Abstract
Objective
We evaluated the clinical relevance of catenins, cadherins and cell cycle regulators in stage IV or recurrent endometrial carcinoma in a multi-center phase II trial (GOG protocol #119).
Methods
Tissue microarrays of metastatic or recurrent (n=42) tumor were developed and immunohistochemistry performed. Average expression (percent staining × intensity) was assessed in tumor epithelium (E) and stroma (S) and categorized into tertiles (T1, T2, T3) for E-cadherinE, N-cadherinE, alpha-cateninE, beta-cateninE, gamma-cateninE, p120-cateninE and Ki-67E; as negative, below median or above median for p16E, p27E and CD44S; or as negative or positive for p53E, Ki-67S and APCS (adenomatous polyposis coli). End points included response and survival.
Results
E-cadherinE, p16E, and p53E varied by race (p=0.003, p=0.024, p=0.002,) and N-cadherinE, Ki-67E, p16E and p27E by tumor type (p=0.015, p=0.011, p=0.005, p=0.021). Correlations were observed among E-cadherinE with p120E (r=0.66), p53E (r=−0.32), alpha-cateninE (r=0.52), beta-cateninE (r=0.58), and gamma-cateninE (r=0.58). High E-cadherinE (T2 or T3) versus low (T1) expression was associated with better survival in unadjusted (hazard ratio [HR]=0.14, 95% confidence interval [CI]=0.06–0.37 or HR=0.17, 95% CI=0.07–0.42) and adjusted models (HR=0.18, 95% CI=0.05–0.59 or HR=0.22, 95% CI=0.07–0.70). High p16E versus negative expression was associated with worse survival in unadjusted (HR=3.87, 95% CI=1.74–8.61) and adjusted (HR=4.18, 95% CI=1.28–13.6) models. Positive versus negative expression of p53E was associated with worse survival in unadjusted (HR=2.31, 95% CI=1.16–4.60) but not adjusted models.
Conclusions
E-cadherinE and p16E appear to be clinically relevant, independent prognostic factors in stage IV or recurrent endometrial cancers treated with T+M, and merit further study.
Keywords: endometrial cancer, cadherin, catenin, p16, p120, p53
INTRODUCTION
Endometrial cancer is the most common gynecological malignancy in the United States. The 5-year survival rate is 96% if the cancer is diagnosed at a local stage, but decreases to 17% if diagnosed at an advanced stage [1]. As invasion and metastasis at the time of diagnosis significantly worsen the prognosis, awareness of the biomarkers that may be clinically relevant in endometrial cancer is warranted.
Invasion may be due to reduced cell to cell adhesion properties. Without strong adhesions between cells, cancer cells can more easily, leave their primary location to invade the surrounding tissue, and spread elsewhere [2]. Cadherin is one of the main adhesion molecules in the cell-cell adherens junction. Cadherin molecules are calcium-dependent transmembrane glycoproteins [3]. E-cadherin is the major cadherin in epithelial cells, while N-cadherin is found in neural tissues. The cytoplasmic domain of cadherin binds to a Catenin which can be of the alpha, beta or gamma subtype [2]. Reduced expression of E-cadherin molecules has been associated with many types of cancer and alterations in E-cadherin proteins and their associated cytoplasmic proteins may have a role in determining differentiation in endometrial adenocarcinomas [4, 5].
Biomarkers that play a role in tumor formation by controlling cell cycle proliferation include p16, p27, p53, and Ki-67. p16 functions as a negative regulator of the cell cycle and the association between p16 and HPV is well documented [6–9]. However, p16 overexpression has also been reported in tumors that have no direct link to Human Papilloma Virus (HPV) [10,11]. A cyclin-dependent kinase inhibitor, p27, also functions as a tumor suppressor gene [12]. One of the most commonly mutated tumor suppressor genes in human cancers is p53 [13]. CD44 is a transmembrane cell surface adhesion glycoprotein with important cell-cell and cell-matrix activities [14]. E-cadherin and APC, being components of the Wnt signaling pathway via aberrant DNA methylation may be important in endometrial carcinogenesis [15,16]. The role of cadherin-catenin complexes has been studied in regards to endometrial cancer, but less so in advanced endometrial cancer [17–20]. Understanding correlations and associations between cadherin-catenin proteins and the additional biomarkers listed above has the potential to predict a patient’s prognosis and survival, even for women with advanced or recurrent disease.
The aim of this study is to evaluate the differential expression of the proteins of the cadherin-catenin complex in advanced endometrial carcinoma and correlate this with clinical outcomes and survival. We also aim to evaluate any interactions between these complexes and other tumor suppressor genes namely p16, p27, p53, APC, and CD44. We hypothesize that the expression profiles of these biomarkers will influence prognosis and clinical outcome.
MATERIALS AND METHODS
Patients
Between 1991 and 1996, the Gynecologic Oncology Group (GOG) conducted a prospective phase II trial of tamoxifen combined with intermittent medroxyprogesterone acetate. This trial included patients with histologically-confirmed, through central review by the GOG Pathology Committee, advanced, persistent or recurrent endometrial carcinoma considered incurable by local therapy or refractory to local therapy. The disease was to be measurable in 2 dimensions by palpation or imaging. If measurable only by imaging, it was to have a minimum diameter of 3 cm. The tumor must not have received radiation therapy within 3 months prior to entry. Prior therapy with cytotoxic drugs or hormonal therapy was not allowed. Normal hepatic, renal and hematologic functions, the absence of significant infection and GOG performance status 0–2 were required. Patients with past or concomitant malignancy were ineligible. A written informed consent conforming to federal, state and local regulations was obtained from all participants prior to study entry.
Treatment
Patients were treated with weekly cycles of 20 mg oral tamoxifen citrate twice daily. On alternating (even-numbered) weeks, patients also received 100 mg oral medroxyprogesterone acetate twice daily. This treatment regimen is abbreviated T+M.
Clinical End Points and Outcomes
The clinical results of the GOG-119 trial have previously been reported [21]. Progression-free interval (PFI), overall survival (OS) and progression-free survival (PFS) were calculated per standard definitions.
Specimens
The GOG-119 protocol required the submission of frozen metastatic or recurrent tumor removed prior to initiation of T+M treatment. Residual frozen tumor specimens were then formalin-fixed and paraffin-embedded to support biomarker evaluations by immunohistochemistry [22] and these were utilized for the creation of tissue microarrays (TMAs).
Tissue Microarray Array Block and Slide Preparation
TMAs were constructed by selecting regions with viable tumor from the hematoxylin and eosin stained slides. TMA assembly proceeded using a tissue-arraying instrument (Beecher Instrument, Silver Springs, MD). A minimum of 3 tissue cores were taken from each patient’s tumor sample. A total of 4 TMA blocks were prepared with 2 of these being duplicates and representing more cores than would have been feasible with 2 TMA blocks alone. Normal controls and navigational aids were also transferred to the recipient block. Multiple 4-µm sections were prepared. Immunohistochemistry (IHC) assays were run on unstained slides.
