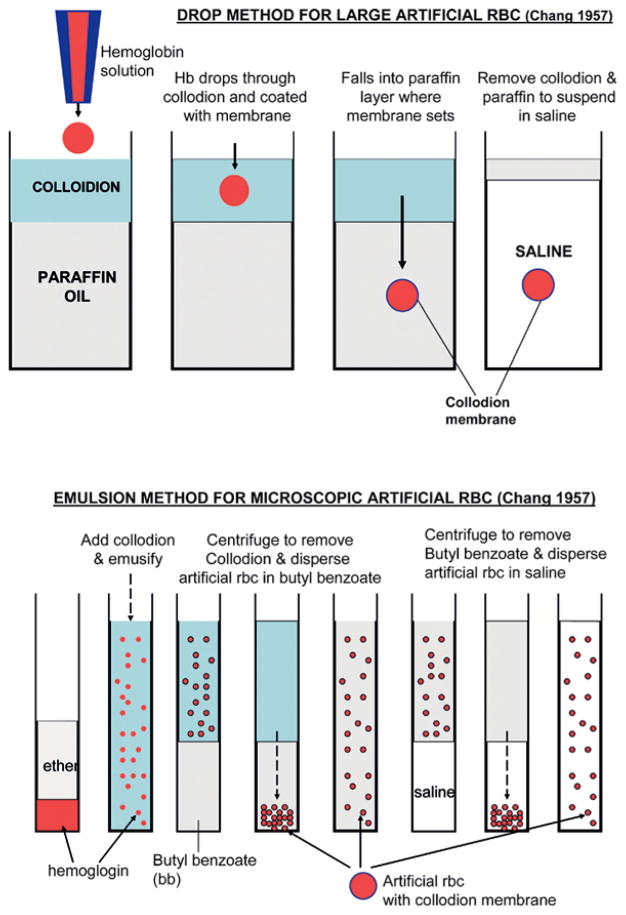
It was 50 years ago that the first “artificial cells” were prepared [1,2]. This was not an attempt to reproduce biological cells, but to use available basic knowledge to prepare simple systems for possible uses in medicine and other areas. The 1957 emulsion method for forming ultrathin polymeric membrane artificial cells containing hemoglobin and red blood cell enzymes (Figure 1) has become the basis for the preparation of other types of microscopic and nanodimension artificial cells. Extensions of the original 1957 drop procedure for forming larger artificial cells (Figure 1) have become the basis for preparing artificial cells to contain islet, hepatocytes, genetic engineered cells, stem cells and other types of cells.
Figure 1.
Original 1957 method of preparing artificial cells (for details see [1,5]). Upper: drop method for preparing large artificial cells. Principle later extended for use in bioencapsulation of cells, stem cells, genetic engineered cells. Lower: emulsion phase separation method for preparing microscopic artificial cells (unlike above, “collodion” prepared by removing most of alcohol and replaced with ether). Principle extended to preparation of microscopic artificial cells and drug delivery systems and nanodimension artificial cells (figure from [5] with copyright permission).
There have been increasing and recently explosive interest and research activities around the world on artificial cells, especially in fields related to biotechnology, nanomedicine, nanoscience, bioencapsulation, cell therapy, blood substitutes, advance drug delivery systems, and even nanoscale robotics and others (Table 1). However, instead of the term “artificial cells,” many use other terminologies, such as liposomes, nanoparticles, microcapsules, blood substitutes, bioencapsulation, and so on.
Table 1.
| Artificial organs: hemoperfusion |
| Drug delivery including biotechnological products |
| Blood substitutes |
| Enzyme and gene therapy |
| Cells therapy: cell/stem cells/genetic engineered cells |
| Agriculture & Industry |
| Nanomedicine |
| Regenerative medicine |
| Bioencapsulation |
| Nanoencapsulation |
| Microencapsulation |
| Liposomes |
| Nanocomputers and nanorobatics |
| Nanosensors |
| Basic research: cell and membrane |
| Others |
As a result, any meaningful literature search for a complete idea of the present status of the whole field of artificial cells is impossible. Furthermore, the fact that papers in this highly interdisciplinary area are published in numerous journals specializing in chemistry, medicine, surgery, bioengineering, nanoscience and others makes a literature search even more difficult. Books in this area are mostly multi-authored, describing very specific and narrow areas. Thus for the 50th anniversary of artificial cells the author has just prepared a monograph on ARTIFICIAL CELLS: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes and Cell/Stem Cell Therapy [5]. This is now such a large area that it needed more than 1000 references just to summarize the present status and future perspectives of artificial cells.
