Abstract
Depletion of tumor associated macrophages and inhibition of tumor angiogenesis have been invoked as the principle mechanisms underlying the antitumor activity of liposomal clodronate (LC). However, previous studies have not examined the effects of LC on systemic antitumor immunity. Here, we used mouse tumor models to elucidate the role of T and NK cells in the antitumor activity elicited by the systemic administration of LC. Strikingly, we found that the antitumor activity of LC is completely abolished in immunodeficient Rag1−/− mice. Moreover, both Cd4−/− and Cd8−/− mice as well as mice depleted of NK cells manifested a significant impaired ability to control tumor growth following LC administration. Treatment with LC did not result in an overall increase in T- or NK-cell numbers in tumors or lymphoid organs, nor was tumor infiltration with T or NK cells altered. However, T and NK cells isolated from the spleen of LC-treated mice exhibited significant increased tumor-specific secretion of interferon γ and interleukin 17 and greater cytolytic activity. We concluded that the antitumor effects of LC are largely dependent on the generation of systemic T-cell and NK- cell activity, most likely owing to the depletion of immune suppressive myeloid cell populations in lymphoid tissues. These findings suggest that the systemic administration of LC may constitute an effective means for non-specifically augmenting the antitumor activity of T and NK cells.
Keywords: cancer, cytokines, innate immunity, liposomal clodronate, macrophages
Introduction
Liposomal clodronate (LC) has been used for years as a research tool to rapidly and efficiently deplete splenic and non-splenic macrophages.1-5 The uptake of LC by myeloid cells results in the delivery of clodronate (dichloromethylene diphosphonate) into the cytoplasm, where it competes for ATP binding and induces cell death via apoptosis.2,3,6-8
Several groups have reported that repeated LC administration can generate significant antitumor activity.5,7-18 For example, the i.p. administration of LC has been shown to significantly inhibit the growth of several different tumor types, in different murine strains.5,7-10,12,18 These studies have generally attributed the antitumor activity of LC to its ability to deplete tumor-associated macrophages (TAMs) and to inhibit tumor angiogenesis.5,7,9,13,15,16,19-21
LC also unspecifically depletes phagocytic myeloid cells other than macrophages, including myeloid cells in the blood and spleen.11,22,23 Thus, the i.v. administration of LC has been shown to deplete monocytes in the blood and bone marrow,22 while i.p. LC reportedly promote the depletion of CD11b+Gr-1+ myeloid derived suppressor cells (MDSCs).11 MDSCs, which consist of immature monocytes and granulocytes, play an important role in regulating inflammatory responses and in inhibiting antitumor immunity.24-31 MDSCs significantly accumulate in the blood, liver and spleen of tumor-bearing mice, and are increased in the blood of cancer patients.26,32-36 MDSCs exert suppressive effects on T-cell functional responses, via a variety of mechanisms (reviewed in Refs37-39).
Given these premises, we wondered whether LC might control tumor growth via mechanisms that depend (at least in part) on systemic T-cell and NK-cell immunity, rather than solely by depleting TAMS and inhibiting angiogenesis. Therefore, we investigated the overall contribution of T cells and NK cells on the antitumor effects of LC-based therapy. Our results suggest a critical and previously unreported role for the systemic generation of both T cell- and NK cell-mediated antitumor activity in the therapeutic efficacy of LC. These findings have important implications for understanding how the unspecific depletion of phagocytic cells can generate specific antitumor immunity.
Results
LC treatment suppresses growth of tumors in mice and enhances overall survival
We first assessed the effects of LC treatment on the growth of tumors in mice. The LC that we used here differed from that used previously in that liposomes contained a mannose receptor-targeting moiety that we found to increase macrophage uptake and in vivo killing (data not shown and ref. 40). In addition, LC was administered by the i.v. route with a once weekly treatment schedule, rather than by more frequent i.p. administration as in earlier studies. The i.v. route was selected because it provided superior antitumor activity as compared with the i.p. route (Figs. 1A–C).
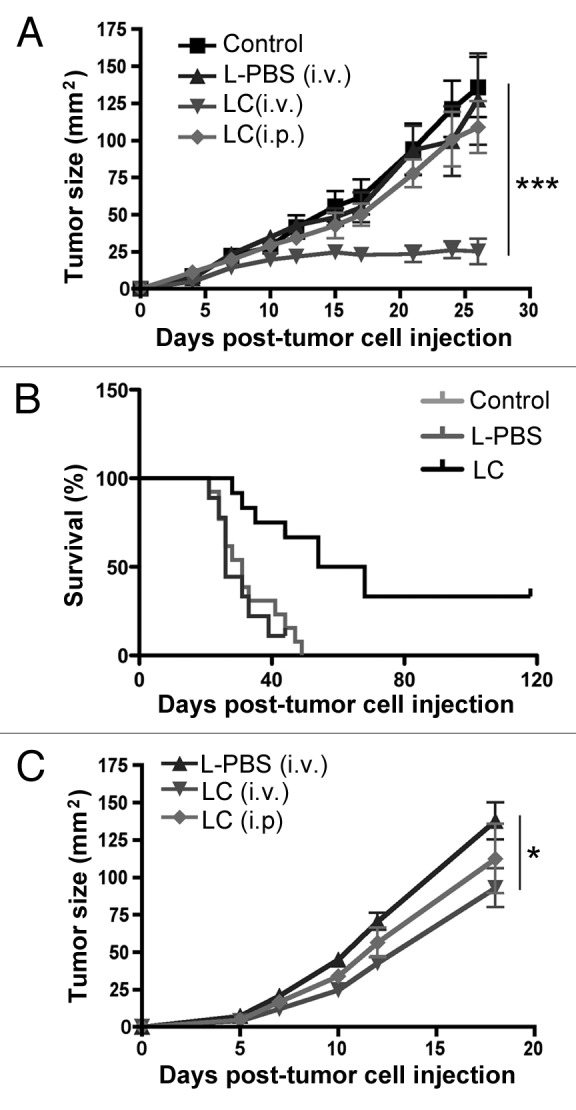
Figure 1. Effects of LC treatment on tumor growth and survival. C57BL/6 mice (n = 5 per group) were injected with 2.5 X 105 MCA205 cells s.c. and 3 d later, mice were treated once weekly with liposomal clodronate (LC) or a control liposomal preparation (L-PBS).. In (A), tumor growth was measured every 2–4 d and growth curves (mean tumor size (mm2), ± SEM) were generated. Tumor growth was significantly reduced (***p < 0.001) in the LC-treated mice, compared with control or L-PBS treated mice, as assessed by ANOVA. Similar results were obtained in 3 additional experiments. In (B), the effects of LC treatment on survival times were assessed. Survival times were significantly longer (p = 0.0032) in LC-treated mice, compared with L-PBS or control mice, as assessed by Kaplan-Meier curve and Logrank test. In (C), tumor growth curves were generated for s.c. injected CT-26 cells treated with either LC (i.v.), LC (i.p.) or L-PBS (i.v.). Tumor growth was significantly reduced (**p < 0.01) in LC-treated mice compared with L-PBS treated mice, as assessed by ANOVA.
