Abstract
Unlike donor red blood cells (RBCs), blood substitutes can be treated to remove infective agents and can be used on the spot or in the ambulance in emergency without the time-consuming typing and cross-matching. Donor RBC requires storage at 4° and is only good for 42 days, but blood substitutes can be stored for much longer time. For example, a bovine polyhemoglobin (PolyHb) can be stored at room temperature for more than 1 year. It has been shown as far back as 1957 that artificial RBC can be prepared with ultrathin polymer membranes of nanodimension thickness. To increase the circulation time, the first-generation engineered hemoglobin (Hb) is formed by using glutaraldehyde to crosslink Hb into soluble nanodimension PolyHb that has been tested clinically in patients. Further extension includes conjugated Hb, intramolecularly crosslinked Hb and recombinant Hb. For certain clinical uses, in addition to engineered Hb, we also need antioxidants to remove oxygen radicals to prevent injury from ischemia reperfusion. Thus, we use nanobiotechnology to prepare second-generation engineered Hb by assembling Hb together with superoxide dismutase (SOD) and catalase (CAT) to form a nanodimension soluble complex of polyhemoglobin (PolyHb)-CAT-SOD. A third generation system is to prepare nanodimension complete artificial RBCs that can circulate for sufficient length of time after infusion. One approach uses lipid vesicles to encapsulate hemoglobin (Hb). Another approach is to use biodegradable polymer-like polylactic acid or a copolymer of polyethylene glycol-polylactide (PEG-PLA) to form the membrane of nanodimension complete artificial RBC (www.artcell.mcgill.ca).
Why do we need blood substitutes? We can just look back into the tragedy of human immunodeficiency virus (HIV) contaminated donor blood in the 1980s. Large number of patients receiving contaminated donor blood were infected. ‘Catchup’ attempts were then started to develop blood substitutes. It has been 20 years and despite major progress in the area, an FDA-approved product is still not yet available in North America. We now know that blood substitute is a complex task, and research and development have to be carried out well in advance of the time when it is needed. One can never be sure that a similar pandemic of another infective agent will not occur again, for example, H1N1 swine flu. Unlike donor blood, blood substitutes can be treated to remove infective agents. There are also other important areas that need blood substitutes. Unlike donor blood, blood substitutes can be given without the time-consuming typing and cross-matching that require special facilities. Thus, it can be used on the spot or in the ambulance in major man-made or natural disasters. Donor blood requires storage at 4° and is only good for 42 days. Blood substitutes can be stored for much longer time. For example, PolyHb prepared from bovine Hb can be stored at room temperature for more than 1 year.
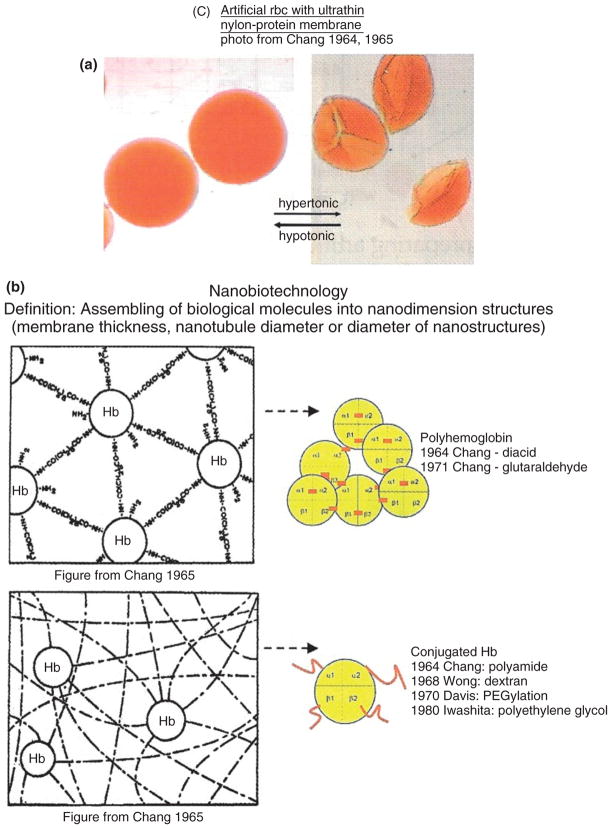
It has been showed as far back as 1957 that the contents of red blood cells (RBCs) can be enveloped in artificial RBC with ultrathin polymer membranes of nanodimension thickness.1,2 This has progressed further into artificial cells for use in biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, gene and drug delivery, and cell and stem cell therapy.2–5 The present review is related to blood replacement with nanobiotechnology-engineered Hb and nanodimension artificial RBC. Nanobiotechnology is the assembling of biological molecules into nanodimension structures, membranes with nanodimension thickness, or nanotubules with nanodimension diameter.
ARTIFICIAL RBC
The first nanobiotechnology approach reported is the crosslinking of Hb into ultrathin nanodimension thickness membrane of crosslinked protein or protein-polymer2 (Figure 1). This is used to form the membrane of artificial RBC.2,3 The nanodimension thickness membranes can be prepared from polymers, crosslinked protein or crosslinked protein-polymers2,3 (Figure 1). In this form, the Hb and the enzyme systems normally present in the RBC are retained inside. At the same time, oxygen and carbon dioxide can equilibrate rapidly across the membrane to interact with the enclosed materials.2,3 The membranes of the artificial RBC would not become fragile after prolonged in vitro storage. The artificial RBC could be transfused without cross-matching, because they do not have any blood group antigens. Because these early artificial RBC are rapidly removed from the circulation after intravenous injection,3 further research has been carried out to solve this problem.
FIGURE 1.
