Nanobiotechnology is the assembling of biological molecules into 1- to 100-nanometer dimensions. These dimensions can be the diameter of nanodimensional artificial cells or particles, membranes with nanodimension thicknesses, or nanotubules with nanodimension diameters. The first nanobiotechnological approach reported in the literature was the cross-linking of hemoglobin (Hb) into ultrathin polyhemoglobin (polyHb) membranes for artificial red blood cell (RBC) membranes.1,2 If the emulsion is made even smaller, then whole artificial cells with their Hb can be cross-linked into polyHb of the nanodimension. Glutaraldehyde can cross-link Hb into soluble polyHb of the nanodimension.3 New generations of this approach include the nanobiotechnological assembly of Hb, catalase (CAT), and superoxide dismutase (SOD) into a soluble nano-dimension complex. This acts as an oxygen carrier and as an antioxidant for those conditions with potential for ischemia-reperfusion injuries.4–7 Another recent novel approach is the assembling of Hb and fibrinogen into a soluble nanodimension polyHb-fibrinogen complex that acts as an oxygen carrier with platelet-like activity.6 This is potentially useful in cases of extensive blood loss requiring massive replacement using blood substitutes, resulting in the need for replacement of platelets and clotting factors. Nanodimension artificial cells can also be formed as nanodimension biodegradable polymeric membrane artificial cells containing Hb and RBC enzymes.4–6
POLYHEMOGLOBIN
Basic Principles
Hb is a tetramer (α1β1α2β2)8 that breaks down into toxic dimers (α1β1 and α2β2) that cause vasoconstriction, renal toxicity, and other adverse effects. Even in the form of tetramers, Hb molecules can cross the intercellular junction of blood vessels to cause adverse vasopressor effects. The author used the principle of nanobiotechnology to assemble Hb molecules into nanodimension polyHb, first using the bifunctional agent sebacyl chloride,1,2 then using glutaraldehyde.3 The glutaraldehyde method was developed independently by other groups for the development of Hb-based oxygen carriers for clinical trials.9,10
Present Status of Polyhemoglobin in Clinical Trials
Phase III clinical trials have been completed on over 171 patients, showing that this product can successfully replace extensive blood loss in patients undergoing trauma surgery and other surgery by maintaining the total Hb level at the 8–10 g/dL range needed for safe surgery, with no reported side effects.9 For example, transfusion of this polyHb in patients with Hb levels as low as 2 g/dL can raise the Hb level to within the 8 to 10 g/dL range, with the patients recovering from surgery. Normally, patients with Hb levels of less than 3 g/dL do not survive. Steven Gould (Northfield Co.) and colleagues have infused up to 10 L of polyHb into individual trauma-surgery patients. In the United States, this product has been approved for compassionate use in patients, and it is awaiting a regulatory decision for routine clinical uses. Gould and colleagues have performed Phase III clinical trials on its use in prehospital emergencies in which no typing and cross-matching is needed, so that it can be used right on the spot.11
Given that the supply of Hb from outdated donor blood is limited, a glutaralde-hyde–cross-linked bovine polyHb has been developed and tested in phase III clinical trials.10 For example, Pearce and colleagues in the United States have performed a multicenter, multinational, randomized, single-blind, RBC-controlled Phase III clinical trial in patients undergoing elective orthopedic surgery. A total of 688 patients were randomized in a 1:1 ratio to receive either the polyHb or RBCs at the time of the first perioperative RBC transfusion decision. Of the patients receiving polyHb, 59.4% required no RBC transfusion all the way to follow up, 96.3% avoided transfusion with RBCs on the first postoperative day, and up to 70.3% avoided RBC transfusion up to day 7 after surgery. In North America, this bovine polyHb has been approved for compassionate use in patients, and in South Africa, this product is approved for use in adult surgery patients to treat acute anemia and reduce allogeneic blood use.
