Abstract
Weak T-cell antigen receptor (TCR)-ligand interactions are sufficient to activate naïve CD8+ T cells, but generally do not result in tumor eradication. How differences in TCR affinity affect the regulation of T-cell function in an immunosuppressive tumor environment has not been investigated. We have examined the functional differences of high- vs. low-affinity CD8+ T cells and we observed that infiltration, accumulation, survival and cytotoxicity within the tumor are severely impacted by the strength of TCR-ligand interactions. In addition, high-affinity CD8+ T cells were found to exhibit lower expression of inhibitory molecules including PD-1, LAG-3 and NKG2A, thus being less susceptible to suppressive mechanisms. Interferon γ and autocrine interleukin-2 were both found to influence the level of expression of these molecules. Interestingly, although high-affinity CD8+ T cells were superior to low-affinity CD8+ T cells in their ability to effect tumor eradication, they could be further improved by the presence of tumor specific CD4+ T cells. These findings illustrate the importance of both TCR affinity and tumor-specific CD4 help in tumor immunotherapy.
Keywords: CD4 help, CD8+ T cells, T cell function, TCR affinity, tumor environment
Introduction
The T-cell repertoire available for immunotherapy of cancer is constrained by central and/or peripheral tolerance mechanisms.1-3 The majority of T cells with high avidity for self/tumor antigens are deleted in the thymus resulting in a T-cell repertoire in the periphery specific for self/tumor antigens that display a relatively low affinity.4,5 In addition, both peripheral tolerance mechanisms and tumor-induced tolerance can contribute to the weakening of T-cell responses against self/tumor antigens.6,7 This raises the question whether we can utilize the natural low-affinity T-cell repertoire for immunotherapy of cancer. Many vaccine approaches have been tested to induce T-cell responses toward a tumor protein. However, in any case the clinical trials show complete tumor eradication, and objective tumor regression is induced in a small percentage of patients.8,9
Using a mouse tumor model in which spontaneous insulinomas arise expressing the influenza hemagglutinin (HA) as a model antigen (RIP-Tag2-HA mice), previous results showed that HA-specific low-affinity Clone 1 CD8+ T cells could not destroy transformed pancreatic islet β cells that express HA, even when activated with a potent viral vaccine. However, the provision of tumor-specific CD4 help within the tumor environment resulted in an increase in both the number and effector function of Clone 1 cells, which resulted in tumor eradication.10,11 As an alternative to the use of the natural T-cell repertoire, tumor-reactive T cells can now be created by genetic engineering. This allows for the selection of the biophysical properties of the T-cell receptor used for immunotherapy12 and for the increase of the functional avidity of TCR-engineered cells.13 Two clinical trials testing MART1-specific T cells of differing avidity showed that superior functional avidity might help to improve objective response rates.14,15 However, it is not clear whether high- and low-affinity CD8+ T cells have major functional differences in the tumor environment and whether high-affinity CD8+ T cells may also benefit from the presence of CD4+ T cells. We compared Clone 1 cells, which express a TCR that was originally isolated from a mouse expressing HA in the pancreas and exhibit low affinity for HA, to Clone 4 cells, in which the TCR was derived from a wild-type mouse and exhibit high affinity for HA.16 We identified several factors involved in tumor rejection that are affected by TCR affinity and studied the role of cytokines and CD4 help on the regulation of these factors.
Results
Functional differences between Clone 1 and Clone 4 CD8+ T cells
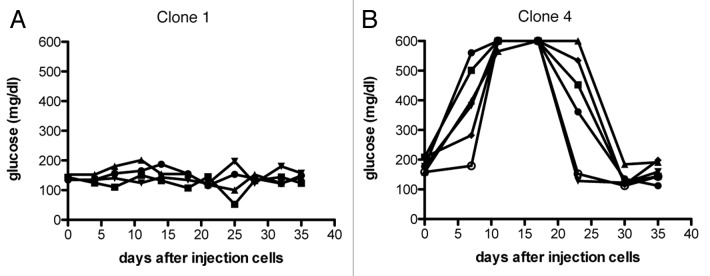
TCR affinity has been frequently reported to be important for the strength of antiviral and antitumor responses.17,18 To asses more specifically the effects of TCR affinity on CD8+ T cell function in the tumor environment we compared HA-specific Clone 1 (low-affinity) and Clone 4 (high-affinity) cells in tumor-bearing RIP-Tag2-HA mice. RIP-Tag2-HA mice received 2 × 105 Clone 1 or Clone 4 cells and were immunized with a vaccine containing cognate peptide and poly(I:C) injected s.c. in Incomplete Freund′s adjuvant. As shown previously,10,11 the activation of Clone 1 cells by a viral or peptide vaccine was not sufficient to induce tumor eradication (Fig. 1A). Clone 4 cells were superior to Clone 1 cells and did induce successful tumor eradication, although this was only short-term, as glucose levels went down again quickly (Fig. 1B).