Immunohistochemistry Assays
The antibody for E-cadherin (1:100 dilution of mouse monoclonal 36, Transduction Laboratories, Lexington, KY), N-cadherin (1:200 dilution mouse monoclonal 32, Transduction Laboratories), p16 (1:200 dilution mouse monoclonal clone 16, Novocastra Laboratories Ltd., Newcastle Upon Tyme, UK), p27 (1:50 dilution of mouse monoclonal SX53D8 Dako, Carpinteria, CA), p53 (1:200 dilution mouse monoclonal clone DO-7 Dako,), p120 (1:200 dilution of mouse monoclonal 15D2, Zymed Laboratories Inc., South San Francisco, CA), alpha-catenin (1:100 mouse monoclonal G-11 Santa Cruz Biotechnology Inc, Santa Cruz, CA), beta-catenin (1:100 dilution of mouse monoclonal clone 14, Transduction Laboratories), gamma-catenin (1:50 dilution mouse monoclonal clone 15, Transduction Laboratories), CD44 (Dako anti-CD44 clone DF1485) and APC (1:50 dilution of rabbit polyclonal C-20; Santa Cruz) was evaluated in conventional sections of formalin-fixed, paraffin-embedded normal endometrium and endometrial carcinoma which served as positive and negative controls. Titration for the appropriate sensitivity and specificity was performed on these tissues. Immunohistochemistry assays were optimized for each biomarker. Briefly, Antigen retrieval was performed in citrate buffer using a Biocare Medical (Walnut Creek, CA) decloaking chamber. TMA slides were incubated with 3% peroxide in absolute methanol, an avidin/biotin blocker, and then with the appropriate primary antibody at 37° C for 1 hour (except p27 I hour at room temperature), the secondary antibody (Dako Biotinylated Multi-Link anti-mouse, anti-goat, and anti-rabbit immunoglobulin in 40% human serum) at room temperature for 30 minutes. The negative control sections were treated in the same manner, except that primary antibody was replaced with IgG sub class matched immune serum.
Interpretation and scoring of IHC
The TMAs were scored independently by study pathologists (MS, JVR, ZW and AA) who were blinded to clinical information. Consistency for interpretation of stain intensity and percentage cells stained amongst the pathologists was first established by jointly reviewing immunostained examples of TMAs and conventional slide sections of the biomarkers evaluated. At least 2 pathologists reviewed each biomarker section and any discrepancies amongst the reviewers were resolved by re-reviewing the tissue cores across a double headed microscope, before assigning a final scoring. We reported percent positive staining cells (0–100%) and staining intensity (0, 1, 2, and 3). Staining intensities were defined as: 0 = no brown staining at high power, 1 = faint brown staining at high power, 2 = moderate brown staining visible at low power, 3 = dark brown staining at low power. An aggregate score was calculated for each core as percent positive staining × staining intensity (0–300). Mean values, for both tumor epithelium (E) and stroma (S), were calculated across all the cores available for a given patient for each biomarker: percent positive staining, staining intensity, and staining index e.g. expression (percent positive staining × staining intensity. Biomarkers were categorized into tertiles (T1, T2, T3) for E-cadherinE, N-cadherinE, alpha-cateninE, beta-cateninE, gamma-cateninE, p120-cateninE and Ki-67E; as negative, below median or above median for p16E, p27E and CD44S; or as negative or positive for p53E, Ki-67S and APCS.
Statistical Analysis and Methods
Biomarkers were categorized as indicated above. Clinical-pathologic characteristics were compared using Fisher’s Exact test, survival probability using the Kaplan-Meier method, and the logrank test to compare groups. Cox proportional hazards models compared groups in unadjusted and adjusted models (with an adjustment for patient age at study enrollment and stratification for performance status and tumor grade). All tests were two-sided, and results considered significant if p<0.05; adjustments were not made for multiple testing as these exploratory analyses were post hoc and are being used to prioritize future testing. Plots of biomarker pairs and rank correlations helped explore correlations between biomarkers. Statistical analyses were performed using SAS (SAS Institute Inc., Cary NC).
RESULTS
Between June 1991 and February 1996, 61 patients were entered into the phase II GOG 119 study [21] and 60 were eligible. Archival metastatic tumor (N=4) and recurrent tumor (N=38) were available for 42 of the 60 eligible women (70%). All of the 12 biomarkers evaluated were expressed in the tumor epithelium but p53 was the only biomarker that was not expressed in the tumor stroma. Figure 1 shows staining for some of the biomarkers. E-cadherin, N-cadherin, p16, alpha-catenin, beta-catenin, gamma-catenin, CD44, APC, and p120 exhibited cytoplasmic staining. p27, p53, p16, and Ki67 displayed nuclear staining. A few tumor cells also presented with nuclear beta and gamma-catenin. Membrane staining was observed for alpha-catenin, beta-catenin, gamma-catenin, and p120.
Figure 1.
Immunohistochemistry photomicrographs for select markers in the tumor epithelium or stroma of representative endometrial carcinomas in the GOG#119 tissue microarrays (TMAs). Representative expression of low E-cadherinE (T1-lowest tertile) [A] compared with high E-cadherinE (T3-highest tertile) [B]. The inset shows a cross section of the TMA core of (A). Representative endometrial carcinoma exhibiting no N-cadherin expression (negative) [C] compared with high N-cadherinE expression [D]. The inset shows that stromal elements are negative for N-cadherin [D]. Representative endometrial carcinoma displaying no p16 expression (negative) [E] compared with high p16E expression in the nucleus and cytoplasm of representative carcinoma cells [F]. The inset shows a cross section of the TMA core in panel F and displays that expression is confined to tumor and non-tumor tissue (bottom of the TMA) is negative for p16.
Table 1 displays the clinicopathologic characteristics and outcomes for the subset of patients included in the TMAs compared with the full cohort of women who participated in the GOG-119 protocol; their characteristics and outcomes were similar. A majority were over 60 years of age, Caucasian and had symptomatic performance scores and recurrent disease. The most common tumor types were endometrioid and uterine serous cancer. Of the women treated with T+M, >30% experienced either a complete response or partial response (Table 1).
Table 1.
Clinicopathologic characteristics and outcomes.
| TMA Cases | All GOG-119 Cases | |||
|---|---|---|---|---|
| Cases | % | Cases | % | |
| Age | ||||
| <=60 | 11 | 26.2 | 15 | 25.0 |
| 61–70 | 16 | 38.1 | 22 | 36.7 |
| 71–80 | 9 | 21.4 | 16 | 26.7 |
| >=81 | 6 | 14.3 | 7 | 11.7 |
| Race | ||||
| Caucasian | 34 | 81.0 | 50 | 83.3 |
| African American | 7 | 16.7 | 8 | 13.3 |
| Other | 1 | 2.4 | 2 | 3.3 |
| Performance Status | ||||
| 0 – asymptomatic | 19 | 45.2 | 26 | 43.3 |
| 1 – symptomatic | 18 | 42.9 | 26 | 43.3 |
| 2 – symptomatic | 5 | 11.9 | 8 | 13.3 |
| Stage | ||||
| IV | 4 | 9.5 | 6 | 10.0 |
| Recurrent | 38 | 90.5 | 54 | 90.0 |
| Histologic Type | ||||
| Endometrioid | 23 | 54.8 | 32 | 53.3 |
| Serous adenocarcinoma | 9 | 21.4 | 14 | 23.3 |
| Adenocarcinoma, not specified | 4 | 9.5 | 6 | 10.0 |
| Mixed epithelial | 2 | 4.8 | 3 | 5.0 |
| Adenosquamous | 2 | 4.8 | 3 | 5.0 |
| Squamous cell carcinoma | 1 | 2.4 | 1 | 1.7 |
| Villoglandular adenocarcinoma | 1 | 2.4 | 1 | 1.7 |
| Tumor Grade | ||||
| 1 | 12 | 28.6 | 15 | 25.0 |
| 2 | 15 | 35.7 | 17 | 28.3 |
| 3 | 15 | 35.7 | 27 | 45.0 |
| Not Specified | 1 | 1.7 | ||
| Prior Radiotherapy | ||||
| No | 17 | 40.5 | 24 | 40.0 |
| Yes | 25 | 59.5 | 36 | 60.0 |
| Tumor Responses | ||||
| Complete Response (CR) | 3 | 7.1 | 6 | 10.0 |
| Partial Response (PR) | 11 | 26.2 | 13 | 21.7 |
| Stable Disease (SD) | 15 | 35.7 | 20 | 33.3 |
| Increasing Disease (ID) | 10 | 23.8 | 16 | 26.7 |
| Not evaluable | 3 | 7.1 | 5 | 8.3 |
| Total | 42 | 100.0 | 60 | 100.0 |
Table 2 displays relationships between categorized expression of the biomarkers and clinicopathologic characteristics. E-cadherinE, p16E, and p53E varied significantly by race. For example, high E-cadherinE expression was only observed in the Caucasian women whereas p16 and p53 expression were observed in Caucasians and African-Americans. N-cadherinE, Ki67E, p16E, and p27E were differentially expressed in histologic types. For example, high N-cadherinE and low Ki-67E expression were more common in non-endometrioid cancers. Serous adenocarcinomas did not exhibit low p16E and always expressed some p27E. None of the biomarkers were associated with tumor grade (Table 2).