Effects of Tetrameric Hemoglobin in Polyhemoglobin on Vasoconstriction and Adverse Cardiac Effects
In addition to the above two polyHbs (one from a human source, one from a bovine source), other modified Hbs have also been prepared and studied. These include intramolecularly cross-linked tetrameric Hb and polyHb containing more than 30% tetrameric Hb that can cause vasoconstriction. This has led to the proposal that the intercellular junctions of the endothelial lining of the vascular wall allow molecular dimension Hb to enter into the interstitial space. There, Hb acts as a sink in binding and removing nitric oxide needed for maintaining the normal tone of smooth muscles. This results in the constriction of blood vessels and other smooth muscles especially those of the esophagus and the gastrointestinal tract. To test this, the author and colleagues prepared polyHbs containing different percentages of unpolymerized Hb molecules using the same glutaraldehyde cross-link and characterized to ensure that they all have the same oxygen affinity.12 The results show that the polyHb with the lowest percentage of unpolymerized Hb molecules does not cause vasoconstriction. With increasing percentages of unpolymerized Hb molecules, there are increasing degrees of vasoconstriction. Rats hearts have high heart rates and are therefore more sensitive to ischemic changes. Recent studies by the author and colleagues in rats show ischemic ECG changes in the form of ST elevation when rats received polyHb with a high percentage of tetrameric Hb. With even higher percentages of tetrameric Hb, there was ECG evidence of cardiac arrhythmia. ST elevation could be due to coronary vasoconstriction, resulting in a decrease in the supply of oxygen to the heart, and this may explain the observation of small subendocardial lesions in some primates and swine after infusion with one type of modified Hb consisting of 100% single-Hb molecules. Thus, to avoid causing adverse vasopressor or cardiac effects, polyHb preparations must contain less than 2% of tetrameric Hb.
NEW GENERATION OF NANOBIOTECHNOLOGY-BASED BLOOD SUBSTITUTES: ASSEMBLING HEMOGLOBIN WITH CATALASE AND SUPEROXIDE DISMUTASE
Rationale
PolyHb is likely to have an important role in certain clinical applications. However, for conditions with the potential for ischemia-reperfusion injuries, the use of a new generation of blood substitute that is an oxygen carrier and an antioxidant should be considered. The reasons for this are that a lack of oxygen supply in heart, sustained hemorrhagic shock, stroke, organ transplantation, and other conditions may result in ischemia. Ischemia leads to alterations in metabolic reactions, producing hypoxanthine and activating the enzyme xanthine oxidase. The level of hypoxanthine increases with the duration and severity of the ischemia. When the tissues are reperfused with oxygen-carrying fluid, xanthine oxidase converts oxygen and hypoxanthine into superoxide. By several mechanisms, superoxide results in the formation of oxygen radicals that can cause tissue injury.
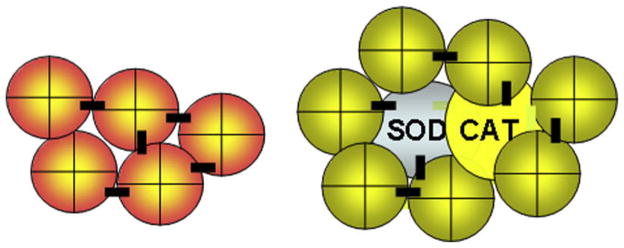
Even in routine surgery, it will be important to rule out patients with cardiac ischemia when using modified Hb with no antioxidants. Otherwise, there could be adverse cardiac effects related to ischemia-reperfusion. Other conditions in which ischemia-reperfusion injuries would be likely include severe sustained hemorrhagic shock, stroke, myocardial infarction, and organ transplantation. For all of the above situations, the author and colleagues used nanobiotechnology to assemble Hb with CAT and SOD into soluble nanodimension polyHb-CAT-SOD (Fig. 1).13 This is an oxygen carrier with the ability to remove oxygen radicals. SOD and CAT convert superoxide into hydrogen peroxide that is in turn converted into water and oxygen.