The antitumor effects of LC were assessed in C57BL/6 mice with day 3 s.c. implanted MCA205 fibrosarcomas. Treatment groups included untreated control mice, mice treated with LC (200 μL i.v. once weekly), and mice treated with control liposomes prepared with PBS instead of clodronate (L-PBS, 200 μL i.v. once weekly). Tumor growth was measured in two dimensions every 2–3 d and data were plotted as mean tumor area in mm2 (Fig. 1A). We found that the weekly administration of LC significantly inhibits tumor growth, and in some cases even completely suppress it, in the MCA205 model (data not shown). Importantly, the administration of control liposomes did not elicit antitumor activity (Fig. 1A). Moreover, LC significantly increased (p = 0.0032) the overall survival of fibrosarcoma-bearing mice (Fig. 1B). Of note, the treatment of mice with free clodronate, at a dose equivalent to that estimated to be incorporated within 200 μL of LC, did not elicit antitumor activity (data not shown). LC also led to a significant decrease in the growth of CT26 colon carcinomas growing in syngenic BALB/c mice (Fig. 1C). Interestingly, in this model, i.p. LC had a modest effect on tumor growth, whereas no effect was seen with i.p. in the MCA205 tumor model (Fig. 1A).
Antitumor activity elicited by LC is T cell-dependent
We hypothesized that the antitumor activity of LC treatment might be mediated by systemic antitumor immunity, rather than by the sole depletion of TAMs. This hypothesis was based on the fact that the systemic administration of LC efficiently depletes not just TAMs, but also other immuno suppressive populations of myeloid cell populations in the spleen, bone marrow and bloodstream.
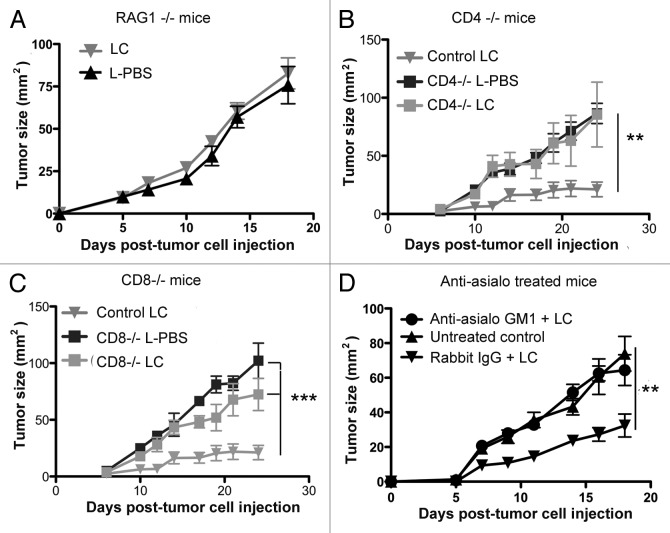
To test this hypothesis, we first investigated whether the antitumor activity elicited by LC treatment requires T cells. Thus, the effects of LC treatment on tumor growth were assessed in Rag1−/− (B6.129S7-Rag1tm1Mom/J) mice. We found that the effects of LC treatment were completely abrogated in Rag1−/− mice as compared with wild type C57Bl/6 animals (Fig. 2A). These results were important because they suggested that the antitumor activity of LC relies on T cells, and therefore is unlikely to depend solely on local TAM depletion and angiogenesis inhibition
Figure 2. Effects of LC treatment in T cell and NK cell deficient mice. (A) MCA205 tumors were established in Rag1−/− mice (n = 5 per group) and half of the mice were treated with LC. Tumor growth was measured and growth curves (mean tumor size (mm2), ± SEM) were generated. Tumor growth was not significantly different (p = 0.95) in LC-treated Rag1−/− mice compared with L-PBS treated Rag1−/− mice. In (B), Cd4−/− mice with MCA205 tumors were treated with LC or L-PBS and tumor growth rates were compared with wild type mice treated similarly. The tumor growth rates in Cd4−/− mice treated with LC or L-PBS were significantly different (**p < 0.01) from wild type mice treated with LC. In (C), tumor growth rates in wild type mice treated with LC were significantly decreased as compared with Cd8−/− mice treated with LC or L-PBS (***p < 0.001). In (D) MCA205 tumor-bearing mice (n = 5/group) were treated weekly with either: 1) LC plus anti-asialo GM1 antibody administered 24h prior to the LC treatment once weekly, 2) LC and an irrelevant control antibody weekly, with the control antibody administered 24 h prior to the LC treatment, or 3) received no treatment. Tumor growth rates in mice treated with asialo-GM1 antibody and LC were not significantly different from untreated control mice, whereas mice treated with irrelevant rabbit IgG and LC had significantly reduced tumor growth rates compared with control and anti-asialo GM1 treated mice (**p < 0.01). Similar results were obtained in one additional experiment. Tumor growth rates were compared using repeated-measures ANOVA followed by Bonferroni post-test.