Table 2.
Associations between biomarker expression and clinicopathologic characteristics in epithelial (E) and stromal (S) elements of metastatic or recurrent endometrial cancers.
| Biomarker | Clinicopathologic Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race (%) | Histologic Type (%) | Tumor Grade (%) | |||||||||||
| n | Caus (n=34) |
AA (n=7) |
Oth (n=1) |
p | End (n=23) |
Ser (n=9) |
Oth (n=10) |
p | G1 (n=12) |
G2 (n=15) |
G3 (n=15) |
p | |
| E-cadherinE | |||||||||||||
| T1: Low | 14 | 21 | 86 | 100 | 0.003 | 39 | 44 | 10 | 0.061 | 33 | 27 | 40 | 0.877 |
| T2: Moderate | 14 | 38 | 14 | 0 | 17 | 33 | 70 | 25 | 40 | 33 | |||
| T3: High | 14 | 41 | 0 | 0 | 43 | 22 | 20 | 42 | 33 | 27 | |||
| N-cadherinE | |||||||||||||
| T1: Low | 14 | 32 | 29 | 100 | 1.000 | 35 | 33 | 30 | 0.015 | 33 | 20 | 47 | 0.250 |
| T2: Moderate | 14 | 32 | 43 | 0 | 52 | 11 | 10 | 33 | 53 | 13 | |||
| T3: High | 14 | 35 | 29 | 0 | 13 | 56 | 60 | 33 | 27 | 40 | |||
| p120-cateninE | |||||||||||||
| T1: Low | 14 | 26 | 57 | 100 | 0.085 | 35 | 33 | 30 | 0.969 | 42 | 27 | 33 | 0.952 |
| T2: Moderate | 14 | 32 | 43 | 0 | 30 | 44 | 30 | 33 | 33 | 33 | |||
| T3: High | 14 | 41 | 0 | 0 | 35 | 22 | 40 | 25 | 40 | 33 | |||
| alpha-catenin E | |||||||||||||
| T1: Low | 14 | 32 | 43 | 0 | 1.000 | 26 | 22 | 60 | 0.403 | 42 | 33 | 27 | 0.952 |
| T2: Moderate | 14 | 32 | 29 | 100 | 39 | 33 | 20 | 33 | 33 | 33 | |||
| T3: High | 14 | 35 | 29 | 0 | 35 | 44 | 20 | 25 | 33 | 40 | |||
| beta-catenin E | |||||||||||||
| T1: Low | 14 | 35 | 29 | 0 | 0.256 | 22 | 44 | 50 | 0.556 | 33 | 20 | 47 | 0.586 |
| T2: Moderate | 14 | 26 | 57 | 100 | 39 | 22 | 30 | 33 | 47 | 20 | |||
| T3: High | 14 | 38 | 14 | 0 | 39 | 33 | 20 | 33 | 33 | 33 | |||
| gamma-catenin E | |||||||||||||
| T1: Low | 14 | 26 | 57 | 100 | 0.256 | 39 | 22 | 30 | 0.199 | 42 | 33 | 27 | 0.814 |
| T2: Moderate | 14 | 38 | 14 | 0 | 35 | 11 | 50 | 33 | 40 | 27 | |||
| T3: High | 14 | 35 | 29 | 0 | 26 | 67 | 20 | 25 | 27 | 47 | |||
| Ki67E | |||||||||||||
| T1: Low | 14 | 32 | 43 | 0 | 0.665 | 17 | 44 | 60 | 0.011 | 33 | 27 | 40 | 0.814 |
| T2: Moderate | 14 | 35 | 14 | 100 | 48 | 0 | 30 | 25 | 47 | 27 | |||
| T3: High | 14 | 32 | 43 | 0 | 35 | 56 | 10 | 42 | 27 | 33 | |||
| p16E | |||||||||||||
| Negative | 21 | 56 | 14 | 100 | 0.024 | 57 | 22 | 60 | 0.005 | 58 | 53 | 40 | 0.120 |
| Below median: low | 10 | 26 | 14 | 0 | 30 | 0 | 30 | 17 | 40 | 13 | |||
| Above median: high | 11 | 18 | 71 | 0 | 13 | 78 | 10 | 25 | 7 | 47 | |||
| p27E | |||||||||||||
| Negative | 19 | 47 | 43 | 0 | 0.552 | 57 | 0 | 60 | 0.021 | 50 | 67 | 20 | 0.144 |
| Below median: low | 11 | 26 | 14 | 100 | 22 | 44 | 20 | 25 | 13 | 40 | |||
| Above median: high | 12 | 26 | 43 | 0 | 22 | 56 | 20 | 25 | 20 | 40 | |||
| p53E | |||||||||||||
| Negative | 29 | 79 | 14 | 100 | 0.002 | 78 | 33 | 80 | 0.052 | 58 | 87 | 60 | 0.196 |
| Positive | 13 | 21 | 86 | 0 | 22 | 67 | 20 | 42 | 13 | 40 | |||
| Ki-67S | |||||||||||||
| Negative | 24 | 56 | 71 | 0 | 0.410 | 57 | 56 | 60 | 1.000 | 75 | 40 | 60 | 0.209 |
| Positive | 18 | 44 | 29 | 100 | 43 | 44 | 40 | 25 | 60 | 40 | |||
| APCS | |||||||||||||
| Negative | 30 | 74 | 71 | 0 | 0.404 | 65 | 67 | 90 | 0.346 | 92 | 60 | 67 | 0.172 |
| Positive | 12 | 26 | 29 | 100 | 35 | 33 | 10 | 8 | 40 | 33 | |||
| CD44S | |||||||||||||
| Negative | 7 | 18 | 14 | 0 | 0.834 | 17 | 11 | 20 | 0.987 | 25 | 13 | 13 | 0.834 |
| Below median: low | 17 | 38 | 57 | 0 | 43 | 44 | 30 | 42 | 33 | 47 | |||
| Above median: high | 18 | 44 | 29 | 100 | 39 | 44 | 50 | 33 | 53 | 40 | |||
Categorized biomarker expression (percent staining × intensity).
N: cases, %: column percentage (e.g., high E-cadherin was observed in 41% of Caucasians and none of the African-American or other racial groups, respectively), Caus: Caucasian, AA: African American, Oth: Other, End: Endometrioid, Ser: Serous, G1: well differentiated, G2 moderately differentiated, G3: poorly differentiated or not graded.
p<0.05 are bolded
Statistically significant correlations were observed among several biomarkers in the tumor epithelium but not in the tumor stroma. For these analyses, average biomarker expression (percent staining × intensity) across replicate cores was examined as continuous rather then categorical variables. Positive correlation were observed between the epithelial expression of E-cadherinE with p120E, alpha-cateninE, beta-cateninE and gamma-cateninE; Ki-67E with p120E, p27E and gamma-cateninE; N-cadherinE with p120E and gamma-cateninE (Table 3). Inverse correlations were observed between E-cadherinE and p53E and between Ki-67E with CD44E (Table 3).
Table 3.