Fig. 1.
Left: PolyHb formed by nanobiotechnological assembling of Hb molecules into soluble nanodimension complex. Right: PolyHb-CAT-SOD formed by nanobiotechnological assembling of Hb, CAT, and SOD into soluble nanodimension complex. (From Chang TMS. Artificial cells: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation. Singapore: World Scientific Publishing Co., 2007; with permission.)
Compared with polyHb, polyHb-CAT-SOD removes significantly more oxygen radicals and peroxides and stabilizes the cross-linked Hb, resulting in a decrease in oxidative iron and heme release.4–6 Cross-linking these enzymes to polyHb is important because otherwise free SOD and CAT are removed rapidly from the circulation, with a half-time of less than 30 minutes. In the form of polyHb-CAT-SOD, these enzymes circulate with a half-time more comparable with polyHb, which is about 24 hours in humans. In the reperfusion of ischemic rat intestine, polyHb-CAT-SOD significantly reduced the increase in oxygen radicals caused by polyHb, as measured by an increase in 3,4 dihydroxybenzoate.14
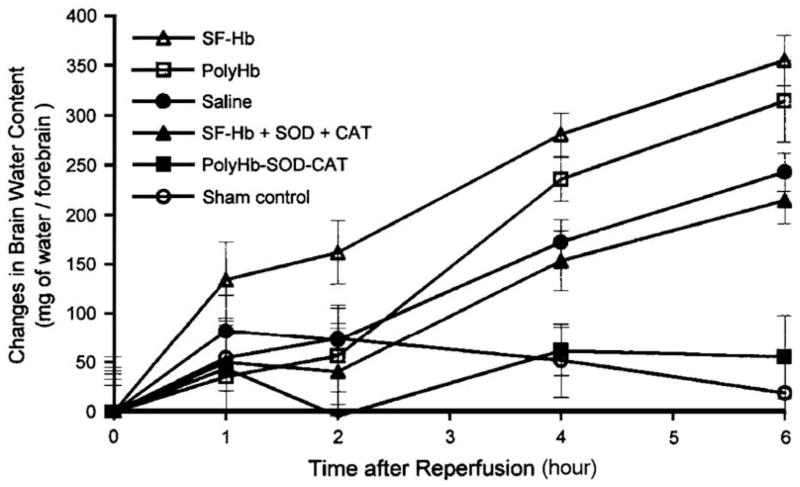
The author and colleagues also performed studies of global cerebral ischemia-reperfusion in a hemorrhagic shock model.15 This was based on bleeding anesthetized rats to a hypotensive level combined with transient occlusion of both common carotid arteries. After different lengths of time, this was followed by the release of the occlusion of the carotid arteries and reinfusion using different types of oxygen-carrying fluids. The effect on the blood-brain barrier was followed by Evans blue extravasation. PolyHb-SOD-CAT, which significantly attenuated the severity of blood-brain–barrier disruption as compared with the use of saline, stroma-free Hb (SF-Hb), polyHb, or a solution of free Hb, SOD, and CAT (P<.01).15,16 In the same study, brain edema was followed based on changes in brain water content. The changes in the brain water content of animals treated with polyHb-SOD-CAT were not significantly different from those of animals in the sham control group (Figs. 2 and 3). The increases in water content of the animals treated with saline, SF-Hb, polyHb, and the solution of free Hb, SOD, and CAT were significantly higher than those of the animals in the sham control and the polyHb-SOD-CAT group by the fourth hour and increased thereafter (P<.01).
Fig. 2.
Brain edema: changes in brain water content. The changes in brain water content of polyHb-SOD-CAT–treated animals are not significantly different from those of animals in the sham control group. The increases in water contents of animals treated with saline, SF-Hb, SF-Hb + SOD + CAT, and polyHb were significantly higher than those of animals in the sham control group and the polyHb-SOD-CAT group by the fourth hour and continued to increase thereafter. Statistical significance is P<.01. (From Chang TMS. Artificial cells: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation. Singapore: World Scientific Publishing Co., 2007; with permission.)