(a) Artificial red blood cell (RBC) with nanodimension thickness nylon-protein membrane. Spherical in hypotonic solution, becoming ‘crenated’ in hypertonic solutions. Reversible when moved from one solution to another. (b) An example of assembling of biological molecules to form polyhemoglobin (PolyHb) and conjugated hemoglobin (Hb) in the form of nanodimension thickness membrane for artificial cells (left) or as soluble nanodimension complexes of PolyHb or conjugated Hb (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
BIOTECHNOLOGICALLY ENGINEERED SOLUBLE HB COMPLEXES
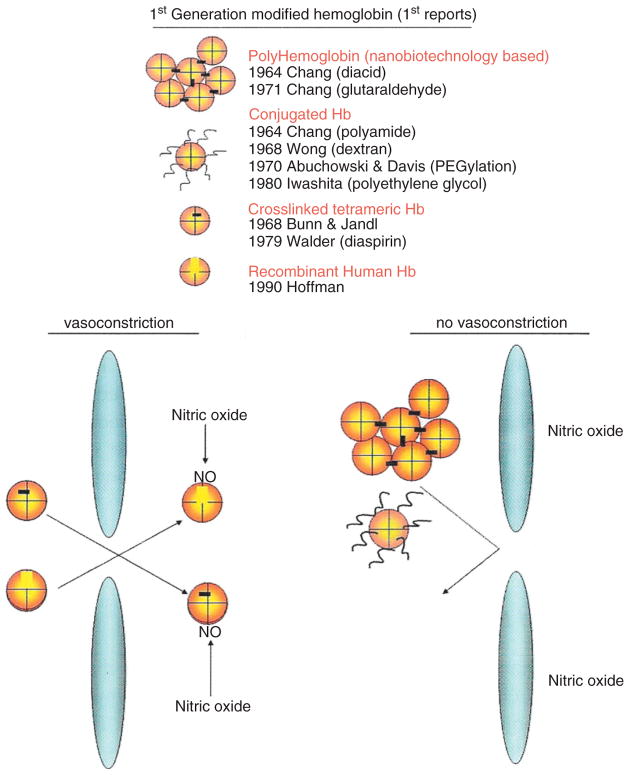
First-Generation Engineered HB
The next step for blood substitute is to modify the above approach of crosslinked Hb using glutaraldehyde to crosslink Hb into soluble nanodimension PolyHb each consisting of an assembly of 4–5 Hb molecules6 (Figure 1). This prevents the breakdown of Hb, a tetramer (α1β1α2β2),7 into toxic dimers (α1β1 and α2β2) that cause renal toxicity and other adverse effects. Engineering of Hb was further extended into intramolecularly crosslinked Hb and recombinant Hb.8 These single Hb molecules are stabilized internally by intramolecular crosslinking or recombinant method and do not break down into dimers. However, the intercellular junctions of the endothelial lining of vascular wall allow this molecular dimension Hb to enter into the interstitial space (Figure 2). There, Hb acts as a sink in binding and removing nitric oxide needed for maintaining the normal tone of smooth muscles. This results in the constriction of blood vessels and other smooth muscles, especially those of the esophagus and the gastrointestinal tract. PolyHb is a larger soluble nanodimension complex that does not cross the intercellular junction (Figure 2).5,8 Even then, PolyHb should not contain significant amounts of single molecule tetrameric Hb. Conjugated Hb in the form of pegylated Hb (PEG-Hb) also does not cross the intercellular junction if free from significant tetrameric Hb. Two groups have independently developed our 1971 basic method of glutaraldehyde-crosslinked Hb for clinical use. One is glutaraldehyde-crosslinked human PolyHb9,10 and the other is glutaraldehyde-crosslinked bovine PolyHb.11
FIGURE 2.
(a) Upper: Four types of engineered hemoglobin (Hb): (1) Polyhemoglobin (PolyHb) based on nanobiotechnology, (2) conjugated Hb, (3) intramolecularly crosslinked Hb, and (4) recombinant Hb. (b) Lower right: Larger complexes, like PolyHb and conjugated Hb cannot cross the intercellular junctions of the endothelial lining of vascular wall. Lower left: Small single Hb molecules, such as intramolecularly crosslinked Hb and recombinant Hb, can cross and bind and remove the nitric oxide needed for maintaining the normal tone of smooth muscles. This results in the constriction of blood vessels (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
Clinical Trials in Patients Using PolyHb and PEG-Hb
Gould’s group has developed and carried out clinical trials in patients showing that their glutaraldehyde-crosslinked human PolyHb with less than 1% tetrameric Hb can successfully replace extensive blood loss.9,10 They have infused up to 10 l of PolyHb into individual trauma surgery patients. They have more recently carried out Phase III clinical trials on 714 pre-hospital emergencies patients.10 In this clinical trial, it is used right on the spot and in the ambulances as unlike donor blood, no typing and cross-matching is needed for PolyHb. The result shows that PolyHb can delay the need for RBC transfusion for 12 h. They propose that PolyHb may be useful ‘for patients at high risk of death when stored RBCs are not available … even though there is some increase in frequency of adverse events’.10 As the supply of Hb from outdated human donor blood is limited, another group has developed a glutaraldehyde-crosslinked bovine PolyHb and tested in a number of phase III clinical trials on different clinical conditions. This includes a recent multicenter, multinational, randomized, single-blind, RBC-controlled Phase III clinical trials in 688 patients undergoing elective orthopedic surgery.11 Half received RBC and the other half received PolyHb. In the PolyHb group, those who did not need RBC transfusion after surgery were 96.3% on the first day, 70.3% up to Day 7 and 59.4% all the way to follow-up.11 This bovine PolyHb has been approved for routine clinical use in patients a number of years ago in South Africa, a region with higher incidence of HIV. Another group has developed conjugated Hb in the form of maleimide-polyethylene glycol-modified Hb and tested this in clinical trials in patients.12 None of these clinical trials have led to FDA’s approval for routine clinical use in North America. The FDA decision came after a controversial meta-analysis by another group that combined the clinical results of different types of engineered Hb, including those intramolecularly crosslinked Hb that consists of 100% single molecules, and concluded that engineered Hb causes vasoconstriction.13 Unfortunately, this meta-analysis did not take into consideration that engineered Hb can be very different in composition and vasoactivity. This meta-analysis and FDA’s recent decision have led to the movement of blood substitutes developments to Europe and Asia. To the confusion of the issue, basic researchers carried out studies on vasoactivity using different types of blood vessels ranging from hamster pouch microcirculation, aortic vessel ring perfusion, systemic blood pressure, pulmonary artery perfusion to others. Further research is needed to clarify the issue of vasoactivity.
