Abstract
Cancer immunotherapy is hampered by the immunosuppression maintained by regulatory T cells (Tregs) in tumor-bearing hosts. Stimulation of the Toll-like receptor 2 (TLR2) by Pam3Cys is known to affect Treg-mediated suppression. We found that Pam3Cys increases the proliferation of both CD4+ effector T cells (Teffs) and Tregs co-cultured in vitro, but did not induce the proliferation of Tregs alone upon CD3 and CD28 stimulation. In a mouse model of RMA-MUC1 tumors, Pam3Cys was administered either alone or in combination with a modified vaccinia ankara (MVA)-based mucin 1 (MUC1) therapeutic vaccine. The combination of Pam3Cys with MVA-MUC1 (1) diminished splenic Treg/CD4+ T-cell ratios to those found in tumor-free mice, (2) stimulated a specific anti-MUC1 interferon γ (IFNγ) response and (3) had a significant therapeutic effect on tumor growth and mouse survival. When CD4+ Teffs and Tregs were isolated from Pam3Cys-treated mice, Teffs had become resistant to Treg-mediated suppression while upregulating the expression of BclL-xL. Tregs from Pam3Cys-treated mice were fully suppressive for Teffs from naïve mice. Bcl-xL was induced by Pam3Cys with different kinetics in Tregs and Teffs. Teff from Pam3Cys-treated mice produced increased levels of Th1 and Th2-type cytokines and an interleukin (IL)-6-dependent secretion of IL-17 was observed in Teff:Treg co-cultures, suggesting that TLR2 stimulation had skewed the immune response toward a Th17 profile. Our results show for the first time that in a tumor-bearing host, TLR2 stimulation with Pam3Cys affects both Tregs and Teffs, protects Teff from Treg-mediated suppression and has strong therapeutic effects when combined with an MVA-based antitumor vaccine.
Keywords: Treg, TLR2, Pam3Cys, cancer, immunotherapy, Th17
Introduction
Effector cellular and humoral immune responses to tumor antigens can be detected in cancer patients1,2 but are unable to control cancer progression. Some tumor-associated antigens administered as therapeutic vaccines show poor efficacy because cancers escape immunological control by establishing tolerance.3 Regulatory T cells (Tregs) contribute to tumor-induced immunosuppression. They have been extensively characterized in mouse models and shown to play a role in human cancer.4 In both species, the presence of tolerogenic CD4+CD25+FOXP3+ Tregs is associated with neoplastic development.5,6 Tregs play a major role in the control of immune responses by modulating the activity of both effector T lymphocytes (Teffs) and antigen-presenting cells.7 There is increasing evidence that both the phenotypes and functions of Tregs and T helper (Th) cells are highly plastic,8 since repolarization has been described from a Treg to a Th17,9-11 from a Th1 to a Treg or a Th17,12,13 and from a Th17 to a Th1 profile.14
Among strategies aimed at inducing or potentiating tumor-specific immune responses, vaccines based on modified vaccinia ankara (MVA) vectors have shown promising activity for the immunotherapy of mucin 1 (MUC1)-positive tumors.15,16 Hypoglycosylated variants of the MUC1 oncoprotein are overexpressed during tumor progression in most cancers of epithelial origin.17 MVA is a non-propagative, highly attenuated vaccinia virus that can mediate antigen expression in infected cells, leading to antigen cross-presentation and Teff responses facilitated by the stimulation of innate immunity.18 Toll-like receptors (TLR) including TLR2 and TLR6 are among the pattern-recognition receptors that are activated during MVA infection.
TLR8 stimulation has been shown to abrogate immunosuppression by human Treg clones.19,20 Other groups have studied the effects of TLR2 ligation by bacterial lipoproteins or the synthetic lipopeptide Pam3Cys21 on the immunosuppressive function of mouse or human Tregs.11,22-26 The immunopotentiating effect of Pam3Cys on cytokine production and proliferation of CD4+ and CD8+ T lymphocytes has been demonstrated in vivo, in murine models, and ex vivo, in human cells.23,26-30 Whether Pam3Cys directly affects the function of Tregs remains a matter of debate. Some studies suggest that TLR2 ligation induces Treg proliferation and directly inhibits their immunosuppressive functions.22,23 Others report that TLR2 ligation does not affect the suppressive activity of Tregs, but rather their survival through Bcl-xL.24
It has been attempted to deplete or inhibit Tregs in cancer patients in order to enhance antitumor immunity.31 Such strategies may be of benefit when combined with tumor-antigen vaccines.32.33 In this study, we have investigated the effect of Pam3Cys in vitro and in a cancer immunotherapy model. We describe the effects of TLR2 stimulation on Treg and Teff functions and on the repolarization of the immune response in vivo, which translate into therapeutic efficacy of the MVA-based vaccines.