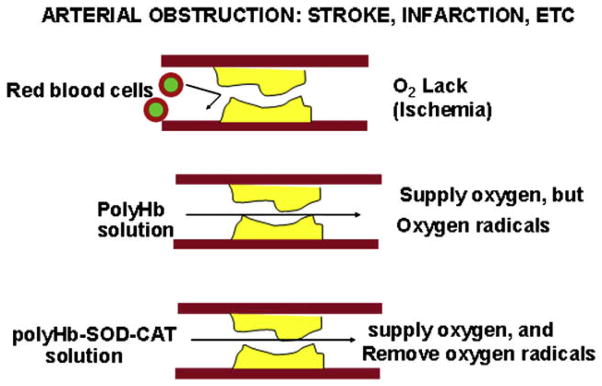
Fig. 3.
In obstructive ischemia, rbc cannot pass through, but polyHb being in solution can supply oxygen but may cause ischemia-reperfusion injuries. On the other hand, polyHb-SOD-CAT can supply oxygen without causing ischemia-reperfusion injuries. (From Chang TMS. Artificial cells: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation. Singapore: World Scientific Publishing Co., 2007; with permission.)
The attenuation in ischemia-reperfusion injuries using polyHb-SOD-CAT shows significant promise for its potential role as a protective therapeutic agent in clinical situations of ischemia and oxidative stress, such as stroke, myocardial infarction, sustained severe hemorrhagic shock, organ transplantation, and cardiopulmonary bypass.
POLYHEMOGLOBIN-FIBRINOGEN: A NOVEL OXYGEN CARRIER WITH PLATELET-LIKE PROPERTIES
In high-blood-volume loss, such as in trauma patients in hemorrhagic shock who require massive blood transfusion, large-volume RBC replacement alone does not replace platelets and coagulation factors, resulting in coagulopathy and thrombocytopenia and promoting ongoing hemorrhage. The author and colleagues therefore used nanobiotechnology to develop a blood substitute that is an oxygen carrier with platelet-like properties. This is a novel blood substitute, polyHb-fibrinogen (polyHb-Fg).17 Briefly, polyHb-Fg was prepared as follows. A fibrinogen solution of 40 mg dissolved in 4 mL of Ringer’s lactate was added to the polymerizing polyHb solution 4 hours after polymerization began. After 24 hours of polymerization, the reaction was stopped by quenching with a 2.0 M lysine solution in a molar ratio of 200:1 lysine to Hb. The solutions were then dialyzed against a Ringer’s lactate solution overnight.
In Vitro Experiments
Glass tubes were prepared with 250 μL or 400 μL of blood substitute. Two-hundred-and-fifty-microliter aliquots of fresh blood were added to the 250-μL aliquots of blood substitute. One-hundred-microliter aliquots of fresh blood were added to the 400-μL aliquots of blood substitute. The timing was started when the fresh blood was added. With polyHb, the clots that formed did not adhere to the glass tubes and no clotting time could be assessed. On the other hand, all of the clots that formed using polyHb-Fg stuck to the walls of the glass tube and could be quantified with a clotting time (P<.01).
In Vivo Experiments
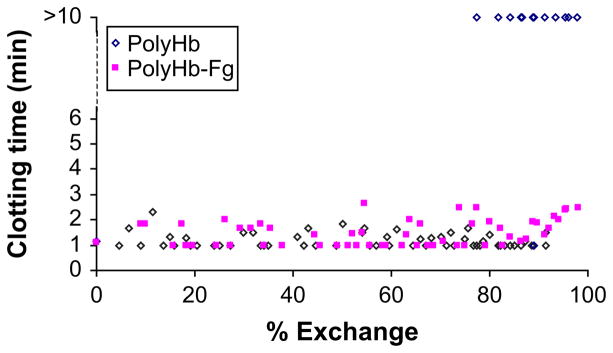
The results of in vivo experiments are shown in Fig. 4. polyHb displayed clotting times similar to those of polyHb-Fg for exchange transfusion of up to approximately 80%. Beyond 80% exchange, polyHb clots that formed did not always stick to the sides of the tubes and would slide freely. Beyond 93% exchange, no clots stuck for polyHb. In contrast, the clotting times for polyHb-Fg remained normal up to 98% exchange, at which point there was only a slight increase in the clotting time (see Fig. 4).