Figure 1. Antitumor efficacy of high and low-affinity CD8+ T cells. (A and B) 8–9 week old RIP-Tag2-HA mice were immunized with cognate peptide and polyI:C in IFA and Clone 1 (A) or Clone 4 cells (B) (2 × 105) were injected i.v. Glucose levels in the blood were measured at the indicated time points, and each line represents one mouse. Data are representative of two independent experiments.
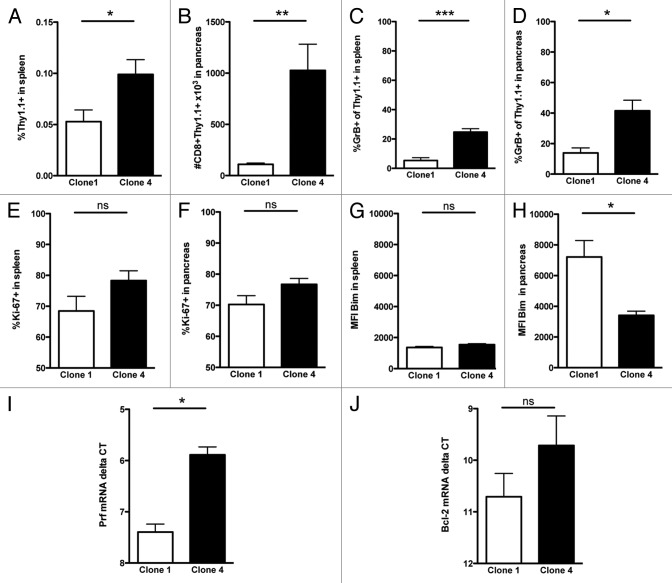
To determine the functional differences between Clone 1 and Clone 4 cells in the tumor environment we examined the expression of granzyme B and perforin (cytolytic function), Ki-67 (proliferative capacity) and molecules promoting apoptosis (Bim) and survival (Bcl-2). Seven days after immunization we observed a 2-fold increase in the percentage of Clone 4 cells in the spleen (Fig. 2A), but tumor eradication by Clone 4 cells was paralleled by an 8-fold to 13-fold increase in the number of Clone 4 cells within the pancreas, as compared with Clone 1 cells (Fig. 2B). The frequency of granzyme B+ Clone 4 cells in the spleen and the pancreas was significantly enhanced compared with Clone 1 cells (Figs. 2C and D). Additionally, perforin expression in the pancreas was analyzed by RT-PCR and was also significantly increased in Clone 4 cells (Fig. 2I). With respect to proliferative capacity, we observed a small increase in the percentage of Ki-67+ Clone 4 cells in the spleen, but both Clone 4 and Clone 1 cells showed approximately 70–80% Ki-67+ cells in the pancreas (Figs. 2E and F). The expression of the pro-apoptotic molecule Bim was significantly reduced and expression of Bcl-2 was increased in Clone 4 cells (Figs. 2H and J), suggesting that Clone 4 cells survive longer than Clone 1 cells in the tumor microenvironment.
Figure 2. High-affinity CD8+ T cells are superior to low-affinity CD8+ T cells in the tumor milieu. 8–9 week old RIP-Tag2-HA mice were immunized with peptide and polyI:C in IFA and Clone 1 or Clone 4 cells (3 × 104) were injected i.v. (A–H) Pancreata and spleens were analyzed at day 7 by flow cytometry to assess percentage of CD8+Thy1.1+ cells, the percentage of cells exhibiting granzyme B, percentage of dividing cells and the expression level of Bim. Data are cumulative from 2 independent experiments with 3 mice per group. I-J, Pancreas derived CD8+Thy1.1+ cells were analyzed by qRT-PCR for perforin and Bcl-2 mRNA levels. Delta Ct values were compared using actin as the normalization control. Data are from 1 experiment with 3 independent samples per group. *p < 0.05, **p < 0.005, ***p < 0.0005.