BASIC FEATURES OF ARTIFICIAL CELLS
The initial research on artificial cell forms the basic principle of artificial cells that has been extended for use in many areas by many groups. Indeed, as stated in the first monograph on Artificial Cells [3]: “Artificial Cell is not a specific physical entity. It is an idea involving the preparation of artificial structures of cellular dimensions for possible replacement or supplement of deficient cell functions. It is clear that different approaches can be used to demonstrate this idea.”
Basic Features of Early Artificial Cells
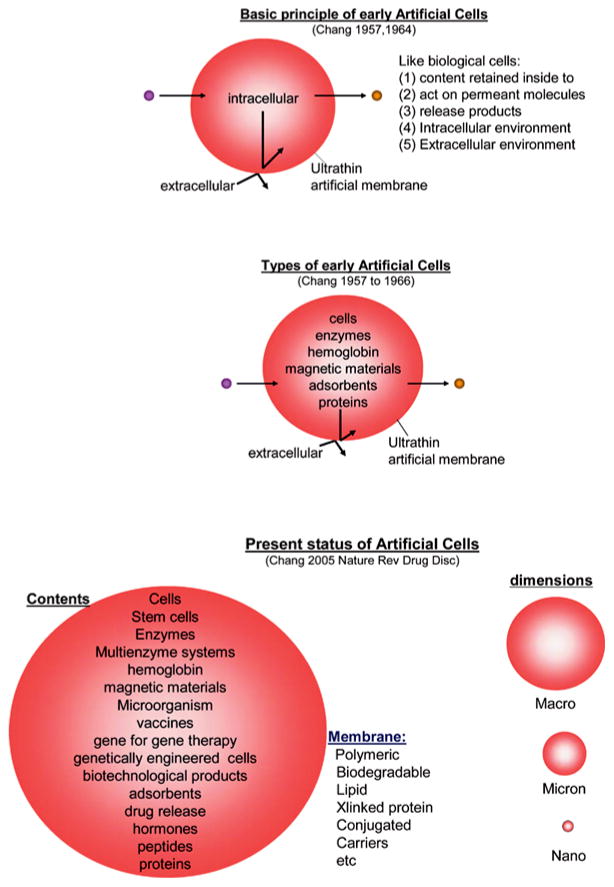
Earlier artificial cells have some of the simpler properties of biological cells (Figure 2). The following are some examples of the basic features:
Figure 2.
Upper: Basic principle of early artificial cells. Middle: Different types of early artificial cells based on this basic principle. Lower: Present status of artificial cells with wide variations in contents, membrane material and dimensions From [5] with copyright permission.
Membrane of artificial cell separates its content from the outside. At the same time, membrane can be prepared to selectively allow different types of molecules to cross. This ranges from membrane that does not allow any molecules to cross to those that allow even very large molecules like proteins to cross. In between this range, one can prepare artificial cell membranes that restrict the movement of molecules according to molecular size, lipid solubility, affinity to carrier mechanisms, etc.
The artificial cell membranes can be very thin and yet strong. There is as well a large surface area. Thus, 10 ml of 20 μm diameter artificial cells has a total surface area of 2,500 cm2. This is the same as the total membrane surface area of an artificial kidney machine. In addition, the artificial cell membrane is 100 times thinner than that of the artificial kidney membrane. This means that smaller molecules can move across 10 ml of 20 μm diameter artificial cells 100 times faster than that across the artificial kidney machine. The microscopic size of artificial cells also allows material to diffuse rapidly inside the artificial cells.
Artificial cells can contain the same biological material as biological cells. In addition, they are more versatile since adsorbents, magnetic materials, cells, drugs and other material can also be included separately or in combination (Figure 2).