Rank correlation coefficients between marker expression in tumor epithelium above the diagonal and in stroma below the diagonal.
| Tumor Epithelium (E) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APC | CD44 | E- cad |
Ki67 | N- cad |
P120 | P16 | P27 | P53 | α-cat | β-cat | γ-cat | ||
| Tumor Stroma (S) | APC | 0.23 | −0.14 | 0.03 | 0.19 | −0.01 | 0.01 | 0.17 | 0.08 | 0.09 | −0.21 | 0.21 | |
| CD44 | 0.17 | −0.04 | −0.33* | −0.10 | 0.08 | −0.20 | 0.06 | 0.00 | −0.24 | −0.19 | −0.03 | ||
| E-cad | −0.17 | 0.14 | 0.17 | 0.16 | 0.66* | −0.16 | −0.19 | −0.32* | 0.52* | 0.58* | 0.58* | ||
| Ki67 | 0.02 | −0.04 | 0.46 | 0.15 | 0.30* | 0.07 | 0.33* | 0.23 | 0.70 | 0.62 | 0.36* | ||
| N-cad | 0.46 | 0.07 | −0.09 | 0.08 | 0.44* | 0.24 | 0.05 | 0.10 | 0.27 | 0.26 | 0.32* | ||
| P120 | 0.17 | 0.04 | 0.25 | 0.15 | 0.21 | −0.04 | −0.10 | −0.21 | 0.54 | 0.71 | 0.65 | ||
| P16 | −0.13 | 0.18 | 0.00 | 0.03 | −0.06 | −0.13 | 0.09 | 0.57 | 0.04 | 0.07 | 0.09 | ||
| P27 | 0.04 | 0.04 | 0.26 | 0.35 | 0.11 | 0.18 | −0.21 | 0.18 | 0.22 | 0.06 | 0.08 | ||
| P53 | NE | NE | NE | NE | NE | NE | NE | NE | −0.06 | −0.09 | −0.12 | ||
| α-cat | 0.04 | −0.04 | 0.30 | 0.05 | 0.01 | −0.13 | 0.19 | −0.08 | NE | 0.77 | 0.68 | ||
| β-cat | 0.21 | 0.02 | 0.03 | 0.09 | 0.47 | −0.04 | 0.22 | 0.25 | NE | 0.39 | 0.51 | ||
| γ-cat | −0.10 | 0.05 | 0.27 | 0.15 | 0.15 | 0.44 | −0.06 | 0.31 | NE | −0.06 | −0.10 | ||
NE: not evaluable, E-cad: E-cadherin, N-cad: N-cadherin, α-cat: alpha-catenin, β-cat: beta-catenin, γ-cat: gamma-catenin
p<0.05