Next, experiments were conducted to determine whether LC antitumor activity is primarily dependent on either CD4+ or CD8+ T cells. When Cd4−/− mice with day 3 tumors were treated with LC, there was a significant loss of antitumor effect as compared with LC-treated wild type animals (Fig. 2B). Similarly, when Cd8−/− mice were treated with LC, they generated significantly less antitumor activity than wild type animals (Fig. 2C). Therefore, we concluded that the antitumor activity of LC is dependent on both CD4+ and CD8+ T cells, consistent with the idea that LC treatment leads to spontaneous activation (or re-activation) of T-cell antitumor activity.
LC antitumor activity also depends on NK cells
It has previously been reported that Rag1−/− mice have a defect in NK cell development.41 In addition, NK cells are known to be inhibited by MDSCs, implying that MDSC depletion might enhance NK cell-dependent antitumor activity.32 To address the role of NK cells in the antitumor activity of LC, experiments were conducted in mice that had been depleted of NK cells by anti-asialo GM1 antibodies, resulting in approximately 80% depletion of splenic NK cells (data not shown).42,43 In NK-depleted mice, the antitumor effects of LC were significantly diminished, compared with LC-treated control animals or animals treated with LC and an irrelevant control antibody (Fig. 2D). These results suggest an important role for NK cells in the antitumor activity of LC.
LC treatment induces spontaneous tumor cytolytic activity and IFNγ and IL-17 production
Since it is known that immunosuppressive cell populations such as TAMs and MDSCs operate by inhibiting effector immune cells, we next examined T-cell and NK-cell functions following LC administration. To this aim, spleen cells were collected from control and LC-treated tumor-bearing mice and assayed for spontaneous tumor cytotoxicity, using a CSFE flow-based CTL killing assay developed by Jedema et al.47 We found that spontaneous tumor killing activity was significantly higher in spleen cells obtained from LC-treated mice than in cells of the same type obtained from untreated tumor-bearing mice (Fig. 3A). Thus, the antitumor cytotoxic activity of spleen cells was increased following LC treatment.
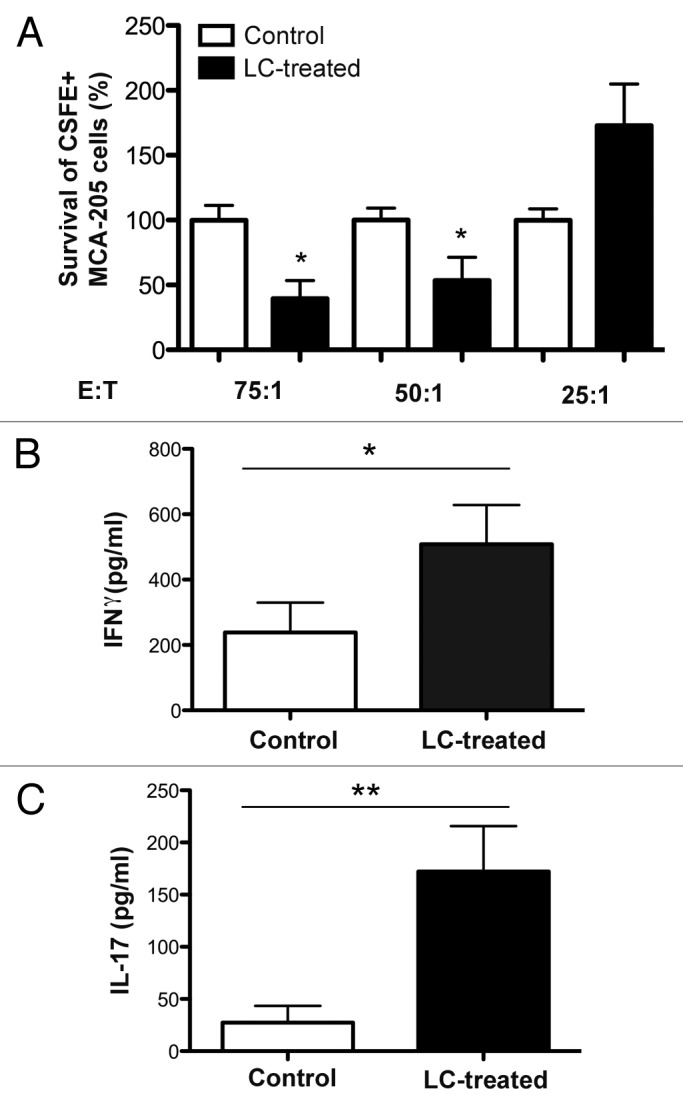
Figure 3. LC treatment induces increased CTL activity against MCA205 tumor cells and ex vivo and LC treatment induces tumor-specific IFNγ and IL-17 production by spleen cells. (A) 5 X 104 CSFE-labeled MCA205 cells (“T,” target) were placed in culture with varying concentrations of freshly isolated spleen cells (“E,” effector) and incubated for 72 h at 37C. Viable CSFE+ MCA205 cells were quantitated using flow cytometry. Viable cell numbers were normalized to those labeled tumor cells quantitated in the wells with control, untreated spleen cells from MCA205 tumor-bearing mice. A significant decrease in viable tumor cells was observed with E:T ratios of 75:1 (*p = 0.02) and 50:1 (*p = 0.04), but not at 25:1 (Mann-Whitney U test). n = 5 mice/group and similar results were seen in two additional experiments. (B and C) Spleen cells were placed in culture for 72hr with MCA205 tumor cells. Supernatants were collected and analyzed by IFNγ (B) and IL-17 (C) ELISA kits. A significant increase (*p = 0.05, Mann-Whitney U test) in IFNγ production and in IL-17 production (**p = 0.03, Mann-Whitney U test) was observed in cultures where the spleen cells were obtained from LC-treated MCA205 tumor-bearing mice as compared with control mice. n = 5 mice/group and similar results were seen in two additional experiments.