Present Status of the Basic Features of Artificial Cells of Macro, Micron, Nano and Molecular Dimensions
The general principle of artificial cells can form the basis of a large number of artificial systems (Figure 2). In addition to being of cellular dimensions in the micron range, they can also be in the macro range, in the nano range or in the molecular range. Furthermore, the membrane material includes polymer, biodegradable polymer, lipid, crosslinked protein, lipid-polymer complex, lipid-protein complex and membrane with transport carriers. The artificial cells can contain an unlimited variety of material individually or in combinations (Figure 2). These include cells, stem cells, enzymes, multienzyme systems, hemoglobin, magnetic materials, microorganism, vaccines, gene for gene therapy, genetically engineered cells, adsorbents, drugs, hormones, peptides, proteins and others.
Importance of Progress in Parallel Areas of Biotechnology, Molecular Biology, and Regenerative Medicine
Most of this author’s earlier original ideas and basic research were related to enzyme and gene therapy, cell therapy, blood substitutes, regenerative medicine, nanomedicine and related areas. Developing these for actual clinical use required parallel developments in molecular biology and biotechnology. More recently, many groups around the world have made exciting progress in biotechnology, molecular biology, genetic engineering and related areas. The outcome is a recent new wave of research and development in artificial cells. Many groups around the world are now working on extensions and modifications of artificial cells for use in nanotechnology, nanobiotechnology, blood substitutes, regenerative medicine, gene therapy, cell/stem cell therapy and other areas [4,5].
Historical Milestone
Table 2 shows examples of the milestone of the first report of original ideas in artificial cells. This is not a complete listing and more details requiring more than 1000 references have been given elsewhere [5].
Table 2.
Artificial Cells (AC): Timeline of ideas first reported (references available from [5])
| 1957 Chang | First artificial cells prepared with a synthetic membrane to replace RBC membrane and containing hemoglobin and red blood cell enzymes (emulsion phase separation, extrusion method or spray coating) |
| 1964 Chang (Science) | Artificial cells (AC) containing enzymes, hemoglobin and cells formed by interfacial coacervation or interfacial polymerization to form membranes of polymer, crosslinked protein, polymer conjugated with protein, also crosslinked protein microspheres |
| 1964, 1965 Chang | Nanobiotechnology: crosslinked protein (polyHb) & conjugated Hb |
| 1964, 1965 Chang 1966 Chang et al. | Extrusion drop method for AC to encapsulate intact cells for immunoisolation in cell therapy. |
| 1965 Bangham et al. | Liquid crystal microspheres of multi-lamellar lipid (liposomes) as membrane model for basic research |
| 1965, 1972a, 1973b Chang | AC for molecular sieve chromatography and separation |
| 1965 Chang 1966 Chang et al. | AC with intracellular multi-compartments |
| 1966 Chang | Silastic AC and microspheres containing protein. |
| 1966 Chang | AC containing magnetic materials and biological materials. |
| 1966, 1969a Chang | Ultrathin membrane AC containing adsorbents for hemoperfusion |
| 1966 Clark & Gollan | Fluorocarbon as oxygen carrier |
| 1967 Chang et al. | AC with polysaccharide complexed membrane for biocompatibility |
| 1968 Chang & Poznansky (Nature) | Implanted enzyme AC for enzyme therapy in inborn error of metabolism (shown in congenital catalase-deficient acatalesemic mice) |
| 1968 Bunn & Jandl | Intramolecularly crosslinked single Hb molecule |
| 1968 Geyer et al. | Fluorocarbon effective in exchange transfusion in animal studies |
| 1969d Chang 1972a Chang | AC with lipid-polymeric membrane or lipid-crosslinked protein membrane containing cyclic transport carrier. (AC contains proteins) |
| 1970–1975 Chang et al. | First clinical use of artificial cells in patients (in hemoperfusion) |
| 1971a Chang (Nature) | Implanted enzyme AC for lymphosarcoma suppression in mice |
| 1971b Chang | Nanobiotechnology: glutaraldehyde crosslinked Hb into polyHb. Later, others used this method for blood substitutes in patients. |
| 1972a Chang | First monograph on Artificial Cells |