Negative correlations indicate an inverse relationship.
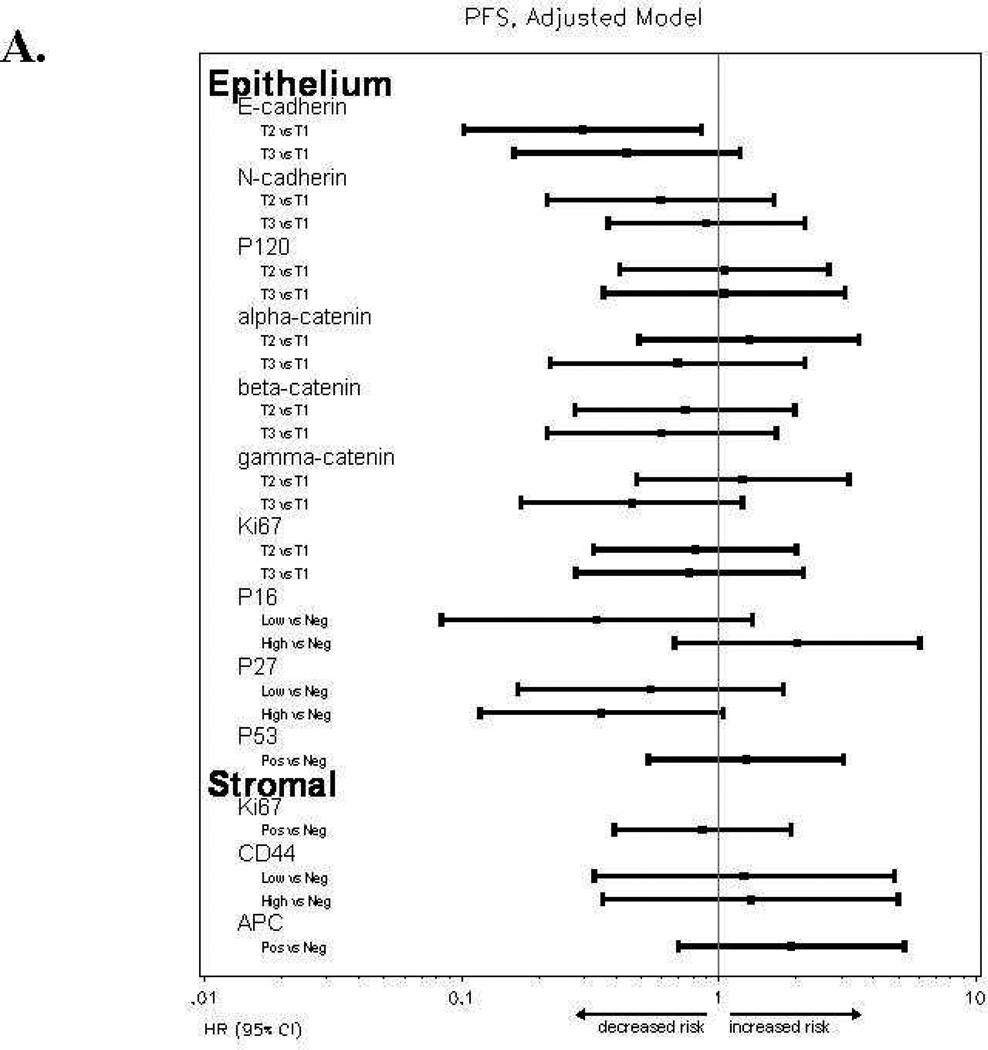
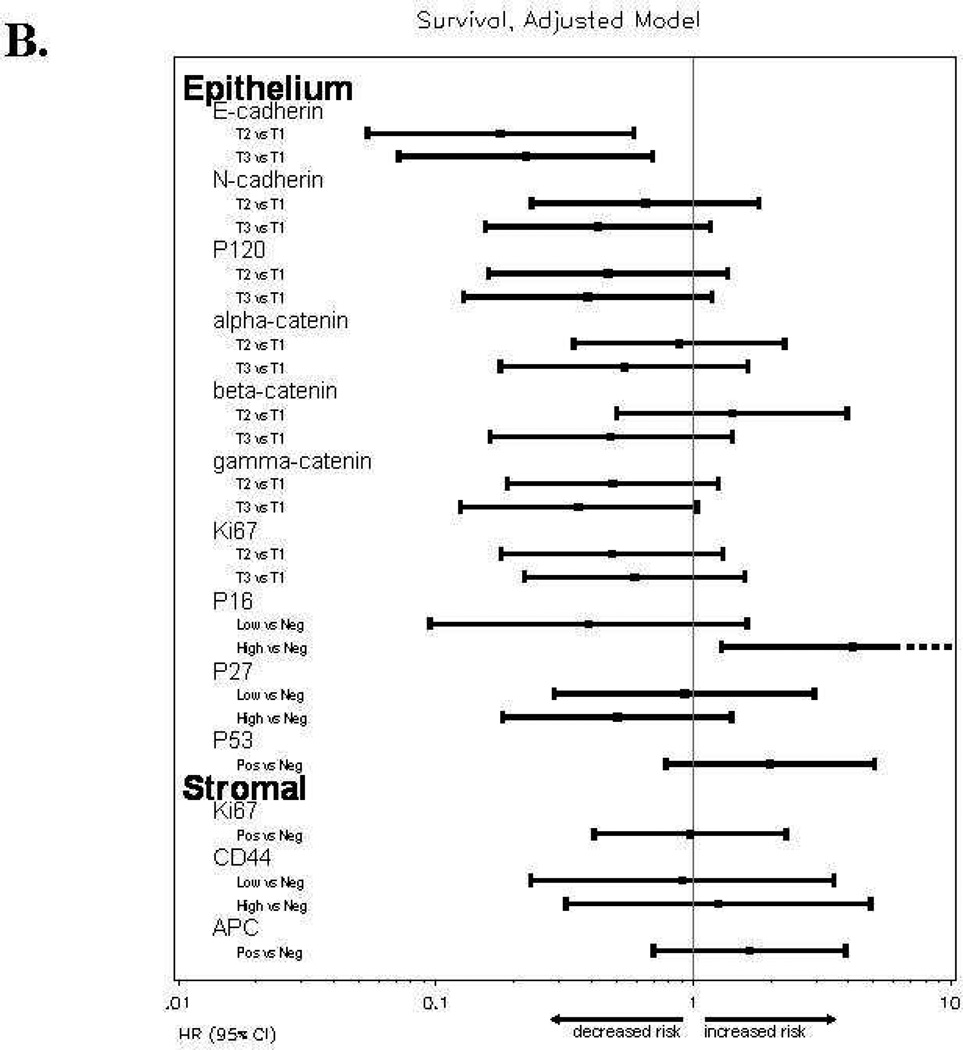
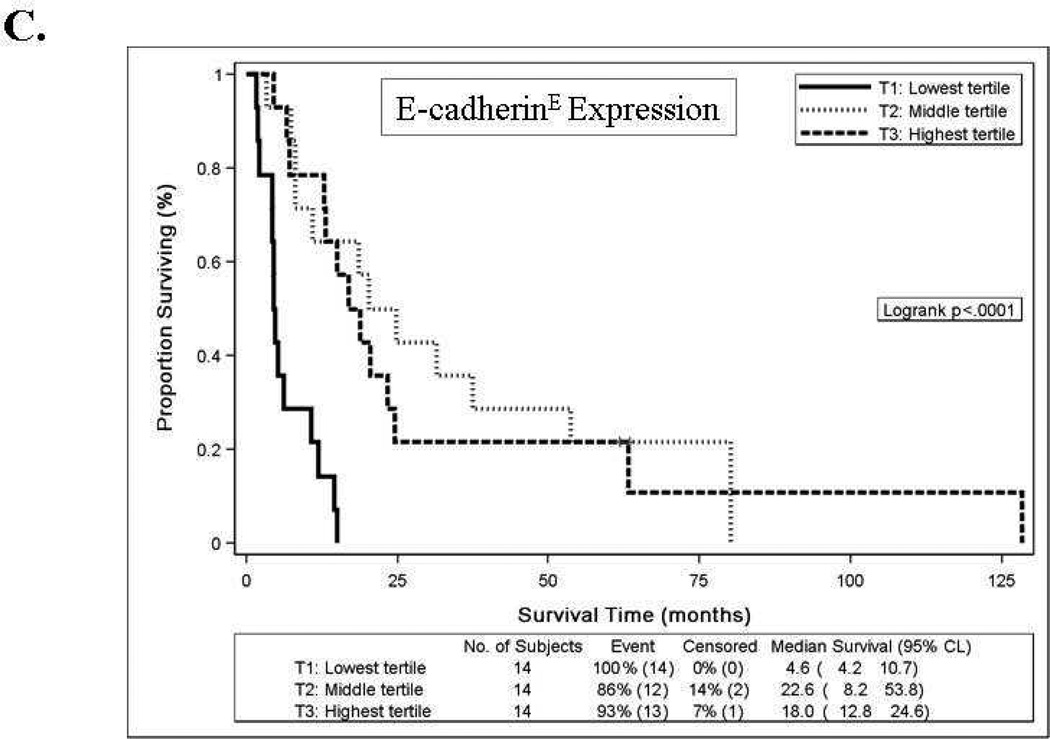
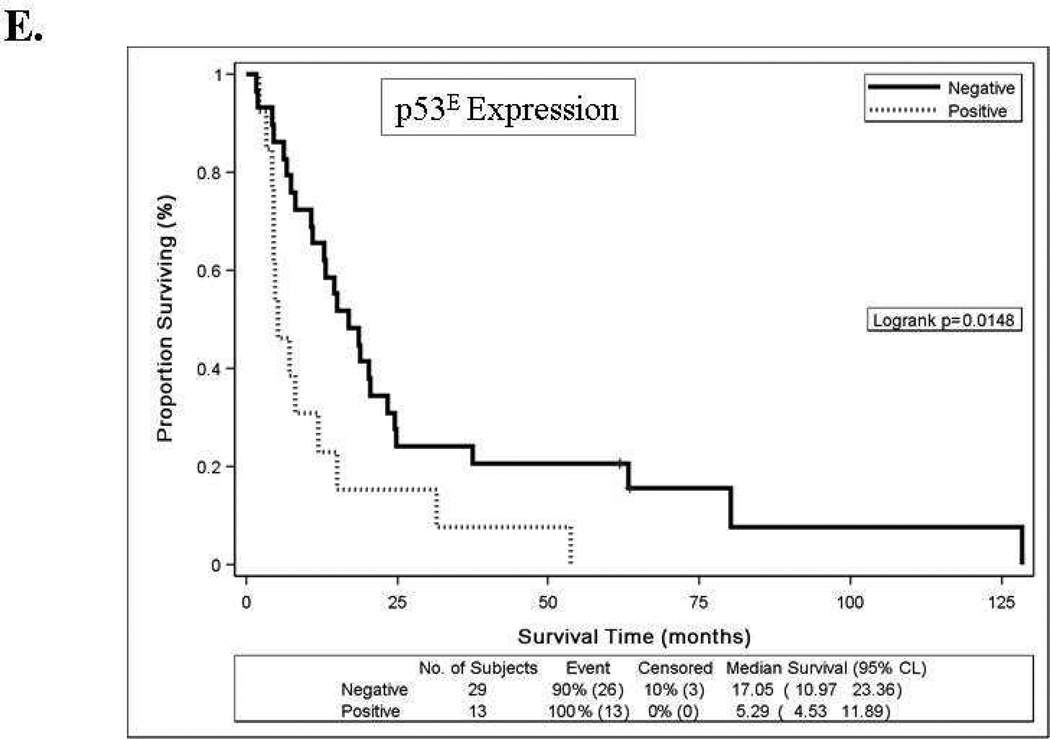
Women with high E-cadherinE expression (T2 or T3) versus low expression (T1) had a reduced risk of disease progression and a reduced risk of death, and the association with better survival persisted after an adjustment for patient age at study enrollment and stratification for performance status and tumor grade (Table 4). The Kaplan-Meier plot in Figure 2C also illustrates the survival benefit associated with high versus low E-cadherinE expression including a 13–18 month difference in median survival time. High p16E expression (above the median) versus negative expression was associated with similar risk of disease progression, an increased risk of death and worse survival (Table 4, Figures 2A, 2B, 2D). Positive versus negative expression of p53E was associated with a similar risk of disease progression, an increased risk of death in unadjusted models but not after adjustment for other factors, and worse OS (Table 4, Figures 2A, 2B, 2E). High (T3) versus low (T1) N-cadherinE and p120E expression were associated with better OS in unadjusted models but not after adjustment for other factors, and neither appeared to be associated with PFS (Table 4, Figures 2A–2B). None of the other biomarkers were associated with either PFS or OS (Table 4, Figures 2A–2B and data not shown) or with response.
Table 4.