The effects of LC on tumor-specific cytokine production were also assessed. Spleen cells from control or LC-treated tumor-bearing mice were co-cultured with live MCA205 tumor cells for 72h at 37°C (without the addition of cytokines or mitogens), after which supernatants were harvested and analyzed for the concentration of tumor necrosis factor α (TNFα), interleukin (IL)-10), interferon γ (IFNγ) and IL-17. Spleen cells isolated from mice treated with LC produced significantly more IFNγ (Fig. 3B) and IL-17 (Fig. 3C) than spleen cells from untreated tumor-bearing mice. In contrast, the production of IL-10 or TNFα by splenic cells did not differ in LC-treated mice and untreated animals (data not shown). Thus, spleen cells from LC-treated animals produced significantly higher amounts of cytokines with antitumor activity (i.e., IFNγ and IL-17) than spleen cells from untreated animals. In order to verify that our results were due to increased cell functionality and not just to changes in cell numbers, we examined tumor tissues for the percentage of infiltrating NK and T cells. We found no significant differences in the frequency of tumor-infiltrating NK, CD4+, CD8+ and regulatory T cells (data not shown). These results indicate that spontaneous T and NK cell activity is increased following LC administration.
Liposomes uptake by TAMs and tumor cells is very inefficient
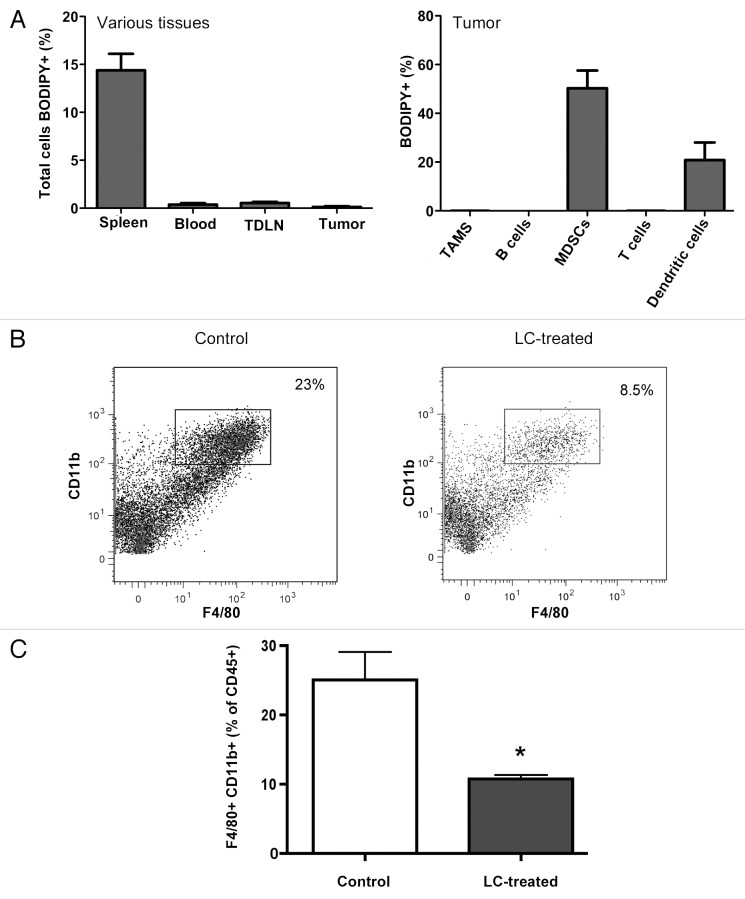
Previous studies on LC-based anticancer therapy suggest that the depletion of TAMs in situ is the primary mechanism responsible for the inhibition of tumor growth.5,7,9,13,15,16,19-21 However, we postulated that the depletion of TAMs by LC is unlikely to represent the primary mechanism of action, as following the i.v. administration of LC, liposome uptake by tumor tissues is very inefficient.6,48 To address this point directly, we administered PBS-containing liposomes (to avoid target cell killing) labeled with the fluorescent dye BODIPY into tumor-bearing mice and assessed the uptake of liposomes by both tumor cells and TAMs. C57BL/6 mice with well-established MCA205 fibrosarcomas were injected i.v. with BODIPY-labeled liposomes and 6h later mice were euthanized, tissues were removed and cells were isolated for cytofluorometric analysis.