Fig. 4.
Exchange transfusion using polyHb-Fg did not change the clotting time. However, exchange transfusion of polyHb of more than 80% resulted in clotting problems because there were insufficient platelets or clotting factors. (From Wong N, Chang TMS. Polyhemoglobin-fibrinogen a novel blood substitute with platelet-like activity for extreme hemodilution. Artif Cells Blood Substit Biotechnol, 2007; with permission.)
BIODEGRADABLE POLYMERIC NANODIMENSION ARTIFICIAL RED BLOOD CELLS
For this study, the author made use of his background in the use of the biodegradable polymers such as polylactides for artificial cells containing Hb and other biologically active material.18 These biodegradable polymers are in routine use in surgical sutures, drug delivery, and other applications. The author is now using them to prepare nano-dimension biodegradable polymer-membrane Hb with a mean diameter of between 80–200 nanometers (Fig. 5).19–22 Polylactides are degraded in the body into lactic acid and then water and carbon dioxide. For a 500 mL suspension, the total lactic acid produced is 83 mEq.5 This is far less than the normal resting-body lactic acid production (1000–1400 mEq/day). This is equivalent to 1% of the capacity of the body to breakdown lactic acid (7080 mEq/day).
Fig. 5.
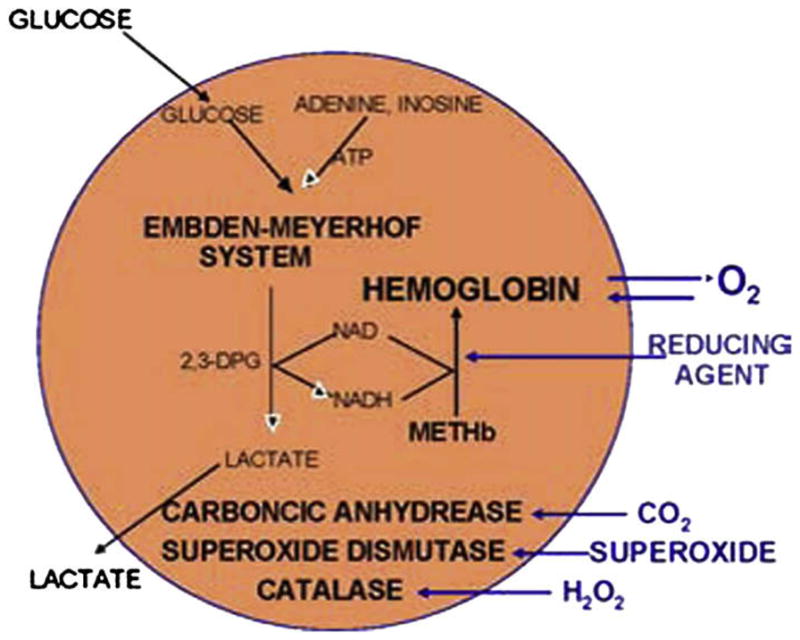
Nanoartificial RBCs with diameters of 80–100 nanometers containing Hb and RBC enzymes. (From Chang TMS. Artificial cells: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation. Singapore: World Scientific Publishing Co., 2007; with permission.)
