yet demonstrated, it seems likely that the full length of GnRH neuron dendrite will receive GABAergic inputs. Studies to date show the density of vesicular GABA transporter-containing appositions on the proximal dendrites of GnRH neurons to be double that found on the soma [52]. Understanding the location of GABAA receptors and their spatial relationship to other receptors, particularly glutamatergic receptors [13], will be essential in defining GABA action on GnRH neurons. For example, it has recently been proposed that shared GABAergic synapses on intertwined magnocellular neuron dendrites play a role in synchronizing oxytocin neuron activity [53].
Effects of GABAA receptor activation on immature GnRH neurons
It is now well established that GABA exerts a depolarising and excitatory influence upon many neurons in the embryonic and perinatal brain in altricial species [54]. Recordings from immature GnRH neurons indicate that they are probably not an exception to the rule. Experiments using the immortalized GT1-7 GnRH neuronal cell line indicate a depolarising effect of GABAA receptor activation as GABA induced action potential firing in GT1-7 cells recorded in the “on-cell” configuration [55]. Calcium imaging further revealed that GABA induced increases in intracellular calcium in GT1-7 cells that were dependent upon action potential generation and cell membrane calcium channels. This was the first indication that activation of GABAA receptors in GnRH neurons might be other than inhibitory, and was followed quickly by a study in which GABAA receptor activation in these GT1-1 and GT1-7 cells was shown to increase GnRH release [56, 57]. The pure nature of GT1 cultures assists interpretation of these studies as secondary effects due to other cell types are precluded. It is arguable, however, that the excitatory response of GT1 cells might be due to an artifact of transformation of the cells, which might result in behaviours different from native GnRH neurons. It is also possible that the cotransporter profile was “fixed” at a relatively “immature” stage by an earlier transformation event despite the derivation of this cell line from an adult mouse.
A depolarising influence of GABAA receptor activation in immature GnRH neurons also receives support from calcium imaging studies undertaken in the embryonic nasal explant model [58, 59]. Mouse embryonic GnRH neurons have been shown to exhibit a sharp increase in intracellular calcium levels in response to GABAA receptor activation; an effect compatible with membrane depolarisation (for review see [60]).
One study using perforated-patch recordings examined the effects of direct GABAA receptor activation on prepubertal GnRH neurons (postnatal day 10 to 30) in the mouse brain slice and found consistent depolarising actions [61]. Together, these findings indicate that GABAA receptor activation exerts a predominant depolarising and excitatory response from embryonic through to pre-pubertal-age GnRH neurons.
Effects of GABAA receptor activation on adult GnRH neurons
Unlike the coherent picture of a depolarising response to GABA in pre-pubertal GnRH neurons, the consequence of GABAA receptor activation in adult GnRH neurons has been controversial, although a more unified view is beginning to emerge.
The first two studies
The development of GnRH-promoter driven reporter genes allowed the question of GABA action to be addressed in acutely-prepared brain slices from mice [14, 100, 101]. Han and colleagues used GnRH neurons expressing beta-galactosidase (GnRH-LacZ), which can convert substrates to a fluorescent state allowing identification of cells expressing this enzyme. In this model, gramicidin-perforated patch recordings suggested a developmental change in the direction of response to GABA from depolarising to hyperpolarising that resembles that in pyramidal neurons [61]. GABA was depolarising in young (day 10–17 postnatal) GnRH neurons but hyperpolarising in cells from more mature mice (day 36–55 postnatal). This suggested a change in response during the peripubertal period and a possible link to puberty onset. However, no such change in response was observed by DeFazio and co-workers using eGFP-identified GnRH neurons [62]. Rather, both on-cell and gramicidin-perforated patch recordings of GnRH neurons revealed an excitatory response to GABAA receptor activation regardless of sex, time of day or age (note: alterations in GnRH neuron response to GABA that might contribute to reproductive senescence have not been studied by either laboratory).
How to account for these different results? One possibility is that of reporter gene (GFP fluorescence vs LacZ). However, this seems unlikely as the same adult hyperpolarising effects of GABAA receptor activation have been observed by the Herbison laboratory using GnRH- eGFP mouse lines [35, 63]; it is perhaps noteworthy that the GnRH-GFP mice used by the two laboratories are different. GFP itself does not appear to confound the data [62]. A further difference was that DeFazio study examined GABAA receptor activation in the presence of ionotropic glutamatergic receptor antagonists and the Han study did not. This should not alter the direction of response to GABA, particularly given the relative paucity of glutamatergic inputs to GnRH neurons, but becomes important in later studies discussed below.