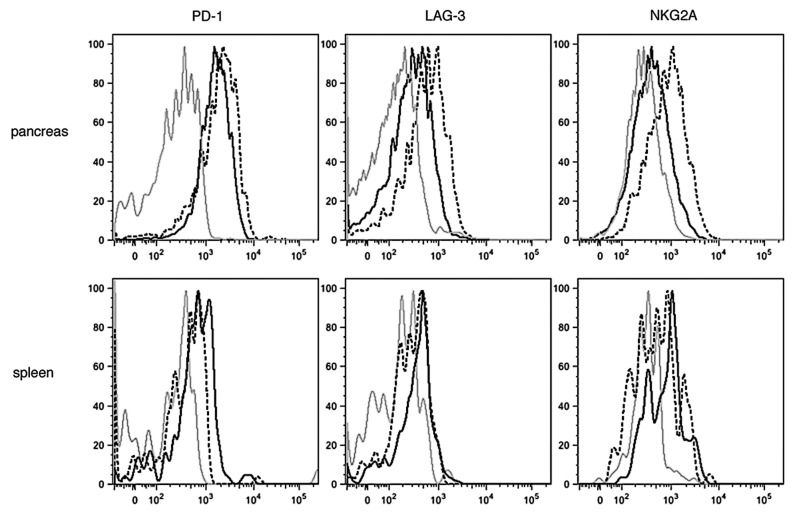
Upregulation of co-inhibitory receptor molecules on tumor infiltrated Clone 1 cells could contribute to the observed impaired effector function. Interestingly, while in the spleen the expression of lymphocyte activation gene-3 (LAG-3), programmed cell death-1 (PD-1) and the inhibitory natural killer cell receptor NKG2a molecules was either no different or even higher on Clone 4 than Clone 1 cells, all these molecules were more expressed by Clone 1 than by Clone 4 cells at day 7 in the tumor environment (Fig. 3). This suggests that high-avidity T cells may be less sensitive to tumor-induced immunosuppressor mechanisms. Taken together, these data suggest that stronger antitumor responses by high-avidity CD8+ T cells may result from enhanced effector functions and lower expression of inhibitory molecules in the tumor environment.
Figure 3. Expression of inhibitory molecules in the spleen and tumor microenvironment. 8–9 week old RIP-Tag2-HA mice were treated as described in Figure 2. Pancreata and spleens were analyzed at day 7 by flow cytometry to assess the expression of PD-1, LAG-3 and NKG2A on CD8+Thy1.1+ cells. Histograms are representative of 3 independent experiments with 3 mice per group. Isotype control = gray line, Clone 4 = black line, Clone 1 = dashed line. Mean MFI ± SD, PD-1: Clone 4 2428.0 ± 216.4, Clone 1 2784.0 ± 234.7. LAG-3: Clone 4 446.5 ± 98.2, Clone 1 664.0 ± 135.76. NKG2A: Clone 4 724.5 ± 151.0, Clone 1 1001.0 ± 186.9.
CD8+ T-cell infiltration into established tumors
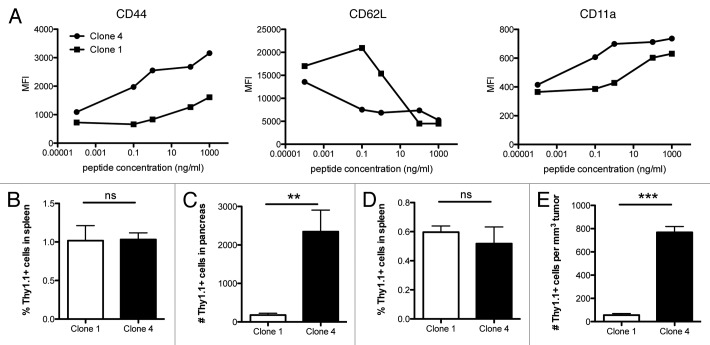
Increased accumulation of Clone 4 cells, compared with Clone 1 cells, in the tumor environment may also be due to differences in their capacity to infiltrate tissues. Activated T cells acquire the ability to infiltrate non-lymphoid sites in which the antigen is localized upon the expression of integrins and selectins.19,20 The stimulation of Clone 1 and Clone 4 cells with cognate peptide in vitro showed a differential expression of CD44, CD62L and CD11a at low peptide doses (Fig. 4A). No differences were observed in the expression levels of CD18 and CD49d (data not shown).
Figure 4. Infiltration of high- vs. low-affinity CD8+ T cells. (A) Purified CD8+ Clone 1 or Clone 4 cells (3 × 105) were incubated with HA110–119 peptide pulsed splenocytes for 15 h. Cells were analyzed for the expression of integrins and lectins by flow cytometry. Data shown are representative of 2 independent experiments. (B–E) Clone 1 or Clone 4 cells were activated in vitro with HA110–119 peptide and after 6 d cells (5 × 106) were injected into InsHA (B and C) or RIP-Tag2-HA mice (14 weeks old, D,E). Pancreata, tumors and spleens were isolated 40 h after injection. In panels B and C, cumulative data are shown from 5 experiments with 15 mice total. Data shown in D and E are representative of 2 independent experiments with 3 mice per group. **p < 0.005, ***p < 0.0005.
To test whether there is a difference in the ability of activated Clone 4 and Clone 1 cells to migrate into tissues, we used 2 different HA-expressing mouse models. First, we examined CD8+ T-cell infiltration into HA-expressing pancreatic tissues in non-tumor bearing mice. Second, we compared the infiltration of CD8+ T cells in HA-expressing insulinomas from the RIP-Tag2-HA mice. Whereas CD8+Thy1.1+ T-cell numbers in the spleen were not different, activated Clone 4 cells were superior in infiltrating pancreatic tissue and tumors (Figs. 4B–E).