Results
In vitro effects of Pam3Cys on T-cell proliferation and suppression
The effects of Pam3Cys on Tregs was investigated in vitro using T cells from naïve mice. CD4+CD25- Teffs and CD4+CD25+ Tregs purified from C57BL/6 splenocytes were incubated either alone or co-cultured in the presence of anti-CD3/anti-CD28 monoclonal antibodies and increasing concentrations of Pam3Cys (Fig. 1A). The proliferation of Teffs alone, measured by BrdU incorporation, was enhanced by Pam3Cys at 0.22, 0.66 and 2 µg/mL (p < 0.05), but this effect was not observed at a higher dose (6 µg/mL). Tregs alone did not proliferate even in the presence of Pam3Cys. When Tregs and Teffs were co-cultured (ratio 1:1), nearly no proliferation was detected in the absence of Pam3Cys, reflecting a Treg-mediated suppression that required direct cell contacts between Tregs and Teffs (Fig. S1). Proliferation was restored in the presence of Pam3Cys at concentrations up to 2 µg/mL (p < 0.05). These results indicated that, at concentrations between 0.22 and 2 µg/mL, Pam3Cys inhibits Treg-mediated suppression.
Figure 1. (A) Pam3Cys affects T-cell proliferation and suppression in vitro. Purified Teffs (6 × 104 cells), Tregs (3 × 104 cells) or Teff:Treg co-cultures (ratio 1:1 for a total of 6 × 104 cells) were stimulated with anti-CD3/anti-CD28 monoclonal antibodies in the presence or absence of the indicated amounts of Pam3Cys. Cell proliferation was measured by BrdU incorporation. The background O.D. value calculated from Teffs cultivated in the absence of anti-CD3/anti-CD28 monoclonal antibodies and Pam3Cys was 0.1 (not shown). Data shown are means ± SD (n = 4) from one out of two independent experiments with similar results. *p < 0.05 compared with the proliferation of control cultures without Pam3Cys. (B) Pam3Cys (0.22 µg/mL) stimulates the proliferation of both Teffs and Treg in co-culture. Either cell population was labeled with CFSE before the suppression assay for flow cytometry analysis (result from 1 experiment representative of 3).
When either Teffs or Tregs were labeled with CFSE before the suppression assay, weak Treg proliferation was detected in unstimulated co-cultures (Fig. 1B), but not when Tregs were cultured alone (not shown). Pam3Cys increased the proliferation of both Tregs and Teffs in co-cultures (Fig. 1B). The Tregs that proliferated upon co-culture remained FOXP3+ (data not shown). Thus, both Tregs and Teffs are induced to proliferate upon TLR2 stimulation, but Treg proliferation requires the presence of stimulated Teffs.
Low therapeutic activity of an MVA-MUC1 vaccine and induction of Tregs in mice bearing RMA-MUC1 tumors
A cancer model based on RMA cells stably expressing the MUC1 tumor antigen was used to study the therapeutic activity of a MVA-MUC1 vaccine. MVA-MUC1 was injected at a sub-optimal dose in C57BL/6 mice two days after implantation of RMA-MUC1 tumors and re-administered at days 9 and 16. As controls, an empty MVA variant (MVA-N33) or vehicle were used. MVA encoding or not the MUC1 antigen had inhibitory effects on tumor progression (Fig. 2A, p = 0.006 and 0.04 respectively), which did not translate into prolonged mouse survival (Fig. 2B). Thus, immunization with MVA-MUC1 in this setting did not lead to a potent therapeutic effect, indicating that specific anti-tumor responses were not sufficiently stimulated. Figure 2C shows that all tumor-bearing mice treated or not with MVA vectors had increased proportions of Tregs among their CD4+ splenocytes at day 23, compared with untreated tumor-free mice.

Figure 2. Low therapeutic effect of MVA-MUC1 and Treg induction in mice bearing RMA-MUC1 tumors. C57BL/6 mice (n = 18 per group) implanted with RMA-MUC1 tumor cells at day 0 were injected subcutaneously with either 5 × 107 p.f.u. of MVA vectors or vehicle at days 2, 9 and 16 (arrows). Animals were monitored every other day, starting on day 6. (A) Tumor volumes are shown as means ± SEM from one out of two independent experiments with similar results. Statistical analyses were performed on tumor volumes measured at day 20, when at least 80% of vehicle-treated mice were still alive. *p < 0.05, **p < 0.01, compared with the tumor volumes of vehicle-treated mice. (B) Percentages of surviving mice in each group. (C) Treg levels were quantified by flow cytometry analysis of pooled splenocytes isolated from tumor-bearing mice one week after each injection of MVA or vehicle, or from naïve mice. Percentages of CD25+FOXP3+ Tregs among the CD4+ cell population were determined after gating of the lymphocyte population on an FSC/SSC dot plot. Data shown are means ± SD from one out of two independent experiments with similar results.