In Vitro Experiments
Bovine Hb in these nanodimension artificial RBCs has the same P50, Bohr, and Hill coefficients.4–6 The content of Hb can match that of RBCs.4–6 One can extract the whole content of RBCs and then nanoencapsulate this extract. Furthermore, additional enzymes can be added to the solution before the nanoencapsulation process. Thus, additional SOD and CAT can also be included with the Hb. The author also used his background in artificial cells containing multienzyme cofactor recycling systems23 to help solve the problem of methemoglobin formation. In nanoRBCs, the biodegradable polymeric membranes can be made permeable to glucose and other molecules. This allows us to prepare Hb nanocapsules containing the methemoglobin reductase system in which external glucose can diffuse into the nanocapsules. Products of the reaction can diffuse out and therefore do not accumulate in the nanocapsules and inhibit the reaction. In vitro study shows that this can convert methemoglobin to Hb.19,24 Furthermore, reducing agents from the plasma can diffuse into the nanocapsules to reduce methemoglobin to oxygen-carrying Hb.19,24
In Vivo Experiments
Rats have been infused with one-third of the total blood volume. Most recently, the author and colleagues used a composite biodegradable polymeric membrane consisting of copolymer of polyethylene glycol (PEG) with polylactic acid (PLA).24 After extensive research using this approach, they have now prepared nanodimension artificial RBCs that can retain their circulating Hb level at double the duration of polyHb.24 They investigated the long-term effects of PEG-PLA nanoartificial cells containing Hb (nanoRBCs) on renal and liver function and also examined the renal, liver, and spleen histologic effects after one-third blood volume top loading in rats.25,26 The experimental rats received one of the following infusions: nanoRBCs in Ringer lactate, Ringer lactate, SF-Hb, polyHb, or autologous rat whole blood (rat RBC). Blood samples for biochemical analysis were taken before infusion and on days 1, 7, and 21 after infusion. Rats were killed on day 21, and the kidneys, liver, and spleen were excised for histologic examination. Infusion of SF-Hb induced significant decrease in renal function, as shown by elevated levels of serum urea, creatinine, and uric acid throughout the 21 days. Histologic examination of the kidneys in the SF-Hb–infusion group revealed focal tubular necrosis and intraluminal cellular debris in the proximal tubules. In all the other groups, there were no abnormalities in renal biochemistry or histology. In conclusion, injection of nanoRBCs did not have adverse effects on renal function or renal histology. NanoRBCs, polyHb, Ringer lactate, and rat RBCs did not have any significant adverse effects on alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatine kinase, and amylase. On the other hand SF-Hb induced significant adverse effect on the liver, as shown by elevation in alanine aminotransferase and aspartate aminotransferase throughout the 21 days. On day 21, the rats were killed and the livers and spleens were excised for histologic examination. NanoRBCs, polyHb, Ringer’s lactate, and rat RBCs did not cause any abnormalities, as seen in the microscopic histologic examination of the livers and spleens. In the SF-Hb group, the livers showed accumulation of Hb in central veins and sinusoids, and hepatic steatosis. In conclusion, injected nanoRBCs can be efficiently metabolized and removed by the reticuloendothelial system, and do not have biochemical or histologic adverse effects on the livers or the spleens.
OTHER DIRECTIONS USING NANOBIOTECHNOLOGY
The above review only includes some examples to show the use of nanobiotechnology for the preparation of blood substitutes. This principle can be extended to other systems. One example is the study conducted by the author and colleagues of another soluble nanodimension complex of polyHb-tyrosinase.27,28 This substance has the combined function of increasing oxygen tension to sensitize melanoma to therapy and lowering systemic tyrosine to retard the growth of melanoma. Many other extensions and modifications of this general principle in nanobiotechnology are possible.28
SUMMARY
There is always the discussion of how safe are blood substitutes. It is reasonable to require that RBC substitutes should be able to replace allogeneic RBCs without causing more adverse effects than allogeneic RBCs. One of the safety concerns regarding RBC substitutes is related to vasoactivity. As discussed above, not all Hb-based blood substitutes have problems related to vasoactivity, and such problems are only seen in those that contain a high proportion of tetrameric Hb. The other potential problem is related to the inappropriate use of Hb-based blood substitutes in those conditions that have potential for ischemia-reperfusion injuries. For these conditions, one needs to consider use of polyHb–CAT-SOD as discussed earlier in the article.