The role of autocrine IL-2 in the tumor environment
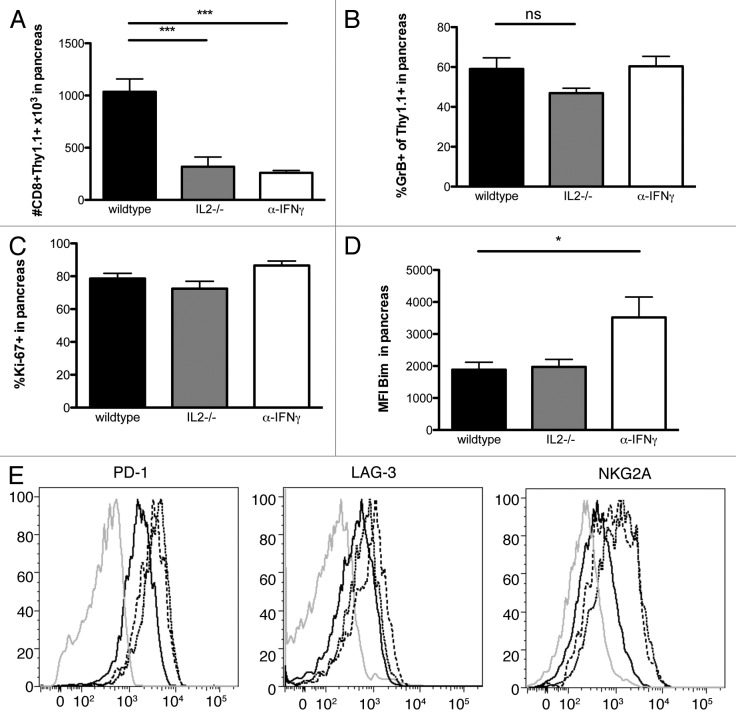
Previous experiments from our laboratory showed that tumor specific Clone 1 CD8+ T cells require interleukin-2 (IL-2) from CD4+ cells at the tumor site to promote cytotoxicity and proliferation, and that interferon γ (IFNγ) is needed to enhance recruitment.10 To examine whether the autocrine production of IL-2 by Clone 4 cells was able to enhance these functions, we analyzed Clone 4 Il2−/− cells 7 d after transfer and immunization of RIP-Tag2-HA. The accumulation of Clone 4 Il2−/− cells in the pancreas was significantly reduced (Fig. 5A). Similarly, blocking IFNγ resulted in the accumulation of far fewer Clone 4 cells. Comparison of Ki-67 expressed by Clone 4 cells indicated that autocrine IL-2 production by Clone 4 cells and IFNγ had no effect on cell division (Fig. 5C). Surprisingly, IL-2 deficiency did not significantly affect the expression of Bim or the ability of Clone 4 cells to express granzyme B in the pancreas (Figs. 5B and D) or the spleen (data not shown), suggesting autocrine IL-2 is crucial for cell expansion but not for the cytotoxic functions of Clone 4. Granzyme B expression by Clone 4 cells was also not affected when IFNγ was blocked. However, we did observe that blocking IFNγ results in an increase of the expression of Bim, suggesting a role for IFNγ in promoting the survival of intratumoral T cells (Fig. 5D).
Figure 5. Effects of autocrine IL-2 and IFNγ on function of Clone 4 cells in the tumor microenvironment. 8–9 week old RIP-Tag2-HA mice were immunized with peptide, polyI:C in IFA and Clone 4 or Clone 4 IL-2−/− (3x104) were injected i.v.. One group receiving Clone 4 cells was also injected with IFNγ neutralizing antibodies at days 4,5 and 6. (A–D) CD8+Thy1.1+ cells from pancreata were analyzed at day 7 by flow cytometry to assess the number of cells in the pancreas, the percentage of cells exhibiting granzyme B, the expression level of Bim and the percentage of dividing cells. Cumulative data are shown from 3 experiments with 2–3 mice per group per experiment. (E) Histograms are representative of 3 independent experiments with 3 mice per group. Isotype control = grey line, Clone 4 = black line, Clone 4 IL2-/- = dashed line, Clone 4 + anti-IFNγ = dotted line.
We also examined whether IL-2 and IFNγ have an effect on the expression of co-inhibitory molecules by Clone 4 cells. As compared with wild type cells, IL-2-deficient Clone 4 cells exhibited increased levels of NKG2a, PD-1 and LAG-3. IFNγ blockade affected NKG2a and PD-1, but only had a minimal effect on the expression of Lag-3 (Fig. 5E).