We found that very few cells, including TAMs, contained BODIPY+ liposomes (Fig. 4A). In contrast, splenic macrophages contained large numbers of BODIPY+ liposomes (Fig. 4A). Significant numbers of BODIPY+ liposomes were also found in monocytes within tumor tissues (Fig. 4A). Therefore, we conclude that the uptake of liposomes by tumor cells and TAMs is inefficient, as compared with the much more efficient uptake by splenic macrophages and blood monocytes. Of note, previous in vitro experiments have shown that MCA205 tumor cells are non-phagocytic and resistant to killing by LC.6
Figure 4. LC is not taken up by tumor cells, but plays a role in depletion of macrophages in the tumor. (A) Mice (n = 5 per group) with established MCA205 tumors were treated with BODIPY-labeled L-PBS and trafficking of the liposomes to various tissues was assessed. Very little uptake of the liposomes was observed in TAMs. (B and C) Mice (n = 5 per group) with established MCA205 tumors were untreated (control) or were treated with LC twice over 2 weeks and then tumor tissues were collected and processed for flow cytometry. In (B), representative flow plots of control vs LC treated tumor tissue. (C), The percentages of CD11b+F4/80+ TAMs were assessed by flow cytometry. In mice treated with LC, there were significantly fewer TAMs (*p < 0.05, Mann-Whitney U test) than in control mice. Similar results were seen in two additional experiments.
Despite the fact that TAMs did not appear to take up liposomes efficiently, the i.v. administration of LC produced significant depletion of TAMs in the tumor tissue (Figs. 4B and C). Altogether, our results suggest that the generation of NK- and T-cell activity by LC is unlikely to be the consequence of TAM depletion. More likely, TAM depletion may be a consequence of the repeated depletion of blood monocytes. These findings also indicate that the NK and T-cell antitumor activity triggered by LC is mediated by the depletion of a phagocytic myeloid cell population other than TAMs.
Effects of LC administration on MDSCs
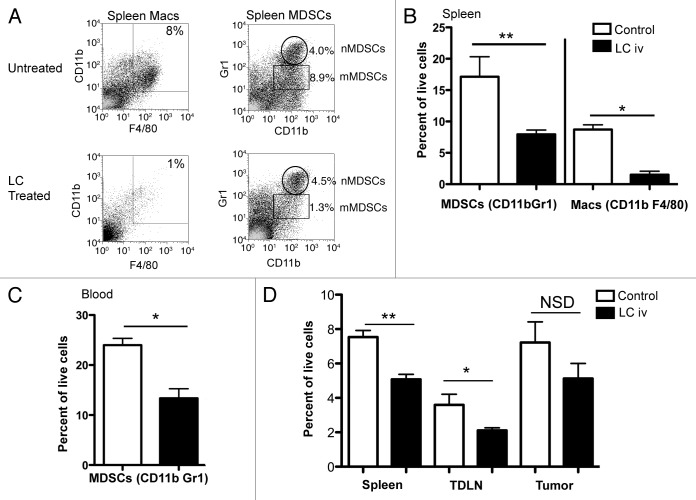
When then determined whether the administration of LC might lead to the depletion of MDSCs, which have been shown previously to potently suppress T-cell35,37,38,49-51 and NK-cell32 functions. Indeed, we found that the i.v. administration of LC induces a significant depletion of CD11b+Gr-1+ MDSCs in the blood and spleen of tumor-bearing mice (Fig. 5). The percentage of total MDSCs in the spleen was reduced by approximately 60% following LC treatment (Fig. 5B), with preferential depletion of monocytic MDSCs (“mMDSCs”) rather than neutrophilic (“nMDSCs”). The percentage of mMDSCs in the blood was reduced by approximately 50% (Fig. 5C). Interestingly, LC treatment had little effects on MDSCs in lymph nodes (data not shown). These findings suggest that LC efficiently depletes immunosuppressive MDSCs in the blood and spleen.
Figure 5. Effects of LC treatment on systemic phagocytic cell populations. Tumor-bearing C57BL/6 mice (n = 5 per group) were treated with three weekly i.v. injections of LC, starting on day 3, and blood and spleen tissues were collected on days 25 and 26 post-tumor cell injection, respectively, and analyzed by flow cytometry, as described in Methods. In (A), Macrophages (“Macs”) and MDSCs in spleen were identified via flow cytometry as CD11b+/F4/80+ (left panel) and either CD11b+/Gr-1hi (neutrophilic (nMDSCs)) or CD11b+/Gr-1lo (monocytic (mMDSCs)) MDSC cells (right panel), respectively. LC treatment induced a significant depletion of total CD11b+Gr-1+ MDSCs and macs in the (B) spleen (**p < 0.01, *p < 0.05) and in the (C) blood (*p < 0.05) as assessed by non-parametric U test. Similar results were obtained in two additional experiments. In (D), total DC populations were examined in LC and control treated mice on day 24 post tumor cell injection. A significant decrease in DCs was seen in the spleen (**p = 0.02) and tumor-draining lymph nodes (*p = 0.05) of the mice, while there was no significant decrease in DCs in the tumor. Similar results were observed in three additional experiments.
Effects of LC treatment on dendritic cells
Finally, we assessed the effects of LC on dendritic cells (DCs) in the tumor, spleen and tumor-draining lymph nodes (TDLNs). As shown in Figure 5D, DCs were significantly reduced in the spleen (p = 0.02) and TDLNs (p = 0.05) following LC administration, while the amount of intratumoral DCs was not significantly altered. Thus, despite the fact that LC administration depletes DCs in secondary lymphoid tissues, LC-treated mice are able to mount an efficient antitumor immune response, perhaps due to the relative sparing of intratumoral DCs.
Discussion
Here, we report that the antitumor activity elicited by the systemic administration of LC is dependent on NK cells, CD4+ and CD8+ T cells (Fig. 2). Notably, the antitumor activity elicited by LC administration was almost completely abrogated in T cell-deficient and NK cell-depleted mice, in spite of concurrent TAM depletion. Thus, our findings suggest that the antitumor effects of i.v. LC are unlikely to be mediated by angiogenesis inhibition following TAM depletion, as other studies have suggested.5,7,9,13,15,16,19-21 While we cannot rule out a contribution for LC-induced TAM depletion to the overall induction of T- and NK-cell antitumor activity, we postulate that the main immunosuppressive cells depleted by LC are MDSCs in the blood and spleen. It was interesting to observe that three different cell populations (CD4+ T cells, CD8+ T cells and NK cells) are required for the antitumor effects of systemic LC. This suggests a significant interaction between these cell populations in generating antitumor immunity.