If these precautions are followed, some of the better Hb-based blood substitutes could possibly be safer that standard allogeneic blood. After all, recent reviews show that liberal blood transfusions are associated with a 20% increase in mortality and a 56% increase in ischemic events when compared with restrictive strategies.29,30 The transfusion of stored packed RBCs is also associated with an increase in ischemic coronary events.29,31 In summary, although it is important for blood substitutes to be as safe as allogeneic blood, it is not reasonable to require that RBC substitutes should have no side effects whereas standard donor RBCs are associated with adverse effects including ischemic coronary events.
Acknowledgments
The author acknowledges ongoing research grants from the Canadian Institutes of Health Research and the Quebec Ministry of Health’s Hemovigilance Program in the form of funding for a FRSQ Research Group (d’equip) on Blood Substitutes in Transfusion Medicine.
References
- 1.Chang TMS. Semipermeable microcapsules. Science. 1964;146(3643):523–5. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 2.Chang TMS. Monograph. Charles C Thomas; Springfield (IL): 1972. [Accessed February 17, 2009]. Artificial cells. Available at: http://www.artcell.mcgill.ca. [Google Scholar]
- 3.Chang TMS. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaral-dehyde. Biochem Biophys Res Commun. 1971;44(6):1531–6. doi: 10.1016/s0006-291x(71)80260-7. [DOI] [PubMed] [Google Scholar]
- 4.Chang TMS. Artificial cells: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, cell/stem cell therapy. Singapore: World Science Publisher; 2007. p. 452. [Google Scholar]
- 5.Chang TMS. Blood substitutes: principles, methods, products and clinical trials. Vol. 1. Basel, Switzerland: Karger; 1997. [Accessed February 17, 2009]. Available at: http://www.artcell.mcgill.ca. [Google Scholar]
- 6.Chang TMS. Nanobiotechnological modification of hemoglobin and enzymes from this laboratory. Biochim Biophys Acta. 2008;1784:1435–40. doi: 10.1016/j.bbapap.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang TMS. Artificial cells: biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation and cell/stem cell therapy. Singapore: World Science Publisher; 2007. pp. 31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perutz MF. Stereochemical mechanism of oxygen transport by hemoglobin. Proc R Soc Lond B. 1980;208:135. doi: 10.1098/rspb.1980.0047. [DOI] [PubMed] [Google Scholar]
- 9.Gould SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human polymer-ized Hb when red cells might be unavailable. J Am Coll Surg. 2002;195:445–52. doi: 10.1016/s1072-7515(02)01335-2. [DOI] [PubMed] [Google Scholar]
- 10.Pearce LB, Gawryl MS, Rentko VT, et al. HBOC-201 (Hb Glutamer-250 (Bovine), Hemopure): clinical studies. In: Winslow R, editor. Blood substitutes. San Diego: Academic Press; 2006. pp. 437–50. [Google Scholar]
- 11.Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hermorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Yu BL, Liu ZC, Chang TMS, et al. PolyHb with different percentage of tetrameric Hb and effects on vasoactivity and electrocardiogram. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:159–75. doi: 10.1080/10731190600580223. [DOI] [PubMed] [Google Scholar]
- 13.D’Agnillo F, Chang TMS. PolyHb-superoxide dismutase catalase as a blood substitute with antioxidant properties. Nat Biotechnol. 1998;16(7):667–71. doi: 10.1038/nbt0798-667. [DOI] [PubMed] [Google Scholar]
- 14.Chang TMS, D’Agnillo F, Razack S. A second generation Hb based blood substitute with antioxidant activities. In: Chang TMS, editor. Blood substitutes: principles, methods, products and clinical trials. Vol. 2. Vol. 1009. Basel Karger Texas Landes; 1998. pp. 175–85. [Google Scholar]