Effects of Engineered Hb on the Heart and Vasoactivity
Vasoconstriction of blood vessels in the heart is the key question raised by the meta-analysis.13 We, therefore, carried out studies in rats to see if there is vasoconstriction of coronary vessels as shown by the ischemic electrocardiogram (ECG) changes of the heart in the form of ST elevation.14 Rat hearts have very high heart rates and therefore more sensitive to ischemic changes because of vasoconstriction. We prepare PolyHb in our laboratory each containing different percentages of unpolymerized Hb molecules using the same glutaraldehyde crosslinking and characterized to ensure that they all have the same oxygen affinity.14 Our analysis of the result shows that PolyHb that contains <2% of single Hb molecules would not cause coronary artery vasoconstriction. With increasing percentage of single Hb molecules, there is an increasing degree of vasoconstriction and ECG changes. With even higher percentage of tetrameric Hb, there was ECG evidence of cardiac arrhythmia. ST elevation could be due to vasoconstriction resulting in a decrease in the supply of oxygen to the heart and this may explain the observation of small subendocardial lesions in some primates and swine after infusion with one type of modified Hb consisting of 100% single Hb molecules. This result shows that vasoactivity varies extensively among different types of engineered Hb. This means that we have to reconsider the validity of combining the clinical results of different types of engineered Hb in the meta-analysis.13
Another group has showed that inhalation of nitric oxide could prevent vasoconstriction in rats that received a type of engineered Hb that would otherwise resulted in vasoconstriction.15 This has important implication especially for use in surgery. It may also allow the possible use of those engineered Hb that would otherwise have significant vasoactivity.. Furthermore, their result also support the role of nitric oxide in vasoactivity related to some type of engineered Hb. Although vasoactivity is an important factor, there is another factor to be considered in those clinical conditions with potential for ischemia-reperfusion injuries as discussed in the next section.
NANOBIOTECHNOLOGICAL ENGINEERING OF HB TO INCORPORATE ANTIOXIDANT ENZYMES
Principles and Characteristics
First-generation engineered Hb as discussed above is likely to have implications in some clinical applications. There are other conditions with sustained lack of oxygen supply, as in coronary artery diseases, sustained hemorrhagic shock, stroke, myocardial infarction, organ transplantation, and other conditions. Engineered Hb alone will supply oxygen, but for these ischemia situations this will also result in the production of oxygen radicals that can cause tissue injuries—a condition called ischemia-reperfusion injury.16 We, therefore, use nanobiotechnology to assemble Hb together with antioxidant enzymes like SOD and CAT to form PolyHb–CAT–SOD (Figure 3).17–20
FIGURE 3.
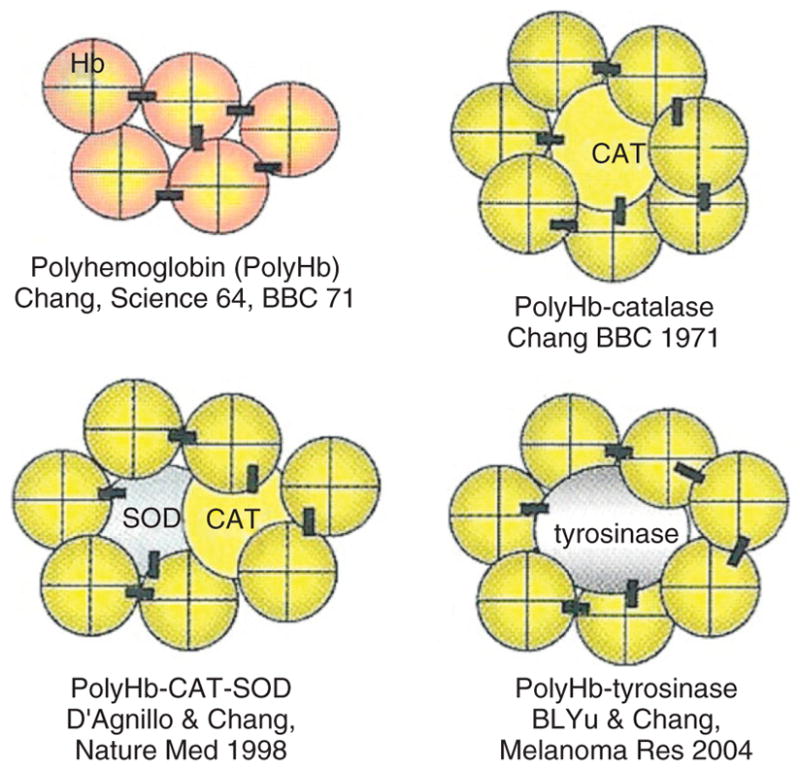
(Upper left) Polyhemoglobin (PolyHb) formed by nanobiotechnological assembling of hemoglobin (Hb) molecules into soluble nanodimension complex. (Upper right) Soluble PolyHb-catalase (CAT) containing Hb and CAT. (Lower left) Soluble PolyHb-CAT-superoxide dismutase (SOD) containing Hb, CAT, and SOD. (Lower right) PolyHb-tyrosinase as oxygen carrier that also remove tyrosine to inhibit the growth of melanoma (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
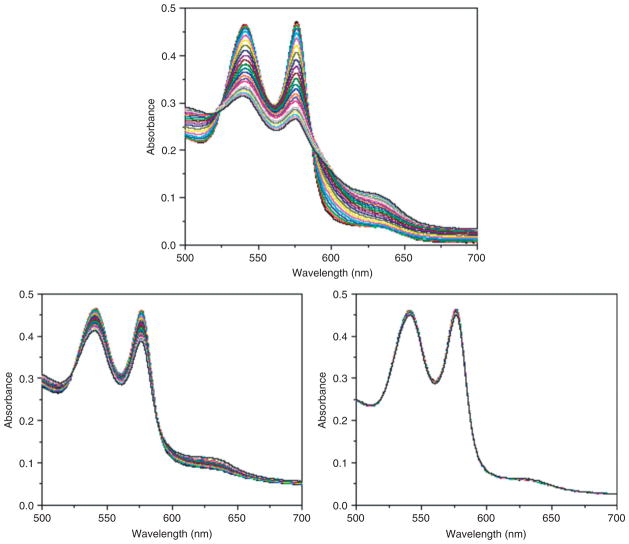
This is an oxygen carrier with the ability to remove oxygen radicals. SOD and CAT convert superoxide into hydrogen peroxide that is in turn converted into water and oxygen. This can be prepared by using glutaraldehye to crosslink an excess of ultrapure Hb with a small amount of crystalline CAT and SOD.17,20 Using the method as described, the percentage of the original enzyme activities after crosslinking is 95–99% for SOD and 85–90% for CAT.17 Crystallized enzymes are extremely expensive and in order to allow for scale up production, we recently were able to crosslink hemolysate (SFHb) to form PolySFHb.21 SFHb is the soluble content of RBC that contains both Hb and enzymes including CAT and SOD. If needed, more CAT and SOD can be extracted from the hemolysate and added to the hemolysate to prepare PolySFHb–CAT–SOD that contains a higher concentration of the enzyme.21 Unlike PolyHb, PolyHb-antioxidant enzymes can remove oxygen radicals and peroxides, and stabilize the crosslinked Hb resulting in a decrease in the oxidative iron and heme release17–21 (Figure 4). For clinical applications, further developments are needed for scale up, but this can be based on the standard scale up method used for PolyHb. Removal of infective agents should not be a problem because methods are available to prepare intravenous SOD and PEG-SOD preparations that are already being used in patients.