Clone 4 cells benefit from CD4 help
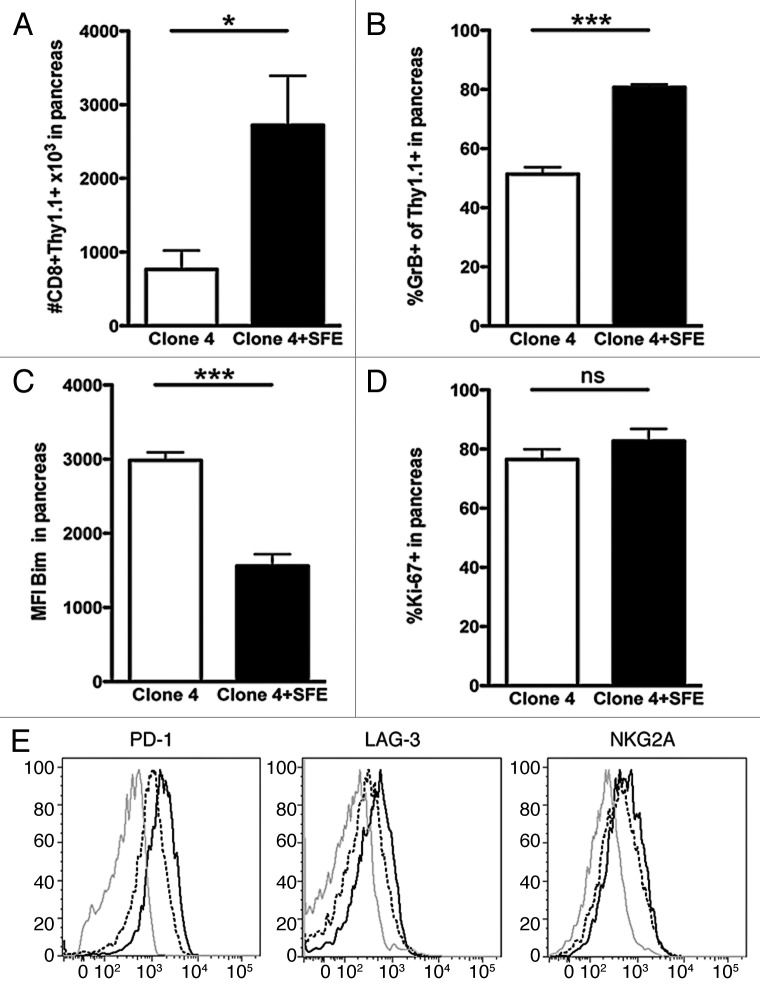
Considering the increased accumulation and function of high-affinity Clone 4 cells within the tumor, it was of interest to determine whether CD4 help would further increase tumor killing. Thus, we compared Clone 4 cells in the presence or absence of tumor-specific CD4+ SFE cells. RIP-Tag2-HA mice received either 3 × 104 Clone 4 cells alone or together with SFE cells and then were immunized as in Figure 1. Accumulation of Clone 4 cells in the pancreas at day 7 was greatly enhanced in the presence of SFE cells (Fig. 6A). High frequencies of granzyme B+ Clone 4 cells were observed also in the absence of SFE cells, but this was greatly increased in the presence of CD4 help (Fig. 6B). In addition, the presence of SFE cells significantly reduced the expression of Bim by Clone 4 cells (Fig. 6C). Most Clone 4 cells stained positive for Ki-67 in the absence of CD4 help and this was not further increased in the presence of SFE cells (Fig. 6D). Additional benefits of CD4 help were observed on the expression of inhibitory receptors by Clone 4 cells in the tumor microenvironment. Thus, the presence of SFE cells promoted a reduction in the expression of PD-1, LAG-3 and - to a lesser extent - of NKG2A (Fig. 6E).
Figure 6. High-affinity CD8+ T cells benefit from CD4 help in the tumor milieu. 8–9 week old RIP-Tag2-HA mice were immunized with peptide, polyI:C in IFA and 3 x 104 Clone 4 cells with or without 2 × 105 SFE cells were injected i.v.. (A–D) Pancreata were analyzed at day 7 by flow cytometry to assess the number of cells in the pancreas, the percentage of cells exhibiting granzyme B, the expression level of Bim and the percentage of dividing cells. Data are cumulative of 2 experiments with 2–3 mice per group and are representative of 4 independent experiments. (E) Pancreata were analyzed at day 7 by flow cytometry to assess the expression of PD-1, LAG-3 and NKG2A on CD8+Thy1.1+ cells. Histograms are representative of 3 independent experiments with 2–3 mice per group. Isotype control = gray line, Clone 4 = black line, Clone 4 + SFE = dashed line. Mean MFI ± SD, PD-1: Clone 4 2428.0 ± 216.4; Clone 4+SFE 1569.0 ± 72.1. LAG-3: Clone 4 446.5 ± 98.2; Clone 4 + SFE 353.0 ± 33.9. NKG2A: Clone 4 724.5 ± 151.0; Clone 4 + SFE 681.0 ± 84.4.