Our findings suggest that TAM depletion following i.v. LC administration is not mediated by a direct cytotoxic effect, but rather occurs as a result of repeated monocyte depletion that, over time, leads to the reduction of newly emigrating TAM precursor cells. Indeed, we and others have found that the i.v. administration of LC elicits a marked depletion of monocytes in the blood and bone marrow (Fig. 5).52,53 A recent report also demonstrates that monocytes develop into TAMs under conditions of hypoxia.54
Expanded populations of MDSCs have been described in tumor-bearing mice and humans and these cells appear to play a key role in suppressing adaptive immunity to tumors.26-31,55,56 We found that the treatment of tumor-bearing mice with LC induces significant depletion of CD11b+Gr-1+ MDSCs (Fig. 5). Administration of LC primarily depleted mMDSCs as opposed to nMDSCs. The preferential depletion of mMDSC is important given that mMDSCs have been shown to be more immunosuppressive than neutrophilic MDSCs.57 Augier et al.58 have shown that inflammatory Gr-1+ monocytes not only support tumor growth, but also provide precursors to tolerogenic DCs involved in IL-10 production and regulatory T-cell stimulation.
One drawback to the use of an unspecific cell-depleting agent such as LC is that many different types of phagocytic cells are depleted, including DCs.5 Depletion of DCs by a drug like LC potentially inhibits the development of robust tumor-immunity. However, although there was a significant decrease in DCs in the spleen and TDLNs following LC administration (Fig. 5D), we observed that weekly treatments with LC did not lead to overt immune suppression, but instead stimulated robust T cell-mediated antitumor immunity. Thus, the net positive effects of systemic LC administration indicate that the elimination of immunosuppressive MDSCs and TAMs outweighs the potential suppressive effects of DC depletion.
The primary effect of LC was to increase T-cell and NK-cell functionality, as reflected by enhanced cytokine secretion and cytotoxicity responses. The fact that LC treatment increased the production of IL-17 (Fig. 3C) suggests a novel pathway by which MDSCs may potentially inhibit antitumor immune responses. IL-17 has been demonstrated to play a role in the development of antitumor immunity by reducing large tumor burdens in an IFNγ-dependent manner59 and by other T cell-dependent mechanisms.60 Interestingly, some LC-treated MCA205 tumor-bearing mice underwent complete regression and become resistant to a subsequent tumor challenge with MCA205 tumor cells (data not shown). This observation suggests that restoration of T- and NK-cell functions upon myeloid cell depletion may also lead to the development of robust memory T-cell responses.
In our model, we observed enhanced immune activity against unmodified and relatively non-immunogenic tumor cells. The tumor cells used in this study (MCA205 and CT26 cells) are resistant to in vitro killing by LC (unpublished observations) and thus the antitumor effects observed following LC administration appear to reflect the activation of an antitumor immune response. Thus, we propose that the treatment with an unspecific myeloid cell-depleting agent could be effective if administered together with chemotherapy or radiation therapy, as MDSC depletion may enhance antigen presentation to T cells and their functional activation.
Materials and Methods
Ethics statement
All research involving animals in these studies was conducted in accordance with guidelines and animal protocols (11–2635A and 11–2817A) approved by the Animal Care and Use Committee at Colorado State University.
Mice
Specific-pathogen-free, 6–8 week old female C57BL/6, BALB/c, Rag1−/− (B6.129S7-Rag1tm1Mom/J) and Cd4−/− (B6.129S2-Cd4tm1Mak/J) and Cd8−/− (B6.129S2-Cd8a tm1Mak/J) mice were purchased from Jackson Laboratories. Mice were housed in sterile microisolater cages in the laboratory animal resources facility at Colorado State University and provided sterile water and food ad libitum.
Tumor model
For the sarcoma model, C57BL/6 mice (n = 5 per group) were injected s.c. with 2.5 or 5 x 105 MCA205 fibrosarcoma cells (MCA205 cell lines were a gift from Dr. Jack Routes).61 Tumor growth was monitored using calipers and typically reported as a measurement of the longest diameter multiplied by the tumor measurement 90 degrees to the first measurement and reported as mean tumor size in mm2. Tumor growth was typically recorded every 2–4 d. Cell lines used in this study were routinely treated with Mycoplasma removal agent (MP Biomedicals). The MCA205 cells were maintained in MEM (Invitrogen, Carlsbad, CA) with essential and non-essential amino acids, L-glutamine, penicillin/streptomycin and 10% FBS (Gemini).
Preparation of liposomal clodronate and PBS and mouse treatment
Liposomal clodronate (LC) was produced as previously described by our laboratory.2,6 Briefly, phosphotidylcholine, cholesterol and mannose were dried as thin films in round bottom tubes and then rehydrated with a 0.7 M solution of dichloroethylene bisphosphonate (clodronate). After rehydration, the liposomes were washed in PBS to remove the unincorporated clodronate, filtered and stored in HEPES buffer at 4°C. Control PBS-containing liposomes (L-PBS) were prepared similarly, using a 1.5 M solution of PBS to rehydrate the liposomes. For tracking studies, BODIPY-labeled liposomes were prepared as described above, except that BODIPY-cholesterol (Invitrogen) was added to unlabeled cholesterol during liposome preparation. Mice were treated with 200 μL of LC or L-PBS injected i.v. via the lateral tail vein, administered once weekly. In some experiments, mice were treated with 200 μL LC administered by the i.p. route.