FIGURE 4.
Effect of superoxide on the stability of: (Upper) PolyHb; (Lower left) crosslinked hemoglobin (Hb) and enzymes obtained directly from red blood cell (RBC) hemolysate; (Lower right) crosslinked Hb and enzymes obtained directly from RBC hemolysate but with more enzymes extracted from RBCs added. (Reprinted with permission from Ref 21. Copyright 2009 Taylor & Francis).
Animal Studies
Free SOD and CAT are removed rapidly from the circulation with a half-time of less than 30 min. In the form of PolyHb–CAT–SOD, these enzymes circulate with a half-time more comparable to PolyHb in rats which is equivalent to about 24 h in human.17 In the reperfusion of ischemic rat intestine, PolyHb–-CAT–SOD significantly reduce the increase in oxygen radicals caused by PolyHb as measured by an increase in 3,4-dihydroxybenzoate.19 We have also carried out studies on global cerebral ischemia reperfusion in a hemorrhagic shock model.18 PolyHb–CAT–SOD significantly attenuated the severity of blood brain barrier (BBB) disruption when compared with saline, SF-Hb, PolyHb, or a solution of free Hb, SOD, and CAT (P < 0.01).18 In the same study, there was no evidence of brain edema in the PolyHb–CAT–SOD-treated animals and the normal control. There were significant brain edema in those treated with saline, SF-Hb, PolyHb, and the solution of free Hb, SOD, and CAT when compared with the normal control.18 Even in routine surgery, patients with coronary artery diseases may require PolyHb–CAT–SOD to remove oxygen radicals to avoid possible damage to the heart as observed in some cases. Other conditions where ischemia-reperfusion injuries would be more likely include organ transplantation because stored donor organs are in a state of oxygen lack. One group found that the use of this PolyHb–CAT–SOD improved the success rate of kidney and liver transplantation in animal studies.22 The potential of engineering Hb with antioxidant enzymes is now being extended to the use of Hb with chemical antioxidants.23
PolyHb-Fibrinogen and PolyHb-Tyrosinase
In addition to PolyHb-antioxidant enzymes, nanobiotechnological complexing of PolyHb with other enzymes or proteins are also possible. For example, high blood volume loss, large volume RBC replacement alone would not replace the platelets and coagulation factors and there is a need to replace these also. We, therefore, use nanobiotechnology to develop a blood substitute that is an oxygen carrier with platelet-like properties (Fig. 8). This is a novel blood substitute, where Hb is crosslinked with fibrinogen to form a soluble nanobiotechnological complex of PolyHb-fibrinogen (PolyHb-Fg).24 Replacement of more than 80% of total blood volume in rats using PolyHb-Fg did not result in clotting problems. On the other hand, the use of PolyHb alone resulted in some clotting problems after 80% replacement and even worse clotting problems with further increase in the blood replaced. In another study, we use PolyHb-tyrosinase (Fig. 8) to remove tyrosine needed for growth by melanoma, a skin cancer. The PolyHb component increases the oxygen tension of the melanoma to increase its sensitivity to the decrease in systemic tyrosine level. The result is a significant decrease in the rate of growth of the melanoma in a rat model.25 Both concepts have been showed in animal studies but the final test would require scale up and development for clinical trials in human.
NANODIMENSION COMPLETE ARTIFICIAL RBCS
Engineering a Complete Artificial RBC
As discussed in the previous sections, the basic principle of nanobiotechnology-based blood substitutes1–6 has been developed into clinically useful PolyHb, conjugated Hb and PolyHb with antioxidant activities. Are we now ready to continue with the earlier approach of a complete artificial RBC?1–3 The first step is to carry out research to increase the low circulation time that is the single major obstacle to the practical realization of the original complete artificial RBC.1–3
Lipid Membrane Hb Vesicles
Extensive research has been carried out by many groups using lipid membrane to encapsulate Hb into 200 nm lipid-encapsulated Hb (LEH).26–30 The use of PEG-lipid membrane to form 200-nm diameter PEG-lipid membrane artificial RBC has increased the circulation time to clinically useful level.27 This allows the development of LEH for animal studies. The result shows that these are effective in replacing loss blood in hemorrhage shock and in exchange transfusion.28–30 This is an important and major step in the development toward a more complete artificial RBC for clinical use. As in all new concepts, clinical trial will be needed in order to show the feasibility. LEH is useful for some clinical applications, but for other applications, two further developments are needed. (1) Amount of lipid in LEH: The smaller the particle, the larger will be the total surface area in the same volume of suspension. Thus, 1 ml suspension of 200-nm diameter LEH would have more than 10 times the total surface area of 1 ml suspension of RBC. As both of them have bilayer lipid membrane, the total amount of lipid would be 10 times higher for the 1 ml suspension of 200 nm lipid membrane artificial RBC compared with 1 ml suspension of RBC. Large amount of lipid can decrease the phagocytic function of the reticuloendothelial systems (RESs). In traumatic hemorrhagic shock, the RES needs to be efficient in removing contaminating microorganisms. Furthermore, in ischemia reperfusion, lipid may induce lipid peroxidation. (2) MetHb formation: The other problem with the use of LEH is the oxidation of Hb into MetHb that cannot carry oxygen. Reducing agents present in the circulating blood cannot cross the lipid membrane to reduce the rate of MetHb formation. Glucose that is needed for the MetHb reductase system inside RBC also cannot cross the lipid membrane of LEH.