- 15.Powanda D, Chang TMS. Cross-linked polyHb-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cells Blood Substit Immobil Biotechnol. 2002;30:25–42. doi: 10.1081/bio-120002725. [DOI] [PubMed] [Google Scholar]
- 16.Chang TMS. A nanobiotechnologic therapeutic that transport oxygen and remove oxygen radicals: for stroke, hemorrhagic shock and related conditions. In: Chang TMS, editor. Artificial cells: biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation and cell/stem cell therapy. Singapore: World Science Publisher; 2007. pp. 62–92. [Google Scholar]
- 17.Wong N, Chang TMS. Polyhemoglobin-fibrinogen: a novel blood substitute with platelet-like activity for extreme hemodilution. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:481–9. doi: 10.1080/10731190701586210. [DOI] [PubMed] [Google Scholar]
- 18.Chang TMS. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioeng. 1976;1:25–32. [PubMed] [Google Scholar]
- 19.Chang TMS. Nanotechnology based artificial red blood cells. In: Chang TMS, editor. Artificial cells: biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation and cell/stem cell therapy. Singapore: World Science Publisher; 2007. pp. 93–128. [Google Scholar]
- 20.Yu WP, Chang TMS. Submicron biodegradable polymer membrane Hb nanocapsules as potential blood substitutes: a preliminary report. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:889–94. doi: 10.3109/10731199409117926. [DOI] [PubMed] [Google Scholar]
- 21.Yu WP, Chang TMS. Submicron biodegradable polymer membrane Hb nanocapsules as potential blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:169–84. doi: 10.3109/10731199609117433. [DOI] [PubMed] [Google Scholar]
- 22.Chang TMS, Yu WP. Nanoencapsulation of Hb and RBC enzymes based on nano-technology and biodegradable polymer. In: Chang TMS, editor. Blood substitutes: principles, methods, products and clinical trials. Vol. 2. Basel: Karger; 1998. pp. 216–31. [Google Scholar]
- 23.Chang TMS. Artificial cells with cofactor regenerating multienzyme systems. Methods Enzymol. 1985;112:195–203. doi: 10.1016/s0076-6879(85)12017-3. [DOI] [PubMed] [Google Scholar]
- 24.Chang TMS, Powanda D, Yu WP. Ultrathin polyethylene-glycol-polylactide copolymer membrane nanocapsules containing polymerized Hb and enzymes as nano-dimension RBC substitutes. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:231–48. doi: 10.1081/bio-120023155. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZC, Chang TMS. Effects of PEG-PLA-nano artificial cells containing hemoglobin on kidney function and renal histology in rats. Artificial Cells Blood Substit Biotechnol. 2008;36:421–30. doi: 10.1080/10731190802369763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu ZC, Chang TMS. Long-term effects on the histology and function of livers and spleens in rats after 33% toploading of PEG-PLA-nano artificial red blood cells. Artificial Cells, Blood Substitutes & Biotechnology. 2008;36:513–24. doi: 10.1080/10731190802554224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu BL, Chang TMS. In vitro and in vivo effects of polyHb–tyrosinase on murine B16F10 melanoma. Melanoma Res. 2004;14:481–9. doi: 10.1097/01.cmr.0000131013.71638.c0. [DOI] [PubMed] [Google Scholar]
- 28.Chang TMS. Enzyme artificial cells in substrate-dependent tumours and activation of prodrug. In: Chang TMS, editor. Artificial cells: biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation and cell/stem cell therapy. Singapore: World Science Publisher; 2007. pp. 160–94. [Google Scholar]
- 29.Hill S, Carless P, Henry D, et al. Cochrane Database Syst Rev. 2006;2:1–41. [Google Scholar]
- 30.Rao S, Harrington R, Califf R, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2005;293:673–4. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 31.Rao SV, Jollis JG, Harrington RA, et al. Blood transfusion in patients with acute coronary syndrome. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]