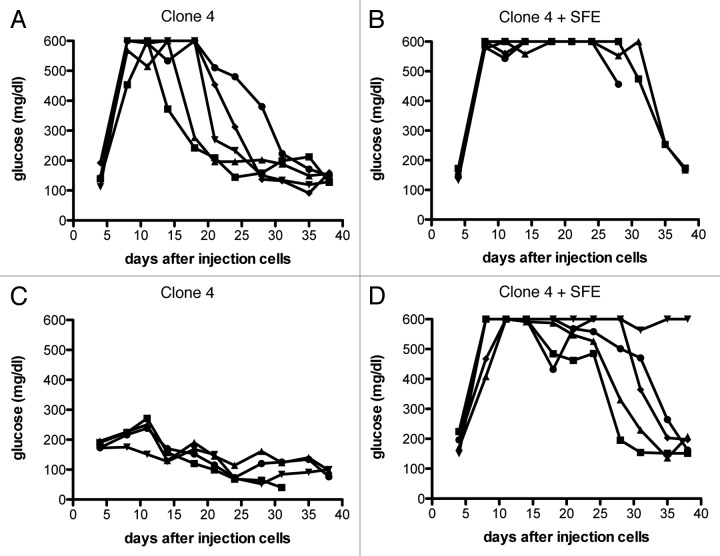
To examine whether SFE cells contribute to tumor eradication, we tested the antitumor efficacy of Clone 4 cells in RIP-Tag2-HA mice that received either Clone 4 alone or both Clone 4 and SFE cells. As shown in Figure 1A, 2 × 105 Clone 4 cells exhibit tumor-killing abilities, but tumors quickly start growing again, reflected by the rapid decrease in blood glucose levels (Fig. 7A). When tumor-bearing mice received both Clone 4 and SFE cells tumor growth was controlled significantly longer (Fig. 7B). When we tested the antitumor effect of lower numbers of Clone 4 cells, we observed an even stronger impact of the CD4 help. Thirty-thousand Clone 4 cells resulted in a minimal elevation of glucose levels, but in the presence of SFE cells long-term tumor eradication was observed in 5/5 mice (Fig. 7C and D).
Figure 7. Improvement of antitumor efficacy of high-affinity CD8+ T cells by the presence of tumor-specific CD4+ T cells. 8–9 week old RIP-Tag2-HA mice were immunized with peptide and polyI:C in IFA and Clone 4 cells (A–B: 2 × 105, C–D: 3 × 104) with or without 2 × 105 SFE cells were injected i.v.. Glucose levels in the blood were measured at the indicated time points, and each line represents one mouse. Data are representative of two independent experiments. (A) compared with (B): p < 0.05, (C) compared with (D): p < 0.0005.
Discussion
It has been previously reported that weak TCR-ligand interactions are sufficient to activate naïve T cells, induce proliferation and generate effector and memory cells.21 Consistent with these results, we found that, following immunization, low-affinity Clone 1 cells expand, demonstrate effector functions and produce cytokines.10,11 However, Clone 1 cells cannot effect tumor eradication. High-avidity CD8+ T cells exhibit improved antitumor efficacy (this manuscript and Refs 22,23), but previous studies have not eludicated the factors that are required for such tumor eradication. Our data show that increased tumor eradication by high-avidity CD8+ T cells is paralleled by increased accumulation of CD8+ T cells within the tumor microenvironment. Whereas the number of Clone 4 cells in the spleen was only doubled as compared with Clone 1 cells, far greater numbers of Clone 4 cells were found in the pancreas. This is unlikely to be explained by increased T cell proliferation as Clone 1 and Clone 4 cells showed little difference in the percentage of Ki-67+ cells. However, early infiltration into tissues was greatly increased in in vitro activated Clone 4 cells as compared with low-affinity Clone 1 cells (Fig. 4). This might in part be attributed to the expression levels of integrins and lectins such as CD62L and CD11a.
Work by others shows that homing of islet-specific CD8+ T cells is abrogated in mice that lack MHC class I expression and that CD8+ T cells directly recognize antigens expressed on pancreatic endothelial cells,24 which perhaps also contributes to increased infiltration by high-affinity T cells. It was further demonstrated that IFNγ affects the homing of CD8+ T cells into the pancreas.25 We and others have reported that cytokines and chemokines produced by T cells and other immune cells in the tumor microenvironment are important for the homing and recruitment of immune cells to the tumor site10 and here we report that blocking IFNγ inhibits the intratumoral accumulation of high-affinity Clone 4 cells.
The death of T cells could also influence accumulation. In fact, we observed lower expression of Bim and higher expression of Bcl-2 by Clone 4 cells, consistent with improved survival relative to Clone 1. In the absence of IFNγ, Clone 4 cells upregulated Bim expression. This finding was somewhat unexpected as work from others26-28 has shown that IFNγ is required for the death phase of CD4+ and CD8+ lymphocytes. These differences in Bim expression were only detectable in the tumor microenvironment and not in the spleen.