Nanodimension Biodegradable Polymeric Membrane Artificial RBC (Nanoartificial RBC)
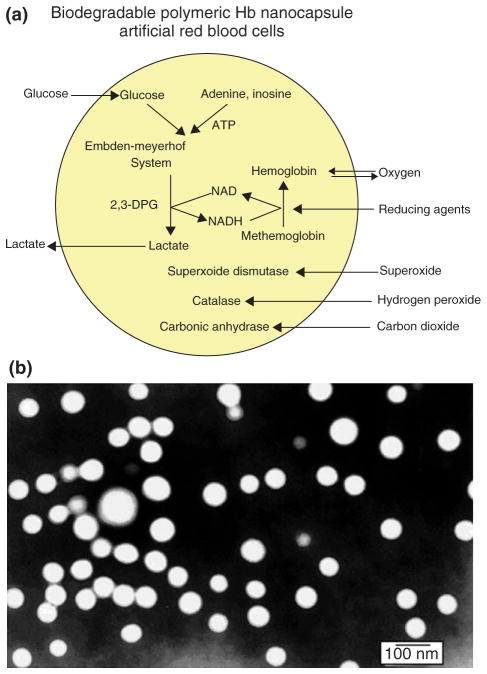
The next step in the development is to cut down as much as possible the amount of lipid needed for the membrane and replacing it by biodegradable polymeric membrane. Biodegradable polymeric membrane can be made permeable to reducing agent and glucose thus allowing them to reduce metHb formation. Since 1976, we have been using biodegradable polymers like polylactic acid (PLA) for the microencapsulation of Hb, enzymes and other biologically active material.31 More recently, we use our basic background to prepare nanodimension artificial RBC (nano-RBC) of 100 nm mean diameter using PLA, PEG-PLA membrane, and other biodegradable polymers.32–35
Biodegradable Polymer Membrane of Nanoartificial RBC
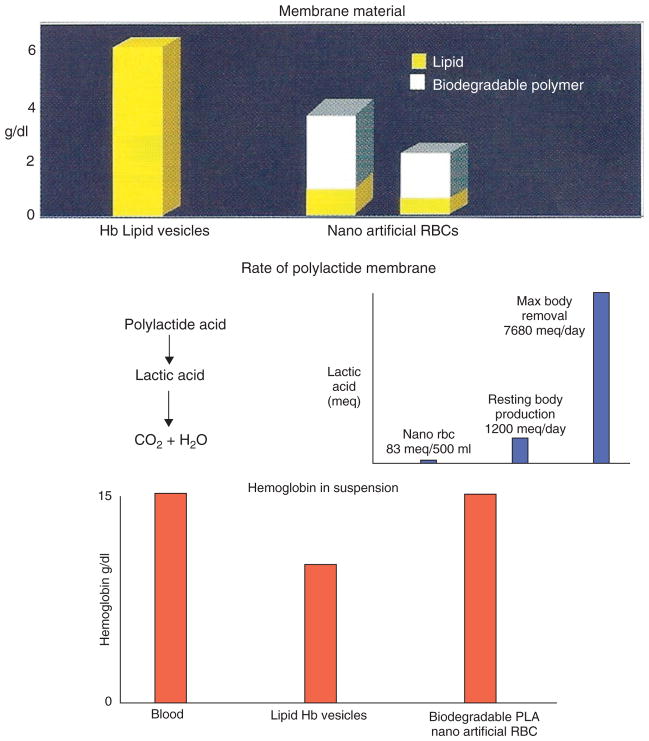
A typical electron micrograph for the biodegradable polymer nano-RBC prepared with D,L-PLA shows that they are spherical and homogeneous. Their diameter ranges from 40 to 120 nm with a mean diameter of 80 nm. The membrane thickness is 5–15 nm. As polymer is stronger than lipid and is also porous, much less membrane material is required. Thus, we can replace most of the 6 g/dl of lipid membrane in LEH with 1.6 g/dl of biodegradable polymeric membrane material (Figure 5). This marked decrease in the lipid component would lessen the effects on RES and lessen the lipid peroxidation in ischemia reperfusion. PLA is degraded in the body into lactic acid and then to carbon dioxide (Figure 5). These are all normal body metabolites. A 500 ml suspension of PLA nanoartificial RBC produced 83 mEq of lactic acid.. The normal resting body lactic acid production is 1000–1400 mEq/day, and the maximal body capacity to breakdown lactic acid is 7080 mEq/day.
FIGURE 5.
(Top) Amount of membrane material in lipid-encapsulated Hb (LEH) and polylactic acid (PLA) nano-red blood cell (RBC); (middle) fate of polylactide membrane in PLA nano-RBC compared with PLA metabolism; (bottom) hemoglobin (Hb) concentration reported (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
Characteristics of Nanoartificial RBC Including MetHb Reduction
We can increase the Hb content in the PLA nanoartificial RBC suspension from 3 to 15 g/dl (same as the whole blood) (Figure 5). This has normal P50, Hill’s coefficient, and Bohr coefficients. The characteristics of the preparation containing 10.97 g/dl of Hb prepared with D,L-PLA is listed in Table 1.
TABLE 1.
Characteristics of Nanoartificial RBC
| Hb concentration: 10.97 g/dl (up to 15 g/dl) |
| Polymer concentration: 1.2 g/dl |
| Phospholipid concentration: 0.6 g/dl |
| Specific gravity (22°C): 1.0047 |
| Viscosity (37°C): 3.7–3.8 cp |
| Bohr effect: −0.22 to −0.24 |
| Hill coefficient: 2.4–2.9 |
| P50: 26 mm Hg |
(Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
A number of enzymes such as carbonic anhydrase, CAT, and SOD, and the metHb reductase system, normally present in RBC, have been encapsulated within nanoartificial RBC and retained their activities (Figure 6).8,34,35 Unlike lipid membrane, biodegradable polymeric membrane is permeable to glucose. Thus, the inclusion of RBC metHb reductase system prevents MetHb formation even at 37°C and we can also convert MetHb to Hb at 37°C. In addition, unlike lipid membrane, the nanocapsule membrane allows plasma factors like ascorbic acid to enter the nanocapsules to prevent MetHb formation (Figure 6).34,35
FIGURE 6.