A main difference between these two studies was duration and type of GABA application. In the Han study, GABA (10–100 μM) was bath-applied for ~30 sec to 1 min; in the DeFazio study, GABA (1 mM) was applied briefly and locally for a couple milliseconds (GABA concentrations in the synapse are estimated to be in the low millimolar range [64, 65]). Bath application of the GABAA receptor agonist muscimol for 1–3 min invariably generated an initial excitatory response of a barrage of action potentials from GnRH neurons [62]. This was followed by a failure to respond to exogenous GABA with either a change in firing rate or membrane potential, and GnRH neuronal quiescence that persisted for several minutes. This quiescence was accompanied by a marked reduction in the membrane (input) resistance of GnRH neurons, typically near 1 GΩ, to ~100 MΩ during and for a period after muscimol treatment. Together these data suggest the suppression of GnRH neuron activity and lack of response to GABA after prolonged activation of the GABAA receptor could be due to a combination of pharmacologically-induced collapse of the chloride gradient, shunting inhibition due to reduced membrane resistance and/or GABAA receptor desensitization. This cannot, in and of itself, account for the membrane hyperpolarisation observed in the Han study in response to GABA. Activation of GABAB receptors would be one possible explanation for this hyperpolarisation; neither study included blockers of GABAB receptors when examining action potential generation although inclusion of GABAB antagonists did not alter EGABA [62]. However, the hyperpolarisation in the Han study was blocked by a GABAA receptor antagonist, suggesting activation of GABAB receptors does not explain these findings.
As mentioned above, cation-chloride cotransporters play a major role in setting internal chloride and hence the response to activation of these receptors. Messenger RNA and protein for the chloride accumulating NKCC1 have been demonstrated in murine GnRH neurons [62]. Some studies have reported protein for the chloride extruding transporter KCC2 in a subpopulation of GnRH neurons [66], whereas others have not detected this protein [62]. In terms of function, the specific NKCC1 inhibitor bumetanide hyperpolarises EGABA in murine GnRH neurons [62] and in terminal nerve GnRH neurons of teleosts [67]. This action of bumetanide provides functional evidence for chloride accumulation via NKCC1 at least within GnRH neuronal cell bodies. Thus, at present, data on chloride co-transporters in GnRH neurons at the expression level are somewhat mixed and not especially helpful in resolving the controversy, however functional data indicate GnRH neurons in brain slices actively accumulate chloride, consistent with a depolarising/excitatory response to GABAA receptor activation.
The second two studies
The first studies performed in our laboratories used exogenous GABA to evaluate the impact of GABAA receptor activation on GnRH neuron excitability. This is a valid approach, used widely by electrophysiologists, but it does not answer the important question of how GnRH neurons respond to endogenous GABA. Ideally to examine this question, one would activate GABAergic afferents and monitor the response of GnRH neurons using one of the approaches that does not alter intracellular chloride milieu. This approach is complicated by our relative lack of understanding of the location of GABAergic neurons that are afferent to GnRH neurons. An alternative approach is to block endogenous signaling via the GABAA receptor using a specific antagonist and observe the response of GnRH neurons. This is a rather poor surrogate for the following reasons. Bath application of GABAA receptor blockers effectively treats the entire GnRH neuron, which local application would likely not achieve given the extensive dendrites in some of these cells [49]. However with bath application, all cells in a brain slice are affected, including non-GnRH neurons presynaptic to the recorded neurons. This effectively removes GABAergic signaling via the A-type receptor, which is inhibitory to many hypothalamic neurons in the same brain slice [68]. In the cortex and hippocampus, this approach is used as an in vitro model of epilepsy because such treatment can cause widespread “disinhibition” of many neurons in the slice, including excitatory inputs to one’s cell of interest [109–111].