NK cell depletion
Tumor-bearing mice were depleted of NK cells by weekly i.p. administration of 50 μL anti-asialo-GM1 antibody (Wako Bioproducts). Injections were initiated 24h prior to the first administration of LC, and were repeated weekly, 24h prior to each successive LC administration. This treatment protocol induced depletion of more than 80% of NK1.1+ cells (data not shown). Control mice were injected weekly i.p. with an equivalent amount of irrelevant rabbit IgG (Jackson ImmunoResearch).
Ex vivo splenic cell assays for CTL activity and cytokines
Mice bearing MCA205 tumors were treated once weekly with LC, as described above, and sacrificed one week after receiving the third dose of LC. To assess CTL activity, a CSFE CTL assay was performed following a novel published protocol.47 Briefly, cultured MCA205 tumor cell line cells (“target cells”) were labeled with CSFE and incubated for 3 d in culture with ex vivo spleen cells (“effector cells”) from the MCA205 tumor-bearing mice treated with LC or untreated (control), as described above. The CSFE-labeled tumor cells were removed via trypsinization and mixed with 7-AAD (eBiosciences) 5 min before and Caltag Counting beads (Invitrogen) immediately prior to analysis via flow cytometry. Numbers of viable tumor cells in the cultures containing spleen cells from LC-treated mice were calculated and compared with numbers of viable cells obtained from cultures of CSFE-labeled MCA205 cells and spleen cells derived from untreated, tumor-bearing mice to calculate percent survival.
To assess tumor-specific cytokine release by T cells, spleen cells were harvested from control or LC-treated MCA205 tumor-bearing mice and placed in culture with MCA205 cell line cells without any additional cytokines or mitogens. Cultures were incubated at 37°C for 72h before removal of supernatants for analysis of IFNγ via ELISA (IFNγ ELISA DuoKit, R&D Systems, Minneapolis, MN) or IL-17 via ELISA (IL-17 ELISA DuoKit, R&D).
Cell isolation and flow cytometry
Single cell spleen suspensions were prepared by gently pressing spleen tissue through a screen followed by lysis of erythrocytes using ACK solution (150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM Na2EDTA). For preparation of single cell suspensions of tumor tissues, tumor tissues were minced and placed in 1–2 mL of collagenase solution and incubated at 37°C for 20 min prior to manual dissociation, as described previously.62 Cells were immunostained using antibodies diluted in FACs buffer (PBS with 2% fetal bovine serum and 0.05% sodium azide) following a 5 min incubation with normal mouse serum to block non-specific binding. Directly conjugated antibodies used for these analyses were purchased from Becton Dickinson, Invitrogen, or eBiosciences.
The following antibodies were used for analysis of the indicated cell populations: 1) Monocytes and macrophages: anti-CD45 (biotin or Pacific Orange conjugated, clone 30-F11), anti-F4/80 (APC, clone BM8), anti-CD11b (Pacific Blue or eFluor 450, M1/70clone), anti-Ly6G (FITC, clone 1A8), anti-CD115 (PE, clone FMS) and anti-Ly6C (biotin, clone AL-21); 2) T cells and NK cells: anti-CD45, anti-CD4 (PB, clone RM4–5), anti-CD8 (PECy7, clone 53–6.7), anti-CD3 (biotin or FITC-conjugated, clone eBio500A2), anti-NK1.1 (PE or APC, clone PK136) and anti-CD25 (PE, clone PC61); 3) MDSCs: anti-CD45, anti-CD11b, anti-Gr-1 (PECy7, clone RB6–8C5); and 4) endothelial cells: anti-CD45, anti-CD11b and anti-CD31 (FITC, clone 390). Endothelial cells were identified as CD45-CD11b-CD31+ cells, as described previously by our group;63 and individual DC populations were pooled for analysis using the following markers: anti-CD11c (PE, clone N418), anti-CD11b, anti-F4/80, anti-B220 (eFluor 780, clone RA3–682), anti-CD8, anti-NKG2D, clone CX5). For analysis of tumor cell populations, cells were stained with propidium iodide (PI) to exclude dead cells from analysis and cell populations (excluding endothelial cells) were calculated based on the percentage of CD45+ cells analyzed.