(a and b) Biodegradable polymeric membrane nanoartificial red blood cell (RBC) with diameters of 80–100 nm containing hemoglobin (Hb) and RBC enzymes (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
Circulation Time of Nanoartificial RBC
In order to increase the circulation time, we synthesized a new PEG-PLA copolymer for the nano-RBC membrane.34 After extensive research, we now have a circulation time in rats which is double that of the glutaraldehye-crosslinked PolyHb. As the RES in rat is much more efficient in removing particulate matter when compared with human, it is likely that the half-time would be even longer in human.
The detailed composition and circulation time are shown in Table 2. Briefly, the final PEG-PLA is prepared based on (a) using the PolyHb (17:1), (b) using a 1.5× concentration of the PLA-co-PEG copolymer, (c) using a higher molecular weight PLA (15K), and (d) crosslinking of the newly formed Hb nanocapsules with glutaraldehyde.
TABLE 2.
Analysis of Results
| Preparation | Max Hb (g/dl) | Time to 1.67 g/dl (h)
|
|
|---|---|---|---|
| Rats | Human | ||
| PolyHb-10 | 3.10 | 10.4 | 17 |
| PolyHb-17 | 3.35 | 14.0 | 24 |
| Nanocap-10-1-5K | 3.05 | 12.3 | 21 |
| Nanocap-17-1-5K | 3.58 | 17.1 | 29 |
| Nanocap-17-1.5-5K | 3.60 | 20.0 | 34 |
| Nanocap-17-1-5K-XL | 3.60 | 20.3 | 35 |
| Nanocap-17-1-15K | 3.57 | 21.2 | 36 |
| Nanocap-17-1.5-15K | 3.65 | 23.3 | 39.9 |
| Nanocap-17-1.5-15K-XL | 3.66 | 24.2 | 41.5 |
The numbers 10 or 17 are the ratios of molecular glutaraldehyde: Hb used in the preparation of PolyHb. 1 or 1.5 are the original and 1.5 times the original amount of copolymer used. 5K and 15K are the molecular weights of the copolymer used. XL is the crosslinking of nanocapsules after their formation to increase their stability further. The results obtained from rats are from actual experiments carried out in rats. These results are then extrapolated to human as described in the text of this article. (Reprinted with permission from Ref 5. Copyright 2007 Taylor & Francis).
Two minutes after a 30% blood volume toploading using PolyHb (10 g/dl), the best PolyHb-17 can only attain a maximal Hb concentration of 3.35 g/dl. The best PEG-PLA artificial nano-RBC, on the other hand, can reach a maximal Hb concentration of 3.60 g/dl. Calculation shows that in order to reach 3.60 g/dl, all the infused nanocapsules have to remain in the circulation when the first samples are taken at 2 min after infusion. PolyHb has a circulation half-time in human of more than 24 h, when compared with 14 h in rats. As the circulation half-time of the best type of PEG-PLA nanoartificial RBC is 24.2 h in rats, we extrapolated this to about 41.5 h in human (Table 2). However, this extrapolation will have to be evaluated in actual clinical use in human. This is a significant and important increase that would allow longer function after infusion and thus decrease the need for donor blood and further increase the avoidance of donor blood. There is a further advantage of PEG-PLA nanoartificial RBC containing PolyHb. Even if PolyHb leaks slowly out after infusion, it would continue to act and, unlike tetrameric Hb, it would not cause adverse effects.
Long-Term Effects of Nanoartificial RBC on the Histology and Function of Livers, Spleens, and Kidneys in Rats
We studied the long-term effects of PEG-PLA nanoartificial RBC containing Hb and RBC enzymes on the liver, spleen, and kidneys in rats.36,37 The experimental rats received one of the following infusions: nanoartificial RBC in Ringer lactate, stroma-free Hb, PolyHb, and autologous rat whole blood. Blood samples were taken before infusions and on Days 1, 7, and 21 after infusions for analysis.
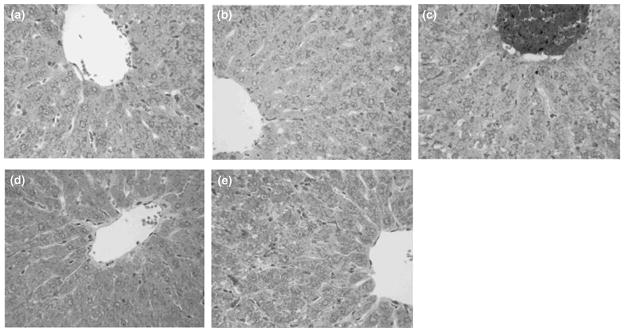
Nanoartificial RBC, PolyHb, Ringer lactate, and rat RBC did not have any significant adverse effects on alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatine kinase, amylase, and creatine kinase. On Day 21 after infusions, rats were sacrificed and livers and spleens were excised for histological examination. Nanoartificial RBC, PolyHb, Ringer lactate, and rat RBC did not cause any abnormalities in the microscopic histology of the livers and spleens. In conclusion, injected nanoartificial RBC can be efficiently metabolized and removed by the RES, and do not have any biochemical or histological adverse effects on the livers or the spleens (Figure 7).
FIGURE 7.
Histology of liver in rats toploaded with nanoartificial red blood cell (RBC) (a), LactRing (b), SF-Hb (c), polyhemoglobin (PolyHb) (d), or RBC (e). Under light microscope, there was no abnormality in Groups a, b, d, and e. For Group c infused with SF-Hb, there is hemoglobin (Hb) accumulation and steatmotosis. H&E staining, × 400 (Reprinted with permission from Ref 37. Copyright 2008 Taylor & Francis).