Using this approach with gramicidin perforated-patch and without blocking ionotropic glutamate receptors, the Herbison laboratory found that approximately 70% of GnRH neurons were depolarised by the GABAA receptor antagonist bicuculline, 20% showed no response and 10% were hyperpolarised [63]. Using extracellular recordings after pretreatment with ionotropic glutamate receptor blockers, the Moenter laboratory found that GABAA receptor antagonists reduced the firing rate of 80% of active GnRH neurons, had no effect on 10% and increased firing in 10% [69]. No increase in firing was observed in quiescent GnRH neurons, indicating quiescence was not due to GABAA receptor activation. When the response of GnRH neurons to GABAA blockers was tested in the absence of the glutamate receptor antagonists, 100% showed an increase in firing rate [69]. These results were interpreted in two ways. One interpretation was that this demonstrated the key importance of on-going glutamatergic signaling to the direction of GABAA receptor responses in GnRH neurons as found elsewhere in the brain [13]. The other interpretation was that the removal of GABAA receptor actions throughout the brain slice resulted in the disinhibition of excitatory inputs to GnRH neurons, again a plausible explanation given work in other brain areas [109–111].
More recent studies
It is important that many different experimental angles and models are used to address difficult questions in a field. A major contribution was made when Kato and colleagues reported that GABAA receptor activation excited GnRH neurons cultured from a rat GnRH-eGFP model [70]. Using perforated-patch electrophysiology, those authors found that GABA exerted a dose- dependent, GABAA receptor-mediated depolarising action on all rat GnRH neurons tested. A caveat of this study is that the membrane preserved in these adult neurons in short-term culture is primarily perisomatic. Other studies from this group using the same model have shown that GABAA receptor activation also increases intracellular calcium levels in GnRH neuron somata [71], supporting their electrophysiological findings.
A recent study from the Herbison laboratory has, to their surprise, identified predominant stimulatory effects of GABAA receptor activation upon intracellular calcium levels in GnRH neurons obtained from the GnRH-pericam transgenic mouse line [72]. This experimental model enables the real-time measurement of intracellular calcium levels in GnRH neurons without any manipulation of the cell in its native environment within the acute brain slice preparation [73]. In this study, GABAA receptor activation elevated calcium levels in approximately 70% of prepubertal as well as adult GnRH neurons. This effect involved activation of L-type calcium channels and suggested that GABAA-receptor mediated depolarisation of GnRH neurons was sufficient to activate these channels.
Another new study from the Herbison laboratory agrees with these more recent results. This study used an angled horizontal brain slice preparation to examine the effects of endogenous GABA inputs originating from the AVPV on GnRH neurons [7]. That investigation demonstrated that low frequency (<1Hz) electrical stimulation of the AVPV could evoke monosynaptic responses from GnRH neurons that were mediated predominantly by GABAA receptor activation. Of the GnRH neurons that were activated by AVPV stimulation, ~60% of GABAergic responses were excitatory, ~25% neutral and ~15% inhibitory to GnRH neuron activity. Thus, it is apparent that endogenous GABA inputs to GnRH neurons can exert both excitation and inhibition, but that excitation predominates.
Together, these more recent studies support the original proposal by DeFazio and colleagues that GABA can provide an excitatory input to adult GnRH neurons. While the majority of GnRH neurons in coronal, sagittal and angled horizontal slice preparations are excited by GABAA receptor activation, others exhibit the more classical inhibitory response. The subcellular and physiological reasons for this heterogeneity remain to be established. It is interesting to note that researchers examining the GABA responses of hypothalamic suprachiasmatic nucleus neurons have long been involved in a similar controversy as to whether GABAA receptor activation is excitatory or inhibitory [116–118] with current perspective being that both responses exist [74]. One source of variability among studies and among individual cells is that chloride ion transporter activity can be labile. Changes in transporter activity could alter a cell’s response to GABAA receptor activation. In several cases, acute activity-dependent changes in KCC2 function have been found to switch the GABA responses of neurons [22, 120–123]. Furthermore, in the context of studies that have been undertaken in culture or in the acute brain slice preparation, it is important to note that cellular damage can rapidly modulate KCC2 activity promoting depolarising effects of GABAA receptor activation [123–125]. Of note, as mentioned above, only a subpopulation of GnRH neurons have been reported to express KCC2 protein [62, 66]. It is also important to point out that cellular damage results in reduced membrane resistance and other changes that can alter the ability of a cell to respond to inputs and intrinsic changes that are independent of chloride cotransporters; cellular damage in slices with poor health also impairs the ability to perform the high-quality recordings needed to test these responses.