Associations between biomarker expression and clinical outcome in epithelial (E) and stromal (S) elements of metastatic or recurrent endometrial cancers.
| Biomarker | Progression-Free Survival | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | |||||||||
| HR | (95% CI) |
p | HR | (95% CI) |
p | HR | (95% CI) |
p | HR | (95% CI) |
p | |
| E-cadherin E | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 0.34 | (0.15, 0.76) | 0.008 | 0.30 | (0.10, 0.86) | 0.025 | 0.14 | (0.06, 0.37) | <.001 | 0.18 | (0.05, 0.59) | 0.004 |
| T3: High | 0.32 | (0.14, 0.73) | 0.006 | 0.44 | (0.16, 1.21) | 0.112 | 0.17 | (0.07, 0.42) | <.001 | 0.22 | (0.07, 0.70) | 0.010 |
| N-cadherin E | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 0.48 | (0.21, 1.08) | 0.076 | 0.60 | (0.22, 1.64) | 0.316 | 0.54 | (0.25, 1.18) | 0.122 | 0.65 | (0.24, 1.80) | 0.408 |
| T3: High | 0.63 | (0.29, 1.36) | 0.236 | 0.90 | (0.37, 2.16) | 0.807 | 0.33 | (0.14, 0.75) | 0.009 | 0.43 | (0.16, 1.17) | 0.097 |
| p120-cateninE | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 0.67 | (0.31, 1.44) | 0.302 | 1.05 | (0.41, 2.69) | 0.913 | 0.54 | (0.24, 1.20) | 0.129 | 0.47 | (0.16, 1.36) | 0.161 |
| T3: High | 0.76 | (0.35, 1.64) | 0.482 | 1.05 | (0.36, 3.11) | 0.928 | 0.44 | (0.20, 0.98) | 0.045 | 0.39 | (0.13, 1.18) | 0.096 |
| alpha-catenin E | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 1.47 | (0.68, 3.18) | 0.330 | 1.31 | (0.49, 3.52) | 0.587 | 1.27 | (0.56, 2.89) | 0.575 | 0.88 | (0.34, 2.26) | 0.790 |
| T3: High | 0.88 | (0.42, 1.86) | 0.738 | 0.69 | (0.22, 2.16) | 0.523 | 0.99 | (0.45, 2.20) | 0.981 | 0.54 | (0.18, 1.63) | 0.274 |
| beta-catenin E | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 1.03 | (0.48, 2.19) | 0.946 | 0.74 | (0.28, 1.98) | 0.549 | 1.37 | (0.62, 3.02) | 0.442 | 1.41 | (0.50, 3.95) | 0.511 |
| T3: High | 0.69 | (0.32, 1.48) | 0.342 | 0.60 | (0.22, 1.67) | 0.329 | 0.67 | (0.30, 1.50) | 0.333 | 0.48 | (0.16, 1.41) | 0.181 |
| gamma-cateninE | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 0.77 | (0.35, 1.70) | 0.519 | 1.24 | (0.48, 3.21) | 0.659 | 0.53 | (0.23, 1.20) | 0.128 | 0.48 | (0.19, 1.25) | 0.134 |
| T3: High | 0.55 | (0.26, 1.19) | 0.131 | 0.46 | (0.17, 1.24) | 0.125 | 0.59 | (0.27, 1.28) | 0.182 | 0.36 | (0.12, 1.03) | 0.057 |
| Ki67E | ||||||||||||
| T1: Low | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| T2: Moderate | 0.82 | (0.38, 1.76) | 0.615 | 0.81 | (0.33, 2.01) | 0.649 | 0.65 | (0.29, 1.46) | 0.294 | 0.48 | (0.18, 1.30) | 0.149 |
| T3: High | 1.07 | (0.51, 2.27) | 0.859 | 0.77 | (0.28, 2.14) | 0.618 | 1.06 | (0.49, 2.32) | 0.883 | 0.59 | (0.22, 1.59) | 0.299 |
| p16E | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Below median: low | 0.83 | (0.38, 1.80) | 0.636 | 0.34 | (0.08, 1.36) | 0.125 | 0.99 | (0.44, 2.22) | 0.975 | 0.39 | (0.09, 1.62) | 0.196 |
| Above median: high | 1.54 | (0.73, 3.27) | 0.258 | 2.02 | (0.67, 6.05) | 0.209 | 3.87 | (1.74, 8.61) | <.001 | 4.18 | (1.28, 13.6) | 0.018 |
| p27E | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Below median: low | 1.12 | (0.53, 2.39) | 0.766 | 0.54 | (0.17, 1.79) | 0.316 | 1.70 | (0.79, 3.70) | 0.177 | 0.92 | (0.29, 2.95) | 0.893 |
| Above median: high | 0.64 | (0.30, 1.37) | 0.248 | 0.35 | (0.12, 1.04) | 0.058 | 0.68 | (0.30, 1.54) | 0.350 | 0.51 | (0.18, 1.41) | 0.191 |
| p53E | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Positive | 1.28 | (0.66, 2.50) | 0.461 | 1.28 | (0.53, 3.07) | 0.584 | 2.31 | (1.16, 4.60) | 0.018* | 1.99 | (0.78, 5.07) | 0.150 |
| Ki-67S | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Positive | 1.35 | (0.72, 2.55) | 0.349 | 0.87 | (0.39, 1.91) | 0.723 | 1.03 | (0.54, 1.95) | 0.934 | 0.97 | (0.41, 2.30) | 0.947 |
| APCS | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Positive | 1.97 | (0.97, 4.00) | 0.060 | 1.92 | (0.69, 5.28) | 0.210 | 1.41 | (0.70, 2.80) | 0.334 | 1.65 | (0.70, 3.90) | 0.254 |
| CD44S | ||||||||||||
| Negative | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA | 1.00 | NA | NA |
| Below median: low | 1.55 | (0.61, 3.96) | 0.356 | 1.26 | (0.33, 4.81) | 0.739 | 1.13 | (0.43, 2.96) | 0.809 | 0.91 | (0.23, 3.51) | 0.886 |
| Above median: high | 2.23 | (0.85, 5.85) | 0.105 | 1.33 | (0.36, 5.00) | 0.670 | 1.22 | (0.48, 3.11) | 0.681 | 1.25 | (0.32, 4.88) | 0.749 |
Categorized biomarker expression (percent staining × intensity).
NA: not applicable
Estimated hazard ratio (HR) and 95% confidence interval (95% CI) for Cox regression analysis.
with an adjustment for patient age at study enrollment and stratification for performance status and tumor grade.
When the 95% CI does not overlap a HR of 1.0, the association was significant (p<0.05 are bolded).
Figure 2.
Forest plots for catenins, cadherins and cell cycle regulators in tumor epithelium (E) and stroma (S) for progression-free survival [A] or overall survival [B] with hazard ratios (HR) and 95% confidence intervals (CI) based on Cox models with an adjustment for patient age at study enrollment and stratification for performance status and tumor grade. When the 95% CI does not overlaps a HR of 1.0 (center line), the association was significant. Biomarkers were categorized into tertiles (T1-lowest tertile, T2-middle tertile, T3-highest tertile) for E-cadherinE, N-cadherinE, alpha-cateninE, beta-cateninE, gamma-cateninE, p120-cateninE and Ki-67E; as negative, below median or above median for p16E, p27E and CD44S; or as negative or positive for p53E, Ki-67S and APCS. Kaplan-Meier survival distributions provided for tumor epithelial expression of E-cadherinE [C], p16E [D], or p53E [E]. Logrank test was used to test the equality in the survival distributions [C–E]. Significant associations (p<0.05) with survival were observed for E-cadherinE [C], p16E [D], or p53E [E]. Death is the event and censored indicates patients who were alive at last contact [C-E]. Median survival times are provided in months with 95% CI.
DISCUSSION
Members of the cadherin-catenin complex play significant roles in cell-cell adhesion and tumor progression in solid tumors including endometrial cancer [4]. The aim of our study was to explore the expression of biomarkers related to cell-cell adhesion and cell cycle in endometrial carcinoma for associations with clinicopathologic characteristics, clinical response, PFS and OS in women with advanced or recurrent disease who participated in a multi-institutional phase II trial of T+M conducted by the GOG [21].
Biomarker Associations with Clinicopathologic Characteristics
Our study showed that high E-cadherinE expression was only observed in the Caucasian women whereas p16E and p53E expression were observed both in Caucasians and African-Americans. In addition, high p16E expression and any p53E expression were both more common in African-American women compared with Caucasians. In contrast, Schimp et al. and Lukes et al., showed that p53 expression did not vary significantly by race [23,24]. Differences in samples size, evaluation criteria for the individual biomarkers and the inclusion versus exclusion of recurrent tumors may contribute to the disparities observed between studies.