In the kidney study, unlike SF-Hb, nanoartificial RBC, PolyHb, Ringer lactate, and rat RBC had no abnormalities in renal biochemistry or histology. Thus, injection of nanoartificial RBC did not have adverse effects on renal function nor renal histology. These promising results have led others to develop similar nanoartificial RBC.38
Promising progress is being made. However, the complexities of studies required to evaluate all of the elements of a complete nanoartificial RBC are not an easy task. This cannot be attained in a ‘one shot’ type of study. For instance, further studies will be needed to analyze the redox reactivities, and NO-related vasoactivity.
GENERAL DISCUSSIONS AND CONCLUSIONS
Regional differences and the potentials of unknown infective agents must be included in any discussions of the future prospect of blood substitutes and also in the degree of regulatory requirements. Blood substitute is urgently needed in regions with high incidences of infective agents such as HIV and thus higher potential for contaminated donor blood. It is less urgent in regions with less incidences of HIV and where costly screening tests are being used to avoid the use of contaminated donated blood. Even here, increase in the aging population has resulted in higher transfusion requirement that has led to inadequate donor blood supply. Irrespective of the regional needs, there are needs for emergency or natural or man-made disasters. In addition, we need to be prepared if some yet unknown infectious or chemical agents should suddenly appear.
Despite much progress in this area, there is nothing currently available for patient use to fulfill the functions of an RBC transfusion if there is an issue with the supply for whatever reason. We now know that blood substitute is a complex area that requires much basic research and development and cannot be made available on demand. Thus, blood substitutes have to be developed immediately and not wait until when it is too late.
In view of the above discussions, what should be a valid strategy for the future development of blood substitutes? We need to carry out basic research to analyze the validity of the proposed issues and theories. This basic information will be important in our continuing search for effective and safe Hb-based blood substitutes. In the meantime, we cannot wait forever and we should analyze the risk/benefit of available engineered Hb for different conditions including regulatory requirements in different regions. For instance, for some region, the benefit of PolyHb may be a matter of life and death when compared with the risk of some adverse effect. We should also analyze the possible use of available engineered Hb for all regions in well-controlled elective surgery for patients with no potentials for ischemia-reperfusion problems. This includes the use of nitric oxide for those HBOC that has some problems with vasoactivity. In carrying out the risk–benefit analysis, we should aim for blood substitutes to be as safe as donor blood, but it will not be realistic to expect something that is safer than donor blood. In this regard, recent reviews show that liberal donor blood transfusions or stored packed RBC have increased the ischemic coronary events.39,40 This degree of adverse effect has not been reported in the best type of PolyHb. PolyHb with antioxidant activity like PolyHb–SOD–CAT or complete nanoartificial RBC is likely required in some clinical conditions. These include severe sustained hemorrhagic shock, cerebral ischemia, stroke, head trauma, myocardial infarction, and others. However, with increase in the complexity of the preparation, there will be an increase in cost and manufacturing challenges.
Artificial cells including blood substitutes and nanomedicine is a very large and rapidly developing area. Further readings and updating are available on the public service website of this research center.41
Acknowledgments
The author acknowledges the many years of ongoing research support from the Medical Research Council of Canada that is now called Canadian Institutes of Health Research. He also acknowledges the earlier support from Bayer/Canadian Red Cross Society followed by the more recent 6 years (2003–2009) developmental award from the Quebec Ministry of Health’s Hemovigillance Program in the form of a FRSQ Research Group (d’equip) on Blood Substitutes in Transfusion Medicine.
References
- 1.Chang TMS. ‘Hemoglobin Corpuscles’ {Honours Physiology Research Report}, 1957 Medical Library, McGill University (Also reprinted as part of ‘30th Anniversary in Artificial Red Blood Cells Research’) Biomater Artif Cells Artif Organs. 1988;16:1–9. [Google Scholar]
- 2.Chang TMS. Semipermeable microcapsules. Science. 1964;146(3643):524. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 3.Chang TMS. Artificial Cells (Monograph) Springfield, IL: Charles C. Thomas; 1972. Available at: www.artcell.mcgill.ca. [Google Scholar]
- 4.Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 5.Chang TMS. Monograph on ARTIFICIAL CELLS: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. Singapore & London: World Science Publisher & Imperial College Press; 2007. pp. 1–452. Available at: www.artcell.mcgill.ca. [Google Scholar]
- 6.Chang TMS. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Common. 1971;44:1531–1536. doi: 10.1016/s0006-291x(71)80260-7. [DOI] [PubMed] [Google Scholar]
- 7.Perutz MF. Myoglobin and Hb: role of distal residues in reactions with haem ligands. Trends Biochem Sci. 1989;14:42–44. doi: 10.1016/0968-0004(89)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.Chang TMS. Blood Substitutes: Principles, Methods, Products and Clinical Trials. Vol. 1. Basel: Karger; 1997. Available at: www.artcell.mcgill.ca. [Google Scholar]
- 9.Gould SA, Moss GS. Clinical development of human polymerized hemoglobin as a blood substitute. World J Surg. 1996;20:1200–1207. doi: 10.1007/s002689900183. [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopaedic surgery. J Trauma. 2008;64:1484–1497. doi: 10.1097/TA.0b013e318173a93f. [DOI] [PubMed] [Google Scholar]