Modulation of GABAergic transmission to GnRH neurons
Another type of evidence that can inform this debate is the study of how GABAergic transmission to GnRH neurons varies with different reproductive states. The frequency of GABAergic postsynaptic currents (p.s.c.s.) reveals how often afferent GABAergic neurons are signaling to the GnRH neuron being recorded. The amplitude of p.s.c.s. is attributable to both presynaptic changes, such as how much transmitter is released, and postsynaptic changes, such as number and type of receptors and any posttranslational modifications that alter conductance through the pore or open probability.
Effects of steroid milieu, nutritional status and neuromodulators on GABAergic transmission to GnRH neurons in sum indicate that those conditions that favor increased GnRH release (mild hyperandrogenemia, oestradiol positive feedback, kisspeptin) increase GABAergic transmission to GnRH neurons and p.s.c. amplitude [53, 55, 61, 126–128]. In contrast, conditions that reduce GnRH release (progesterone, fasting, oestradiol negative feedback) reduce GABAergic transmission and p.s.c. amplitude [16, 55, 61, 62]. The sole exception to a direct relationship between the frequency of GABAergic transmission and GnRH activity is a study of the effects of long-term treatment with supraphysiological levels ofsynthetic androgens to mimic anabolic steroid abuse, in which an indirect relationship between these parameters was observed provides the sole exception to date to this direct relationship between the frequency of GABAergic transmission to GnRH neurons and the activity of GnRH neurons [34]. An interesting topic for future study will be to see if androgen abuse alters chloride cotransporter function in GnRH and other neurons.
It is interesting that amplitude and frequency of GABAergic p.s.c.s. in GnRH neurons tend to move in the same direction. The smaller p.s.c.s. detected in GnRH neurons with reduced activity would be more likely to generate subthreshold depolarisations rather than depolarisations that result in spike generation. This could compound any effect of reduced frequency of GABAergic drive. Reduced temporal summation of low frequency GABAergic p.s.c.s. would also reduce the occurance of GABA-driven depolarisation that is sufficiently large to reach action potential threshold. Although data from studies such as these provides circumstantial evidence rather than direct evidence of the consequence of GABAA receptor activation, they are largely consistent with an excitatory role.
6. Summary and perspective
Over roughly the past decade, the thinking on the consequence of GABAA receptor activation on GnRH neurons has evolved from a fairly stark controversy to a consensus. It appears that considering GABA as purely inhibitory or excitatory is to some extent an oversimplification as both responses can be observed. Further, the integration of GABAergic with other ionotropic and neuromodulatory inputs [56, 57, 129–131] and intrinsic conductances will sculpt the ultimate membrane response. For example, on-going glutamatergic activity in the vicinity of the GABAA receptor synapse may influence the GABAA receptor response. Equally, it is possible that GABAA receptor activation at the cell body could have different net effects on GnRH neuron activity compared with activation of GABAA receptors located on the dendrites [75].
GnRH neurons are regulated by a number of influences and the importance of continued function of this system for passing on the genome of an organism suggests multiple regulatory systems, and also that redundant systems will operate in the absence of specific signaling. In this regard, a recent study demonstrated continued fertility in a mouse model with knockdown of GABAA receptor mediated signaling in GnRH neurons via germline deletion of the γ-2 subunit [76]. Likewise there is GnRH neuronal activity even in the absence of the most potent stimulator identified to date in these neurons-kisspeptin [77, 78]. Together these observations point to a need to move away from all-or-none statements to more integrated viewpoints when considering the impact of specific neurotransmitters on GnRH neurons. They also point to the importance of cellular level observations in examining mechanisms. In this regard, the relationship between the predominantly excitatory actions of GABAA receptor activation on GnRH neurons and the primarily suppressive effects of GABA on LH secretion in vivo [2] are most likely explained by indirect influences of even narrowly focused drug treatments in vivo.
The present status of the GABA story, with mainly excitatory but also inhibitory responses to this transmitter, suggests several questions that need to be addressed in future studies. Is GnRH neuron intracellular chloride differentially regulated in various subcellular compartments (dendrites, soma, terminals)? Are chloride cotransporters modulated by reproductive state, neural inputs or on-going patterns of electrical activity? What happens with aging? Does GABA potentially have a role in synchronizing GnRH neurons as it does in some hippocampal networks [79]? Answers to these and other questions will further evolve our understanding of the role of GABA in sculpting GnRH neuronal activity. And finally, how might our present view of GABAA-receptor mediated signaling in GnRH neurons be revised when in vivo recordings of this response become possible?