High p16 expression was identified more often in serous carcinomas than in endometrioid or other types. This is supported by Engelson et al, who found p16 expression to correlate with serous/clear cell histologic subtypes [25]. We also showed that uterine serous adenocarcinomas expressed at least some p27E. However, Nycum et al did not find any association between p27 expression and histologic type of endometrial cancer [26]. The observation that high N-cadherinE was more common in non-endometrioid cancers is an intriguing, novel finding considering that N-cadherin has been described to originate in neural tissues and merits further study [2].
None of our biomarkers were associated with tumor grade, unlike some other reports [18–20]. We did not observe any evidence to suggest that any of the biomarkers was associated with grade even when the serous papillary tumors were excluded from the analysis. Associations with grade may require larger sample sizes to detect the relationship. Alternatively, inclusion of low and intermediate risk patients with stage I, II or III disease may be required to detect associations between these biomarkers and grade.
Associations between Biomarkers
We report many positive and negative correlations between the biomarkers evaluated Others have shown correlations between the expression of p27 and Ki-67 [27] and absent/low expression of p27 and absent/low expression of p53 [12]. These interactions have the potential to shed light on the pathobiology of endometrial cancer.
Biomarker Associations with Survival
Increased E-cadherin expression was associated with longer median survival and reduced risks of disease progression and death. Removal of the serous cancers from the Cox regression analysis did not alter the association between E-cadherin and PFS or survival (data not shown). Our findings support the hypothesis that cell adhesion molecules when expressed in tumor cells will influence prognosis and outcomes, and are in agreement with those of Mell et al. [28]. It is important to note that the patients in our study had stage IV or recurrent endometrial carcinoma and in theirs had stage I-III patients. Combined results from both studies would suggest that E-cadherin expression is associated with a reduced risk of progression and death in all stages of endometrial cancer (I-IV) as was also reported by Kim et al. [29]. Our results are also in line with those of Scholten et al. who report that in a univariate analysis, 5 and 10-year survival rates were 88% each for patients with E-cadherin positive tumors, while the survival rates were 79% and 71% for patients with negative E-cadherin expression [18].
We report that high versus negative expression of p16 in the tumor epithelium was associated with worse survival and an increased risk of death, and appeared to have independent prognostic value. These findings are supported by those of Engelsen et al [25]. The 5-year survival rate for patients with normal expression of p16 was 85%, but decreased to 50% among patients with increased p16 expression. p16 is used as a surrogate for HPV and for differentiating adenocarcinomas of endocervical origin from those of endometrial origin [30]. Our results, and those of Engleson et al., raise questions about the validity of using p16 for this role since endometrial carcinomas did express p16 in our hands and may serve as an indicator of bad prognosis [25]. Ignatov et al., did not find a correlation between cancer-specific 5-year survival and p16 expression [31]. This remained true even when they used different cut off values for p16 expression in cells (15%, 25%, or 35%). However, they did not take intensity into consideration and the differences between the studies may possibly be explained by this. Expression of p53 in the tumor epithelium was associated with a worse survival but was not an independent prognostic factor. This is concordant with the results of Engelsen et al [25]. The 5-year survival rates dropped from 85% with normal expression of p53, to 52% in patients with pathologic expression. p53 is one of the few IHC stains in addition to ER and progesterone receptor (PR) that is utilized in endometrial cancer pathology work ups, therefore its correlation with decreased survival lends it additional significance as a prognostic indicator. We have previously reported in this patient population that ER was associated with objective clinical response to treatment with T+M [22]. However, none of the biomarkers in the current study were associated with response.
We did not find a significant relationship between Ki-67 and PFS or OS. This is supported by Kallakury et al. [32]. Some report that the S-phase fraction of Ki-67 has a stronger prognostic value [33–36]. With respect to α- and β-catenin, Scholten et al.’s findings, deviate from ours, in that they found their expression to correlate with improved cancer specific survival [18]. However, they did not include Stage IV cases, while we have [18]. A significant relationship was not observed between the expression of CD44 and PFS or OS. While our analysis focused on standard CD44, the subject of many has been the splice variants (isoforms), especially CD44 v6) and has yielded contradictory trends, but falling short of statistical significance [37–41].
Study Limitations and Conclusions
The sample size is a limitation that should be addressed in future investigations. In addition, these results pertain to a specific clinical and treatment scenario and caution should be exercised in extrapolating this information to all groups of endometrial carcinoma. In conclusion, E-cadherin and p16 appear to be clinically relevant tumor biomarkers with independent prognostic value in stage IV or recurrent endometrial cancer patients treated with T+M.
Highlights.
High E-cadherin expression is associated with better survival
High p16 expression is associated with worse survival
E-cadherin and p16: independent prognostic factors in advanced endometrial cancers
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Receptor Core Laboratory (CA 27469), the GOG Tissue Bank (CA 27469 and CA 11479) and the GOG Statistical and Data Center (CA 37517), as well as special funding from the NCI Cancer Diagnosis Program for the GOG Tissue Bank in Columbus, Ohio to create a tissue microarray for endometrial cancer research using specimens from women who participated in GOG protocol 119. This work was also supported by NIH R01CA 99908-1 (KKL), by NIH CA 27469 to the Gynecologic Core Laboratory for Receptors, by the Cory/Beach Family Fund (KKL), and by a University of New Mexico Cancer Research and Treatment Center Translational Research Grant (KKL).