As previously reported,29 we found that autocrine IL-2 production by Clone 4 cells is not required for initial proliferation, as the percentage of IL-2-deficient Clone 4 cells in the spleen was not different from that of wild-type Clone 4 cells (data not shown). In contrast, the accumulation in the pancreas of such cells was greatly reduced, which perhaps may be explained by the finding that autocrine IL-2 is critical for CD8+ T cells to mount optimal secondary proliferative responses.30 However, we did not observe decreased expression of Ki-67 or an effect on the expression of the pro-apoptotic molecule Bim in IL-2-deficient Clone 4 cells in the pancreas. Thus, further research is needed to understand the role of autocrine IL-2 on the accumulation of CD8+ T cells in the tumor milieu. Production of IL-2 by CD4 helper cells was previously found to promote the induction of granzyme B via STAT5,31,32 but, surprisingly, our data show that the lack of autocrine IL-2 does not significantly affect the expression of granzyme B by Clone 4 cells in the tumor microenvironment.
T-cell function and accumulation in the tumor milieu can be regulated by co-inhibitory molecules. LAG-3 and PD-1 have been shown to negatively regulate tumor infiltrating CD8+ T cells.33-35 In addition, increased expression of NKG2A has been described to control CD8+ T cell cytotoxicity in the tumor tissue.36 Indeed, we found an increased expression of LAG-3, PD-1 and NKG2A on Clone 1 cells compared with Clone 4 cells only at the tumor site, correlating with a reduced expression of granzyme B and perforin by low-affinity CD8+ T cells. Furthermore, we demonstrate that the expression of LAG-3, PD-1 and NKG2A can be regulated by IFNγ, autocrine IL-2 and by the presence of tumor specific CD4+ T cells. This is consistent with our data showing the effects of cytokines and CD4+ T cells on CD8+ T cell functions.
We have previously shown that paracrine IL-2 derived from tumor-specific CD4+ cells is crucial for the functions of low-avidity Clone 1 cells in the pancreas.10 As discussed above, in the absence of CD4+ cells, high-affinity Clone 4 CD8+ cells are superior to Clone 1 cells in that they exhibit increased accumulation, survival (reduced Bim levels and increased Bcl-2 expression) and cytolytic functions (increased levels of granzyme B and perforin). However, the presence of tumor specific CD4+ T cells also appears to be important for Clone 4 cells, as it further increases their functions. This is most likely due to IL-2 provided by CD4+ cells, as shown previously for Clone 1 cells.10 Thus, even high-affinity CD8+ T cells that demonstrate good effector functions can benefit from CD4 help.
Most adoptive immunotherapy protocols have focused on the transfer of CD8+ tumor-specific T cells. The use of CD4+ T cells is limited due to a lack of well-characterized tumor antigens presented by class II MHC, as the majority of tumor cells are class II negative. However, several laboratories have found ways to overcome these limitations and it has been shown that CD4+ T cells transduced with class I-restricted TCRs can provide antigen-specific helper functions.37-39 Our studies highlight the functional advantages of high-affinity CD8+ T cells and the additional effects of CD4 help, emphasizing the importance of including CD4 help in adoptive cell transfer immunotherapy, even when high-affinity TCRs are expressed by tumor-specific CD8+ T cells.
Material and Methods
Mice
B10.D2 rat insulin promoter (RIP)-Tag2-HA mice have been previously described40 and were used at 8 to 9 weeks of age. B10.D2 Clone 1, Clone 4 and Clone 4 Il2−/− TCR transgenic mice which express a TCR specific for HA518–526 (IYSTVASSL) in the context of HA-2Kd, and SFE TCR transgenic mice, which express TCR that recognizes HA110–119 (SFERFEIFPK) in the context of I-Ed, were bred with the congenic markers Thy1.1 and CD45.1, respectively. All mice were bred in our facility. All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute.
Adoptive transfer, immunization and analysis of T-cell responses
Lymph nodes were collected and purified by magnetic cell sorting using CD8+/CD4+ T-cell enrichment sets (BD Bioscience). Purified lymphocytes (2 × 105 or 3 × 104) were injected into RIP-Tag2-HA mice i.v. Recipient mice were immunized with 10 µg HA518–526-Kd peptide, 50 µg SFE110–119 and 200 µg poly(inosinic-cytidylic acid) (polyI:C, EMD Biosciences in incomplete Freund’s adjuvant s.c. in the right flank. Neutralizing antibodies against IFNγ (500 µg/mouse, Clone R4–6A2 BioXcell) were injected on day 4,5 and 6 after the injection of T cells. Glucose levels in the blood were measured as described before.11
Lymphocytes were purified from the pancreas for in vitro analysis as described previously.10 Prior to isolation of insulinoma’s from 14 week old RIP-Tag2-HA mice, mice were perfused with HBSS. Tumors were isolated and lymphocytes were purified as described above for the pancreas. Cells were stained for fluorescence-activated cell sorting (FACS) analysis in HBSS containing 1% FCS and 2 mmol/L EDTA. Antibodies for FACS were used from eBioscience, BD Biosciences and Alexis Biochemicals (BimS/EL/L).