| 1972b Chang (Lancet) | AC hemoperfusion resulted in Grade IV hepatic coma patient recovering consciousness. |
| 1973 Gregoriadis | First use of liposomes to entrap enzymes & drugs. Led to extensive development of liposomes as delivery systems |
| 1975h Chang | Paper discussing one shot vaccine using AC |
| 1976a Chang | Biodegradable polylactide microcapsules and microparticles containing proteins & hormones |
| 1976 Tam, Blumenstein & Wong | Soluble dextran conjugated hemoglobin |
| 1976 Bonhard et al. | Develop glutaraldehyde crosslinked polyHb as blood substitute |
| 1977–1985 Chang with Campbell, Cousineau, Ilan, Grunwald, Wahl, Yu, etc. | Artificial cells containing multienzyme systems with cofactor recyclying for multistep enzyme reactions. |
| 1978 Naito & Yokoyama | Developed perfluorodecalin as blood substitute towards clinical trials |
| 1980 Lim & Sun (Science) | Alginate-polylysine-alginate AC encapsulated cells |
| 1980 Rosenthal & Chang | AC membrane of lipid-protein-polymer containing Na+K+-ATPase |
| 1980 Djordjevich & Miller | Lipid membrane AC encapsulated hemoglobin |
| 1985 Mitsuno & Ohyanagi | Clinical trials of perfluorodecalin as red blood cell substitute |
| 1986 Yuan & Chang | AC containing microsomes & cytosol |
| 1986 Bourget & Chang | Oral enzyme AC for inborn error of metabolism (Phenyketonuria rat) |
| 1986 Sipehia, Bannard & Chang | AC membrane that exclude small hydrophilic molecules but permeable to large lipophilic molecules |
| 1986 Chang, Bourget & Lister | Novel finding of extensive enterorecirculation of amino acids leading to the use of oral enzyme AC therapy to selectively remove specific unwanted systemic amino acid. |
| 1988 Tsuchida’s group 2002 Tsuchida et al. | Development and in-vivo testing of synthetic heme complex either to liposome or to recombinant albumin as blood substitute. |
| 1989a Chang, 1989 Palmour et al., Chang | Clinical use of oral enzyme artificial cells in a patient (patient with an inborn error of metabolism: Lesch-Nyhan disease) |
| 1989 Moss et al. | Clinical trials with glutaraldehyde crosslinked PolyHb |
| 1990 Hoffmann et al. | Recombinant human hemoglobin |
| 1994 Yu & Chang | Biodegradable polymeric membrane nano-artificial red blood cells |
| 1994 Soon-Shiong et al. | AC encapsulated islet transplantation in a type 1 diabetic patient. Insulin independence reported. |
| 1996 Prakash & Chang (Nature Medicine) | Oral artificial cells containing genetically engineered cells lowers systemic urea in an uremic rats model |
| 1996 Aebischer, Lysagth et al. (Nature Medicine) | Polymeric fiber encapsulation of genetically modified xenogeneic cells for intrathecal delivery of CNTF in amyotrophic lateral sclerosis patients |
| 1998 D’Agnillo & Chang (Nature Biotechnology) | Nanobiotechnology of crosslinking of Hb, catalase and superoxide dismutase to form soluble nanodimension PolyHb-CAT-SOD |
| 1998 Tsuchida | Lipid AC vesicle Hb: Develop and test in animal towards clinical use |
| 1999 Philips et al. | PEG-lipid membrane AC containing Hb increases circulation time |
| 2000 Liu & Chang | AC coencapsulating hepatocytes and adult stem cells |
| 2001 Lörh et al. (Lancet) | Clinical trial of AC microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma in patients |
| 2002 Gould et al. | The life-sustaining capacity of human polyhemoglobin in trauma surgery clinical trials |
| 2002 Sprung et al. | The use of bovine polyhemoglobin in surgical patients: results of a multicenter, randomized, single-blinded trial |
| 2003 Chang, Powanda, Yu | PEG-PLA membrane AC containing Hb & rbc enzymes |
| 2004 Bloch et al., Aebischer | Phase I clinical study for Huntington’s Disease, using encapsulated cells engineered to secrete Human Ciliary Neurotrophic Factor |
| 2004 Yu & Chang (Melanoma Res J) | Nanobiotechnological approach of PolyHb-tyrosinase: delays the growth of melanoma in a rat model |
| 2006 Liu & Chang (J Liver Transplantation) | AC encapsulated bone marrow stem cells regenerate liver resulting in recovery and survival of rats with 90% of liver surgically removed |
FUTURE PERSPECTIVES
In the last 50 years, there has been extension and development of the idea of artificial cells around the world. However, we have barely touched the surface of the potential of this idea. Many other extensions and variations in the membrane material, the configurations and the contents are possible.