The following Gynecologic Oncology Group member institutions participated in this study: Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, The Milton S. Hershey School of Medicine of the Pennsylvania State University, University of Cincinnati College of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Wake Forest University School of Medicine, University of California, Irvine Medical Center, Tufts New England Medical Center, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Columbus Cancer Council, Fox Chase Cancer Center, Medical University of South Carolina, University of Oklahoma, University of Virginia, University of Chicago, Tacoma General Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
William E. Brady has an Advisory Board, $50.00 Honorarium with Angstrom Pharmaceuticals. All other co-authors have no conflict of interest to declare.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hirohashi S. Inactivation of E-Cadherin mediated cell adhesion system in Human Cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 4.Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, et al. Expression of Immunoreactive E-cadherin Molecules in Human Cancers. Am J Pathol. 1991;139:17–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto S, Baba H, Kuroda S, Kaibuchi K, Fukuda T, Maehara Y, et al. Changes in E-cadherin associated with cytoplasmic molecules in well and poorly differentiated endometrial cancer. Br J Cancer. 2000;83:1168–1175. doi: 10.1054/bjoc.2000.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carico E, Fulcinti F, Giovagnoli MR, Losito NS, Botti G, Benincasa G, et al. Adhesion molecules and p16 expression in endocervical adenocarcinoma. Virchows Arch. 2009;455:245–251. doi: 10.1007/s00428-009-0811-1. [DOI] [PubMed] [Google Scholar]
- 7.Kalof A, Cooper K. p16INK4A Immunoexpression: Surrogate Marker for High-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anatomical Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Klaes R, Friedric T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 9.Masumoto N, Fujii T, Isikawa M, Saito M, Iwata T, Fukuchi T, et al. p16INK4A overexpression and human papillomavirus infection in small cell carcinoma of the uterine cervix. Hum Pathol. 2003;34:778–783. doi: 10.1016/s0046-8177(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein A, Fraser M, Struewing J, Hussussian CJ, Ranade K, Zametkin DP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 11.Hansson J. Familial cutaneous melanoma. Adv Exp Med Biol. 2010;685:134–145. doi: 10.1007/978-1-4419-6448-9_13. [DOI] [PubMed] [Google Scholar]
- 12.Ozkara S, Corakci A. Significantly Decreased p27 expression in endometrial carcinoma compared to complex hyperplasia with atypia (correlation with p53 expression) Pathol Oncol Res. 2004;10:89–97. doi: 10.1007/BF02893462. [DOI] [PubMed] [Google Scholar]
- 13.Athanassiadou P, Athanassiades P, Grapsa D, Gonidi M, Athanassiadou AM, Stamati PN, et al. The prognostic value of PTEN, p53, and beta-catenin in endometrial carcinoma: a prospective immunocytochemical study. Int J Gynecol Cancer. 2007;17:694–704. doi: 10.1111/j.1525-1438.2007.00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Afify A, Craig S, Paulino AF, Stern R. Expression of hyaluronic acid and its receptors, CD44s, and CD44v6, in normal, hyperplastic, and neoplastic endometrium. Annals Diag Pathol. 2005;9:312–318. doi: 10.1016/j.anndiagpath.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Banno K, Yanokura M, Susumu N, Kawaguchi M, Hirao N, Hirasawa A, et al. Relationship of the aberrant DNA hypermethylation of cancer-related genes with carcinogenesis of endometrial cancer. Oncol Rep. 2006;16:1189–1196. [PubMed] [Google Scholar]
- 16.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 17.Stefansson I, Salvesen H, Akslen L. Prognostic Iimpact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22:1242–1251. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Scholten AN, Aliredjo R, Creutzberg CL, Smit VT. Combined E-cadherin, α-catenin, and β-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer. 2006;16:1379–1385. doi: 10.1111/j.1525-1438.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakuragi N, Nishiya M, Ikeda K, Ohkouch T, Furth EE, Hareyama H, et al. Decreased E-Cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol. 1994;53:138–139. doi: 10.1006/gyno.1994.1113. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb K, Delatorre R, Pedemonte B, McLeod C, Anderson L, Chambers J. E-Cadherin expression in endometrioid, papillary serous, and clear cell carcinoma of the endometrium. Obstet Gynecol. 2002;100:1290–1295. doi: 10.1016/s0029-7844(02)02391-8. [DOI] [PubMed] [Google Scholar]
- 21.Whitney C, Brunetto V, Zaino R, Lentz SS, Sorosky J, Armstrong DK, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2004;92:4–9. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Singh M, Zaino R, Filiaici V, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106:325–333. doi: 10.1016/j.ygyno.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Schimp V, Ali-Fehmi R, Solomon L, Hammoud A, Pansare V, Morris RT, et al. The racial disparity in outcomes in endometrial cancer: could this be explained on a molecular level? Gynecol Oncol. 2006;102:440–446. doi: 10.1016/j.ygyno.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Lukes AS, Kohler MF, Pieper CF, Kerns BJ, Bentley R, Rodriguez GC, et al. Mutivariable analysis of DNA ploidy, p53, and HER-2/neu as prognostic factors in endometrial cancer. Cancer. 1994;73:2380–2385. doi: 10.1002/1097-0142(19940501)73:9<2380::aid-cncr2820730922>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Engelsen I, Stefansson I, Akslen L, Salvesen HBl. Pathologic expression of p53 or p16 in preoperative curettage specimens identifies high-risk endometrial carcinomas. Am J Obstet Gynecol. 2006;195:979–986. doi: 10.1016/j.ajog.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 26.Nycum L, Smith L, Farley J, Kost ER, Method MW, Birrer MJ. The role of p27 in endometrial carcinoma. Gynecol Oncol. 2001;81:242–246. doi: 10.1006/gyno.2001.6144. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe J, Sato H, Kanai T, Kamata Y, Jobo T, Hata H, et al. Paradoxical expression of cell cycle inhibitor p27 in endometrioid adenocarcinoma of the uterine corpus – correlation with proliferation and clinicopathological parameters. Br J Cancer. 2002;87:81–85. doi: 10.1038/sj.bjc.6600434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mell LK, Meyer JJ, Tretiakova M, Khramtsov A, Gong C, Yamada SD, et al. Prognostic significance of e-cadherin protein expression in pathological stage I–III endometrial cancer. Clin Cancer Res. 2004;10:5546–5553. doi: 10.1158/1078-0432.CCR-0943-03. [DOI] [PubMed] [Google Scholar]
- 29.Kim YT, Choi EK, Kim JW, Kim DK, Kim SH, Yang WI. Expression of E-cadherin and alpha-, beta-, gamma-catenin proteins in endometrial carcinoma. Yonsei Med J. 2002;43:701–711. doi: 10.3349/ymj.2002.43.6.701. [DOI] [PubMed] [Google Scholar]
- 30.McCluggage W, Jenkins D. p16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol. 2003;22:231–235. doi: 10.1097/01.PGP.0000055172.04957.2F. [DOI] [PubMed] [Google Scholar]
- 31.Ignatov A, Bischoff J, Schwarzenau C, Krebs T, Kuester D, Herrmann K, et al. P16 alterations increase the metastatic potential of endometrial carcinoma. Gynecol Oncol. 2008;111:365–371. doi: 10.1016/j.ygyno.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Kallakury BV, Ambros RA, Hayner-Buchan AM, Sheehan CE, Malfetano JH, Ross JS. Cell proliferation-associated proteins in endometrial carcinomas, including papillary serous and endometrioid subtypes. Int J Gynecol Pathol. 1998;17:320–326. doi: 10.1097/00004347-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Friberg L, Noren H, Dell U. Prognostic value of DNA ploidy and S-phase fraction in endometrial cancer stage I and II: a prospective 5 year survival study. Gynecol Oncol. 1994;53:64–69. doi: 10.1006/gyno.1994.1089. [DOI] [PubMed] [Google Scholar]
- 34.Stendahl U, Strang P, Wagenius G, Bergström R, Tribukait B. Prognostic significance of proliferation in endometrial carcinoma: a multivariate analysis of clinical and flow cytometric variables. Int J Gynecol Pathol. 1991;10:271–284. doi: 10.1097/00004347-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Strang P, Stenkvist B, Bergström R, Stendahl U, Valdes del Campo M, Tribukait B. Flow cytometry in endometrial carcinoma: a comparative and prognostic study. Anticancer Res. 1991;11:783–788. [PubMed] [Google Scholar]
- 36.Wagenius G, Bergström R, Strang P, Gerdes U, Rogo K, Tribukait B, et al. Prognostic significance of flow cytometric and clinical variables in endometrial adenocarcinoma stages I and II. Anticancer Res. 1992;12:725–732. [PubMed] [Google Scholar]
- 37.Ayhan A, Tok E, Bildirici I, Ayhan A. Overexpression of CD44 variant 6 in human endometrial cancer and its prognostic significance. Gynecol Oncol. 2001;80:355–358. doi: 10.1006/gyno.2000.6014. [DOI] [PubMed] [Google Scholar]
- 38.Stokes GN, Shelton JB, Jr, Zahn CM, Kendall B. Association of CD44 isoform immunohistochemical expression with myometrial and vascular invasion in endometrioid endometrial carcinoma. Gynecol Oncol. 2002;84:58–61. doi: 10.1006/gyno.2001.6470. [DOI] [PubMed] [Google Scholar]
- 39.Katsura M, Furumoto H, Nishimura M, Kamada M, Aono T. Overexpression of CD44 variants 6 and 7 in human endometrial cancer. Gynecol Oncol. 1998;71:185–189. doi: 10.1006/gyno.1998.5169. [DOI] [PubMed] [Google Scholar]
- 40.Saegusa M, Hashimura M, Okayasu I. CD44 expression in normal, hyperplastic and malignant endometrium. J Pathol. 1998;184:297–306. doi: 10.1002/(SICI)1096-9896(199803)184:3<297::AID-PATH995>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc M, Poncelet C, Soriano D, Walker-Combrouze F, Madelenat P, Scoazec JY, et al. Alteration of CD44 and cadherins expression: possible association with augmented aggressiveness and invasiveness of endometrial carcinoma. Virchows Arch. 2001;438:78–85. doi: 10.1007/s004280000269. [DOI] [PubMed] [Google Scholar]