Acknowledgments
The authors would like to thank all past and present members of their laboratories who have struggled with the difficult issue of GABA’s influence upon GnRH neurons.
Funding: Supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD41469, R01 HD34860 and U54 HD34860 to SMM and New Zealand Health Research Council to AEH.
Abbreviations
- GABA
gamma-aminobutyric acid
- GnRH
gonadotropin-releasing hormone
- NKCC
sodium-potassium-chloride cotransporter
- KCC
potassium-chloride cotransporter
Literature Cited
- 1.Ondo JG. Gamma-Aminobutyric Acid Effects on Pituitary Gonadotropin Secretion. Science. 1974;186(4165):738–739. doi: 10.1126/science.186.4165.738. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. New York: Raven Press; 2006. pp. 1415–1482. [Google Scholar]
- 3.Bilger M, et al. A conditional tetracycline-regulated increase in Gamma amino butyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology. 2001;142(5):2102–14. doi: 10.1210/endo.142.5.8166. [DOI] [PubMed] [Google Scholar]
- 4.Donoso AO, Lopez FJ, Negro-Vilar A. Cross-talk between excitatory and inhibitory amino acids in the regulation of luteinizing hormone-releasing hormone secretion. Endocrinology. 1992;131(3):1559–61. doi: 10.1210/endo.131.3.1354606. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, et al. {gamma}-Aminobutyric Acid B Receptor Mediated Inhibition of Gonadotropin-Releasing Hormone Neurons Is Suppressed by Kisspeptin-G Protein-Coupled Receptor 54 Signaling. Endocrinology. 2009;150(5):2388–2394. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr DIB, Ong J. GABAB receptors. Pharmacology & Therapeutics. 1995;67(2):187–246. doi: 10.1016/0163-7258(95)00016-a. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, et al. Frequency-dependent recruitment of fast and slow neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31(7):2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrant M, Kaila K, James EDAaJPB, Tepper M. Progress in Brain Research. Elsevier; 2007. The cellular, molecular and ionic basis of GABAA receptor signalling; pp. 59–87. [DOI] [PubMed] [Google Scholar]
- 9.Baumann SW, Baur R, Sigel E. Individual Properties of the Two Functional Agonist Sites in GABAA Receptors. J Neurosci. 2003;23(35):11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. The Journal of Physiology. 1987;385(1):243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaila K, et al. Influence of GABA-gated bicarbonate conductance on potential, current and intracellular chloride in crayfish muscle fibres. The Journal of Physiology. 1989;416(1):161–181. doi: 10.1113/jphysiol.1989.sp017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatima-Shad K, Barry PH. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1993;253(1336):69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- 13.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaila K, et al. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. The Journal of Physiology. 1993;464(1):273–289. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport HW. Gastric carbonic anhydrase. J Physiol. 1939;97:32–43. doi: 10.1113/jphysiol.1939.sp003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaesse P, et al. Cation-Chloride Cotransporters and Neuronal Function. Neuron. 2009;61(6):820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Rivera C, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 18.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366(6452):283–6. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 19.Reisert J, et al. Mechanism of the Excitatory Cl- Response in Mouse Olfactory Receptor Neurons. Neuron. 2005;45(4):553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obata K, Oide M, Tanaka H. Excitatory and inhibitory actions of GABA and glycine on embryonic chick spinal neurons in culture. Brain Research. 1978;144(1):179–184. doi: 10.1016/0006-8993(78)90447-x. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Ari Y, et al. Giant synaptic potentials in immature rat CA3 hippocampal neurones. The Journal of Physiology. 1989;416(1):303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hille B. Ion channels of excitable membranes. 3. Sunderland, Mass: Sinauer Associates, Inc; 2001. p. 814. [Google Scholar]
- 24.Staley KJ, Mody Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- 25.Bieda MC, MacIver MB. Major Role For Tonic GABAA Conductances in Anesthetic Suppression of Intrinsic Neuronal Excitability. J Neurophysiol. 2004;92(3):1658–1667. doi: 10.1152/jn.00223.2004. [DOI] [PubMed] [Google Scholar]