Each major progress in other areas has led to stepwise progress in artificial cells. First there is the coming of age of polymer chemistry and biomaterial. Then there is the recognition of the importance and developments in biotechnology. Then there is the present ongoing progress in molecular biology and genomics that will contribute to a quantum leap in the area of artificial cells. One can expect that there will be important future progress in other areas that will contribute to unlimited progress in the area of artificial cells.
The following prediction in my 1972 monograph on Artificial Cells is already out of date: “Artificial Cell is not a specific physical entity. It is an idea involving the preparation of artificial structures of cellular dimensions for possible replacement or supplement of deficient cell functions. It is clear that different approaches can be used to demonstrate this idea.” In the last 50 years [1], artificial cells have progressed way beyond this 1972 prediction. Artificial cells can now be of macro, micro, nano and molecular dimensions. There are also unlimited possibilities in variations for the artificial cell membranes and contents. Even then, we have only just touched the surface of the enormous potential of artificial cells. For instance, there are groups that are working towards a very ambitious aim of creating what they call a “living artificial cells.” Other researchers are working on the next generation of self-repairing computer and robotics technology, which requires the use of intelligent technical systems based on artificial cells. Toward this end, the European Commission is supporting an integrated program of “Programmable Artificial Cell Evolution.” This project focuses on the intelligent technical (IT) potential of artificial cells that are truly “artificial” and not replicates of biological cells.
There is a tendency for new development and extension of “artificial cells” to be hidden under numerous new names. Some of these include nanoparticles, nanotubule, lipid vesicles, liposomes, polymer tethered lipid, polymersome, microcapsules, bioencapsulation, nanocapules, nanosensor, macroenapsulation, polyhemoglobgin, conjugated hemoglobin, etc. The result is a fragmentation of the field of artificial cells into different subdivisions, subdisciplines and societies that do not interact with one another. This is at a time when this very interdisciplinary field needs researchers from different areas coming together to move the field forward. One waits for the time when the many arbitrary subdivisions of “artificial cells” under the guise of different names can come together! When this takes place, the result of the pooling of talents, specialized know-how in this very interdisciplinary and international area will lead to progress beyond anyone’s imagination.
Acknowledgments
The author acknowledges the support of the Canadian Institutes of Health Research, the “Virage” Centre of Excellence in Biotechnology from the Quebec Ministry, the MSSS-FRSQ Research Group award on Blood Substitutes in Transfusion Medicine from the Quebec Ministry of Health’s new Haemovigillance and Transfusion Medicine Programme.
References
- 1.Chang TMS. Hemoglobin Corpuscles (report of a research project for Honours Physiology, Medical Library, McGill University); reprinted in 1988 as part of the 30th Anniversary in Artificial Red Blood Cells Research. J Biomaterials, Artificial Cells & Artificial Organs. 1957;16:1–9. [Google Scholar]
- 2.Chang TMS. Semipermeable microcapsules. Science. 1964;146(3643):524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 3.Chang TMS. Artificial Cells. Springfield, IL: Charles C. Thomas; 1972. (Out of print, but available for free download on www.artcell.mcgill.ca) [Google Scholar]
- 4.Chang TMS. Therapeutic applications of polymeric artificial cells. Nature Review: Drug Discovery. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 5.Chang TMS. Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation and Cell/Stem Cell Therapy. Singapore: World Science Publisher; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]