Quantitative PCR
Relative expression levels of Perforin and Bcl-2 in Clone 1 and Clone 4 cells were measured by Quantitative real-time PCR (qPCR). Clone 1 and Clone 4 cells were isolated from the pancreas as described previously10 and subsequently stained with PE anti-mouse CD8 and APC anti-mouse Thy1.1. Cell sorting was performed on a FACS Aria (BD Biosciences). RNA was extracted in TRIzol (Invitrogen) and total RNA was used to make cDNA with High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). Primer sets were designed for perforin (Pfr-F 5′-TAGCCAATTTTGCAGCTGAG-3′ and Prf-R 5′-GGTTTTTGTACCAGGCGAAA-3′) and Bcl-2 (Bcl-2-F 5′-GGAGAATGGATACGGCAGAA-3′ and Bcl-2-R 5′-TTCCCAGATCTGTCCTGTCA-3′) using ensemble genome browser and Primer3 input41 and primers were purchased from Valuegene Inc. (San Diego, CA). Reactions were performed in duplicate and we used actin as the internal control. The quantitative data analysis was completed using the SYBR Green PCR Master Mix and a 7900HT FAST Real-time PCR System (Applied Biosystems).
In vitro activation of CD8+ T cells and in vivo tissue/tumor infiltration
To generate effector CD8+ T cells, Clone 1 and Clone 4 cells were activated in vitro as described previously.10 Activated Clone 1 and Clone 4 cells (5 × 106) were injected into InsHA or 14 weeks old RIP-Tag2-HA mice. Pancreata and spleens were isolated after 40 h, and the number of CD8+Thy1.1+ cells was analyzed by FACS. To examine the expression levels of integrins and lectins after activation, 3 × 105 purified CD8+ Clone 1 and Clone 4 cells were incubated for 15 h with 2 × 106 splenocytes pulsed with different concentrations of HA518–526-Kd peptide. Cells were analyzed by FACS for the expression of CD44, CD62L and CD11a.
Statistical analysis
Differences between tumor growth curves were determined by a Mann-Whitney test. Differences between means were determined by a unpaired Student’s t-tests. Data are presented as means ± SEM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21285
References
- 1.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–35. doi: 10.1034/j.1600-065X.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller JF, Kurts C, Allison J, Kosaka H, Carbone F, Heath WR. Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens. Immunol Rev. 1998;165:267–77. doi: 10.1111/j.1600-065X.1998.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40. doi: 10.1016/S1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 5.Nugent CT, Morgan DJ, Biggs JA, Ko A, Pilip IM, Pamer EG, et al. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J Immunol. 2000;164:191–200. doi: 10.4049/jimmunol.164.1.191. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 7.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–6. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–77. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180:3122–31. doi: 10.4049/jimmunol.180.5.3122. [DOI] [PubMed] [Google Scholar]
- 12.Merhavi-Shoham E, Haga-Friedman A, Cohen CJ. Genetically modulating T-cell function to target cancer. Semin Cancer Biol. 2012;22:14–22. doi: 10.1016/j.semcancer.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Jorritsma A, Gomez-Eerland R, Dokter M, van de Kasteele W, Zoet YM, Doxiadis II, et al. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110:3564–72. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyman MA, Nugent CT, Marquardt KL, Biggs JA, Pamer EG, Sherman LA. The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice. J Immunol. 2005;174:2563–72. doi: 10.4049/jimmunol.174.5.2563. [DOI] [PubMed] [Google Scholar]
- 17.Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–6. [PubMed] [Google Scholar]
- 18.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 20.Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–89. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- 21.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–4. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 23.Bullock TN, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J Immunol. 2001;167:5824–31. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 24.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–56. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savinov AY, Wong FS, Chervonsky AV. IFN-gamma affects homing of diabetogenic T cells. J Immunol. 2001;167:6637–43. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 26.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–8. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 28.Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–22. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Souza WN, Lefrançois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–35. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 30.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12:908–13. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci U S A. 2008;105:16683–8. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. 2006;176:4834–42. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheu BC, Chiou SH, Lin HH, Chow SN, Huang SC, Ho HN, et al. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Res. 2005;65:2921–9. doi: 10.1158/0008-5472.CAN-04-2108. [DOI] [PubMed] [Google Scholar]
- 37.Kessels HW, Schepers K, van den Boom MD, Topham DJ, Schumacher TN. Generation of T cell help through a MHC class I-restricted TCR. J Immunol. 2006;177:976–82. doi: 10.4049/jimmunol.177.2.976. [DOI] [PubMed] [Google Scholar]
- 38.Morris EC, Tsallios A, Bendle GM, Xue SA, Stauss HJ. A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection. Proc Natl Acad Sci U S A. 2005;102:7934–9. doi: 10.1073/pnas.0500357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005;22:117–29. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172:6558–67. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 41.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]