- 26.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biological Procedures Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verheugen JA, Fricker D, Miles R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J Neuorsci. 1999;19:2546–2555. doi: 10.1523/JNEUROSCI.19-07-02546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. Journal of Neuroscience Methods. 1995;57(1):27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 29.Sim JA, et al. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. European Journal of Neuroscience. 2000;12(10):3497–504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan SD, DeFazio RA, Moenter SM. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. Journal of Neuroscience. 2003;23(24):8578–85. doi: 10.1523/JNEUROSCI.23-24-08578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts CB, Hemond P, Suter KJ. Synaptic integration in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Neuroscience. 2008;154(4):1337–1351. doi: 10.1016/j.neuroscience.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell RE, Suter KJ. Redefining the GnRH neurone dendrite. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.02032.x. in press. [DOI] [PubMed] [Google Scholar]
- 33.Belelli D, et al. Extrasynaptic GABAA Receptors: Form, Pharmacology, and Function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penatti CAA, et al. Altered GABAA Receptor-Mediated Synaptic Transmission Disrupts the Firing of Gonadotropin-Releasing Hormone Neurons in Male Mice under Conditions That Mimic Steroid Abuse. J Neurosci. 2010;30:6497–6506. doi: 10.1523/JNEUROSCI.5383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattarai PJ, et al. Tonic extrasynaptic GABAA receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology. 2011 doi: 10.1210/en.2010-1191. in press. [DOI] [PubMed] [Google Scholar]
- 36.Staley KJ. Quantal GABA release: noise or not? Nat Neurosci. 1999;2:494–495. doi: 10.1038/9139. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch JC, et al. Deficit of quantal release of GABA in experimental models of temporal lobe epilepsy. Nat Neurosci. 1999;2(6):499–500. doi: 10.1038/9142. [DOI] [PubMed] [Google Scholar]
- 38.Jones RSG, Woodhall GL. Background synaptic activity in rat entorhinal cortical neurones: differential control of transmitter release by presynaptic receptors. The Journal of Physiology. 2005;562(1):107–120. doi: 10.1113/jphysiol.2004.076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Research Bulletin. 1997;44(4):321–6. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 40.Farkas I, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourque CW, Renaud LP. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Res. 1991;540:349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- 42.Gentet LJ, et al. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-Aminobutyric Acid A Receptors: Classification on the Basis of Subunit Composition, Pharmacology, and Function. Update. Pharmacological Reviews. 2008;60(3):243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pape JR, et al. Profiling gamma-aminobutyric acid (GABA(A)) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology. 2001;74(5):300–308. doi: 10.1159/000054697. [DOI] [PubMed] [Google Scholar]
- 46.Jung H, et al. Several GABAA receptor subunits are expressed in LHRH neurons of juvenile female rats. Brain Research. 1998;780(2):218–29. doi: 10.1016/s0006-8993(97)01152-9. [DOI] [PubMed] [Google Scholar]
- 47.Sim JA, Skynner MJ, Herbison AE. Direct regulation of postnatal GnRH neurons by the progesterone derivative allopregnanolone in the mouse. Endocrinology. 2001;142(10):4448–53. doi: 10.1210/endo.142.10.8451. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan SD, Moenter SM. Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144(10):4366–75. doi: 10.1210/en.2003-0634. [DOI] [PubMed] [Google Scholar]
- 49.Campbell RE, Han S-K, Herbison AE. Biocytin Filling of Adult Gonadotropin-Releasing Hormone Neurons in Situ Reveals Extensive, Spiny, Dendritic Processes. Endocrinology. 2005;146(3):1163–1169. doi: 10.1210/en.2004-1369. [DOI] [PubMed] [Google Scholar]
- 50.Campbell RE, et al. Dendro-dendritic bundling and shared synapses between gondadotropin-releasing hormone neurons. Proc Natl Acad Sci, USA. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts CB, et al. Dendritic Action Potential Initiation in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2008;149(7):3355–3360. doi: 10.1210/en.2008-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cottrell EC, et al. Postnatal Remodeling of Dendritic Structure and Spine Density in Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006;147(8):3652–3661. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]