Liposome distribution and uptake by tumors and TAMs
To track the distribution and uptake of LC by phagocytic cells in tumor tissues and other tissues, mice were injected with L-PBS labeled with BODIPY cholesterol64 and tissues were collected 6h later for preparation of single cell suspensions and analysis by flow cytometry. Mice with established s.c. MCA205 tumors were injected i.v. with 200 μL labeled liposomes (or diluent) and sacrificed 6h later. In addition, tumor, spleen and draining lymph node tissues were also evaluated to identify cells that had phagocytosed labeled liposomes.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (San Diego, CA). Differences between two groups were compared using a non-parametric U test (Mann-Whitney). Differences between three or more treatment groups were determined using a nonparametric, one-way ANOVA (Kruskal-Wallis), followed by Dunn’s multiple means comparison tests. Tumor growth rates were compared using repeated measures ANOVA followed by Bonferroni post-test. Kaplan-Meier curves were compared with the Logrank test. For all analyses, a p value of < 0.05 was considered statistically significant, unless otherwise noted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to acknowledge the assistance of Dr. Tom Anchordoquy with liposome formulation. This work was supported by a grant from the Colorado State University Supercluster Fund and by a grant by the National Center for Research Resources (K01RR25162) that is currently supported by the Office of Research Infrastructure Programs/OD (8K01OD010911–03) to A.M.G.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21317
References
- 1.van Rooijen N, Kors N, Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989;45:97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- 2.Mathes M, Jordan M, Dow S. Evaluation of liposomal clodronate in experimental spontaneous autoimmune hemolytic anemia in dogs. Exp Hematol. 2006;34:1393–402. doi: 10.1016/j.exphem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 4.van Rooijen N, van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238:355–8. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafeman S, London C, Elmslie R, Dow S. Evaluation of liposomal clodronate for treatment of malignant histiocytosis in dogs. Cancer Immunol Immunother. 2010;59:441–52. doi: 10.1007/s00262-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–41. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 8.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7:788–99. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 9.Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–86. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraoka K, Zenmyo M, Watari K, Iguchi H, Fotovati A, Kimura YN, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008;99:1595–602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15:4391–400. doi: 10.1158/1078-0432.CCR-09-0489. [DOI] [PubMed] [Google Scholar]
- 12.Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147–55. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 13.Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–16. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 15.Piña Y, Boutrid H, Murray TG, Jager MJ, Cebulla CM, Schefler A, et al. Impact of tumor-associated macrophages in LH(BETA)T(AG) mice on retinal tumor progression: relation to macrophage subtype. Invest Ophthalmol Vis Sci. 2010;51:2671–7. doi: 10.1167/iovs.09-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van Rooijen N, et al. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol. 2010;185:3481–8. doi: 10.4049/jimmunol.0903479. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Ibata M, Yu Z, Shikama Y, Endo Y, Miyauchi Y, et al. Rejection of intradermally injected syngeneic tumor cells from mice by specific elimination of tumor-associated macrophages with liposome-encapsulated dichloromethylene diphosphonate, followed by induction of CD11b(+)/CCR3(-)/Gr-1(-) cells cytotoxic against the tumor cells. Cancer Immunol Immunother. 2009;58:2011–23. doi: 10.1007/s00262-009-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonman ZF, Schurmans LR, van Rooijen N, Melief CJ, Toes RE, Jager MJ. Macrophages are vital in spontaneous intraocular tumor eradication. Invest Ophthalmol Vis Sci. 2006;47:2959–65. doi: 10.1167/iovs.05-1427. [DOI] [PubMed] [Google Scholar]
- 19.Lee C-C. Liu, Ko-Jiunn Liu, Huang, Tze-Sing. Tumor-Associated Macrophage: Its Role in Tumor Angiogenesis. Journal of Cancer Molecules. 2006;2:135–40. [Google Scholar]
- 20.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–91. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura YN, Watari K, Fotovati A, Hosoi F, Yasumoto K, Izumi H, et al. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci. 2007;98:2009–18. doi: 10.1111/j.1349-7006.2007.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MB, van Rooijen N, Izui S, Kappler J, Marrack P. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood. 2003;101:594–601. doi: 10.1182/blood-2001-11-0061. [DOI] [PubMed] [Google Scholar]
- 24.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest. 2006;116:2587–90. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 33.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–21. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye XZ, Yu SC, Bian XW. Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis. J Genet Genomics. 2010;37:423–30. doi: 10.1016/S1673-8527(09)60061-8. [DOI] [PubMed] [Google Scholar]
- 38.Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 40.Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–33. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88:107–16. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 42.Kasai M, Yoneda T, Habu S, Maruyama Y, Okumura K, Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981;291:334–5. doi: 10.1038/291334a0. [DOI] [PubMed] [Google Scholar]
- 43.Fujii Y, Sendo F, Kamiyama T, Naiki M. IgG antibodies to asialoGM1 are more sensitive than IgM antibodies to kill in vivo natural killer cells and prematured cytotoxic T lymphocytes of mouse spleen. Microbiol Immunol. 1990;34:533–42. [PubMed] [Google Scholar]
- 44.Liu Z, Kim JH, Falo LD, Jr., You Z. Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol. 2009;182:6160–7. doi: 10.4049/jimmunol.0802664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113:6120–7. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004;103:2677–82. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 48.Kamstock D, Guth A, Elmslie R, Kurzman I, Liggitt D, Coro L, et al. Liposome-DNA complexes infused intravenously inhibit tumor angiogenesis and elicit antitumor activity in dogs with soft tissue sarcoma. Cancer Gene Ther. 2006;13:306–17. doi: 10.1038/sj.cgt.7700895. [DOI] [PubMed] [Google Scholar]
- 49.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–16. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–97. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi JY, Oughton JA, Kerkvliet NI. Functional alterations in CD11b(+)Gr-1(+) cells in mice injected with allogeneic tumor cells and treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int Immunopharmacol. 2003;3:553–70. doi: 10.1016/S1567-5769(03)00046-8. [DOI] [PubMed] [Google Scholar]
- 56.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 58.Augier S, Ciucci T, Luci C, Carle GF, Blin-Wakkach C, Wakkach A. Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. J Immunol. 2010;185:7165–73. doi: 10.4049/jimmunol.0902583. [DOI] [PubMed] [Google Scholar]
- 59.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.V99.6.2114. [DOI] [PubMed] [Google Scholar]
- 61.Routes JM, Ryan S, Morris K, Takaki R, Cerwenka A, Lanier LL. Adenovirus serotype 5 E1A sensitizes tumor cells to NKG2D-dependent NK cell lysis and tumor rejection. J Exp Med. 2005;202:1477–82. doi: 10.1084/jem.20050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163:1552–61. [PubMed] [Google Scholar]
- 63.Sottnik JL, Guth AM, Mitchell LA, Dow SW. Minimally invasive assessment of tumor angiogenesis by fine needle aspiration and flow cytometry. Angiogenesis. 2010;13:251–8. doi: 10.1007/s10456-010-9182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]