- 12.Olofsson C, Torbjo A, Johansson T, Larsson S, Nellga P, et al. A multicenter clinical study of the safety and activity of maleimide-polyethylene glycol–modified hemoglobin (Hemospan) in patients undergoing major orthopedic surgery. Anesthesiology. 2006;105:1153–1163. doi: 10.1097/00000542-200612000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Meta-analysis: risk of myocardial infarction and death: a cell-free hemoglobin-based blood substitutes. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu BL, Liu ZC, Chang TMS. PolyHb with different percentage of tetrameric Hb and effects on vasoactivity and electrocardiogram. Artif Cells Blood Substit Biotechnol. 2006;34:159–175. doi: 10.1080/10731190600580223. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, et al. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alayash AI, D’Agnillo F, Buehler PW. First-generation blood substitutes: what have we learned? Biochemical and physiological perspectives. Expert Opin Biol Ther. 2007;7:665–675. doi: 10.1517/14712598.7.5.665. [DOI] [PubMed] [Google Scholar]
- 17.D’Agnillo F, Chang TMS. PolyHb-superoxide dismutase. Catalase as a blood substitute with antioxidant properties. Nat Biotechnol. 1998;16:667–671. doi: 10.1038/nbt0798-667. [DOI] [PubMed] [Google Scholar]
- 18.Powanda D, Chang TMS. Cross-linked polyHb-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cells Blood Substit Biotechnol. 2002;30:25–42. doi: 10.1081/bio-120002725. [DOI] [PubMed] [Google Scholar]
- 19.Chang TMS. Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation and Cell/Stem Cell Therapy. Singapore: World Science Publisher; 2007. A nanobiotechnologic therapeutic that transport oxygen and remove oxygen radicals: for stroke, hemorrhagic shock and related conditions. In: Chang TMS; pp. 62–92. [Google Scholar]
- 20.Chang TMS. Nanobiotechnological modification of hemoglobin and enzymes from this laboratory. Biochim Biophys Acta: Proteins and Proteomics. 2008;1784:1435–1444. doi: 10.1016/j.bbapap.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Chang TMS. Extraction of erythrocyte enzymes for the preparation of polyhemoglobin-catalase-superoxide dismutase. Artif Cells Blood Substit Biotechnol. 2009;37:69–77. doi: 10.1080/10731190902742240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang EJ, Lee TH, Mun K. Effects of polyhemoglobin-antioxidant enzyme complex on ischemia-reperfusion in kidney. Transplant Proc. 2004;36:1952–1954. doi: 10.1016/j.transproceed.2004.08.146. [DOI] [PubMed] [Google Scholar]
- 23.Buehler PW, Haney CR, Gulati A, Ma L, Hsia CJ. Polynitroxyl hemoglobin: a pharmacokinetic study of covalently bound nitroxides to hemoglobin platforms. Free Radic Biol Med. 2004;37:124–135. doi: 10.1016/j.freeradbiomed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Wong N, Chang TMS. Polyhemoglobin-fibrinogen: a novel blood substitutes with platelet-like activity for extreme hemodilution. Artif Cells Blood Substit Biotechnol. 2007;35:481–489. doi: 10.1080/10731190701586210. [DOI] [PubMed] [Google Scholar]
- 25.Yu BL, Chang TMS. In vitro and in vivo effects of polyHb–tyrosinase on murine B16F10 melanoma. Melanoma Res. 2004;14:197–202. doi: 10.1097/01.cmr.0000131013.71638.c0. [DOI] [PubMed] [Google Scholar]
- 26.Djordjevich L, Miller IF. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp Hematol. 1980;8:584. [PubMed] [Google Scholar]
- 27.Phillips WT, Klipper RW, Awasthi VD, Rudolph AS, Cliff R, et al. Polyethylene glyco-modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J Pharmacol Exp Ther. 1999;288:665–670. [PubMed] [Google Scholar]
- 28.Tsuchida E, editor. Blood Substitutes. Amsterdam: Elsevier; 1998. Present and future perspectives; p. 267. [Google Scholar]
- 29.Tsuchida E, Sakai H, Horinouchi H, Kobayashi K. Hemoglobin-vesicles as a transfusion alternative. Artif Cells Blood Substit Biotechnol. 2006;34:581–588. doi: 10.1080/10731190600973907. [DOI] [PubMed] [Google Scholar]
- 30.Atoji T, Aihara M, Sakai H, Tsuchida E, Takeoka S. Hemoglobin vesicles containing methemoglobin and tyrosine to suppress methemoglobin formation in vitro and in vivo. Bioconjug Chem. 2006;17:1241–1245. doi: 10.1021/bc050349h. [DOI] [PubMed] [Google Scholar]
- 31.Chang TMS. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioeng. 1976;1:25–32. [PubMed] [Google Scholar]
- 32.Yu WP, Chang TMS. Submicron biodegradable polymer membrane Hb nanocapsules as potential blood substitutes: a preliminary report. Artif Cells Blood Substit Biotechnol. 1994;22:889–894. doi: 10.3109/10731199409117926. [DOI] [PubMed] [Google Scholar]
- 33.Yu WP, Chang TMS. Submicron biodegradable polymer membrane Hb nanocapsules as potential blood substitutes. Artif Cells Blood Substit Biotechnol. 1996;24:169–184. doi: 10.3109/10731199609117433. [DOI] [PubMed] [Google Scholar]
- 34.Chang TMS, Powanda D, Yu WP. Ultrathin polyethylene-glycol-polylactide copolymer membrane nanocapsules containing polymerized Hb and enzymes as nanodimension RBC substitutes. Artif Cells Blood Substit Biotechnol. 2003;31:231–248. doi: 10.1081/bio-120023155. [DOI] [PubMed] [Google Scholar]
- 35.Chang TMS. Nanotechnology based artificial red blood cells. In: Chang TMS, editor. Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation and Cell/Stem Cell Therapy. Singapore: World Science Publisher; 2007. pp. 93–128. (monograph) [Google Scholar]
- 36.Liu ZC, Chang TMS. Effects of PEG-PLA-nano artificial cells containing hemoglobin on kidney function and renal histology in rats. Artif Cells Blood Substit Biotechnol. 2008;36:421–430. doi: 10.1080/10731190802369763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu ZC, Chang TMS. Long term effects on the histology and function of livers and spleens in rats after 33% toploading of PEG-PLA-nano artificial red blood cells. Artif Cells Blood Substit Biotechnol. 2008;36:513–524. doi: 10.1080/10731190802554224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rameez S, Alosta H, Palmer AF. Biocompatible and biodegradable polymersome encapsulated hemoglobin: a potential oxygen carrier. Bioconjug Chem. 2008;19:1025–1032. doi: 10.1021/bc700465v. [DOI] [PubMed] [Google Scholar]
- 39.Rao S, Harrington R, Califf R, Stamler J. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. J Am Med Assoc. 2005;293:673–674. doi: 10.1001/jama.293.6.673-a. [DOI] [PubMed] [Google Scholar]
- 40.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, et al. Blood transfusion in patients with acute coronary syndrome. J Am Med Assoc. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 41.Artificial cells, blood substitutes and nanomedicine. Public Service Website of the Artificial Cells and Organs Research Centre of McGill University. Available at: www.artcell.mcgill.ca.