- 53.Popescu IR, et al. Synchronized bursts of miniature inhibitory postsynaptic currents. J Physiol. 2010;588:939–951. doi: 10.1113/jphysiol.2009.181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 55.Hales TG, Sanderson MJ, Charles AC. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1–7) neurons. Neuroendocrinology. 1994;59(3):297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- 56.Martinez de la Escalera G, Choi AL, Weiner RI. Biphasic gabaergic regulation of GnRH secretion in GT1 cell lines. Neuroendocrinology. 1994;59(5):420–5. doi: 10.1159/000126687. [DOI] [PubMed] [Google Scholar]
- 57.Spergel DJ, et al. L-type Ca2+ channels mediate joint modulation by gamma-amino-butyric acid and glutamate of [Ca2+]i and neuropeptide secretion in immortalized gonadodropin-releasing hormone neurons. Neuroendocrinology. 1995;61(5):499–508. doi: 10.1159/000126873. [DOI] [PubMed] [Google Scholar]
- 58.Moore JP, Jr, Shang E, Wray S. In Situ GABAergic Modulation of Synchronous Gonadotropin Releasing Hormone-1 Neuronal Activity. J Neurosci. 2002;22(20):8932–8941. doi: 10.1523/JNEUROSCI.22-20-08932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constantin S, Wray S. Gonadotropin-Releasing Hormone-1 Neuronal Activity Is Independent of Cyclic Nucleotide-Gated Channels. Endocrinology. 2008;149(1):279–290. doi: 10.1210/en.2007-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jasoni CL, et al. Calcium dynamics in gonadotropin-releasing hormone neurons. Front neuroendocrinol. 2010 doi: 10.1016/j.yfrne.2010.05.005. in press. [DOI] [PubMed] [Google Scholar]
- 61.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143(4):1459–66. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 62.DeFazio RA, et al. Activation of A-Type {gamma}-Aminobutyric Acid Receptors Excites Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2002;16(12):2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 63.Han SK, Todman MG, Herbison AE. Endogenous GABA Release Inhibits the Firing of Adult Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 64.Mody I, et al. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 65.Mozrzymas JW, et al. Modulation of GABAA Receptors by Hydrogen Ions Reveals Synaptic GABA Transient and a Crucial Role of the Desensitization Process. J Neurosci. 2003;23(22):7981–7992. doi: 10.1523/JNEUROSCI.23-22-07981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leupen SM, et al. Heterogeneous expression of the potassium-chloride cotransporter KCC2 in gonadotropin-releasing hormone neurons of the adult mouse. Endocrinology. 2003;144:3031–3036. doi: 10.1210/en.2002-220995. [DOI] [PubMed] [Google Scholar]
- 67.Nakane R, Oka Y. Excitatory Action of GABA in the Terminal Nerve Gonadotropin-Releasing Hormone Neurons. J Neurophysiol. 2010;103(3):1375–1384. doi: 10.1152/jn.00910.2009. [DOI] [PubMed] [Google Scholar]
- 68.Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 69.Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite GnRH neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- 70.Yin C, et al. Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurons isolated from adult rats. J Neuroendocrinol. 2008;20:566–575. doi: 10.1111/j.1365-2826.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe M, Sakuma Y, Kato M. GABAA Receptors Mediate Excitation in Adult Rat GnRH Neurons. Biology of Reproduction. 2009;81(2):327–332. doi: 10.1095/biolreprod.108.074583. [DOI] [PubMed] [Google Scholar]
- 72.Constantin S, et al. gamma-Aminobutyric Acid and Glutamate Differentially Regulate Intracellular Calcium Concentrations in Mouse Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2010;151:262–270. doi: 10.1210/en.2009-0817. [DOI] [PubMed] [Google Scholar]
- 73.Jasoni CL, et al. Cell Type-Specific Expression of a Genetically Encoded Calcium Indicator Reveals Intrinsic Calcium Oscillations in Adult Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2007;27(4):860–867. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi HJ, et al. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;21:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of C1- and HCO3 transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee K, et al. Knockdown of GABAA Receptor Signaling in GnRH Neurons Has Minimal Effects upon Fertility. Endocrinology. 2010:en.2010–0314. doi: 10.1210/en.2010-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan YM, et al. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023. doi: 10.1111/j.1365-2826.2009.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X, et al. Frequency-dependent recruitment of fast and slow neurotrnasmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.5759-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonifazi P, et al. GABAergic Hub Neurons Orchestrate Synchrony in Developing Hippocampal Networks. Science. 2009;326(5958):1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 80.Miles R. Neurobiology: A homeostatic switch. Nature. 1999;397(6716):215–216. doi: 10.1038/16604. [DOI] [PubMed] [Google Scholar]