Because of (1) the low efficacy of the therapeutic vaccine in this setting and (2) the increase in Treg/CD4+ T-cell ratios during tumor progression, this model appeared relevant for studying the effect of compounds targeting Treg-mediated suppression combined with a therapeutic tumor-antigen vaccine.
Therapeutic effect of Pam3Cys combined with a MVA-MUC1 vaccine
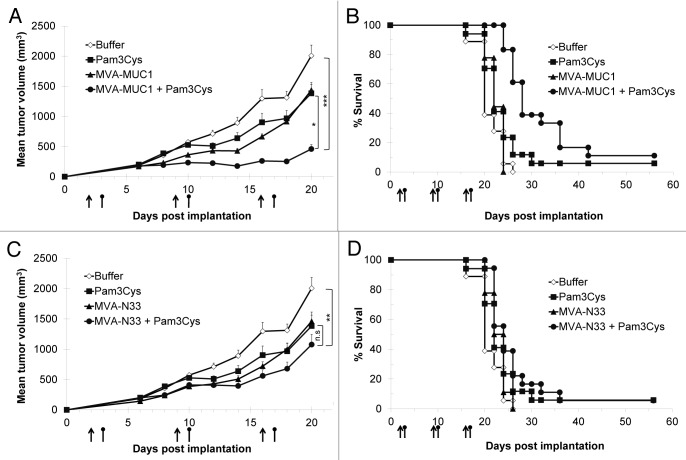
Pam3Cys was administered intraperitoneally 24 h after each injection of MVA or vehicle. Tumor progression was inhibited when Pam3Cys was combined with MVA-MUC1 (Fig. 3A, p = 0.0001 at day 20 compared with vehicle-treated mice). Pam3Cys alone had a moderate effect on tumor progression, as did MVA-MUC1 (p = 0.02). Survival was prolonged only in mice treated with the combination of MVA-MUC1 and Pam3Cys (Fig. 3B, p < 0.0001 compared with vehicle- or MVA-MUC1-treated mice, and p < 0.01 compared mice treated with Pam3Cys alone). Median survival was 20 d in vehicle-treated mice and 22 d for mice treated with MVA-MUC1 or Pam3Cys alone. The combination of these treatments increased median survival to 28 d.
Figure 3. Therapeutic effect of Pam3Cys combined with MVA-MUC1 in RMA-MUC1 tumor-bearing mice. C57BL/6 mice implanted with RMA-MUC1 tumor cells at day 0 were treated with subcutaneous injections of MVA-N33, MVA-MUC1 or vehicle (arrows) combined or not with Pam3Cys (round-headed bars) administered intraperitoneally 24 h later. Animals were monitored every other day, starting on day 6. (A,C) Tumor volumes are shown as means ± SEM from one out of two independent experiments with similar results. Statistical analyses were performed on tumor volumes measured at day 20, when at least 80% of vehicle-treated mice were still alive. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant. (B,D) Percentages of surviving mice in each group.
To verify whether this therapeutic effect was linked to MUC1 vaccination, Pam3Cys was combined with MVA-N33 using the same treatment schedule. No significant therapeutic effect was observed in these conditions, neither on tumor progression (Fig. 3C) nor on mouse survival (Fig. 3D), compared with mice treated with MVA-N33 or Pam3Cys alone. These results show that the MVA-mediated expression of the MUC1 tumor antigen is required, together with the administration of Pam3Cys, to achieve a therapeutic effect.
Cell-mediated immune responses against MUC1 are stimulated in mice treated with MVA-MUC1 + Pam3Cys
The cellular response against MUC1 was analyzed by interferon γ (IFNγ) ELISpot assays using splenic mononuclear cells purified one week after each MVA injection. RMA-MUC1 tumors alone did not induce any detectable specific Teff response against the MUC1 peptide (Fig. 4). Treatment with MVA-MUC1 alone resulted in a low specific response, which was significant only at days 9 and 23. Similarly, Pam3Cys alone did not stimulate a MUC1-specific IFNγ response. Only the combination of MVA-MUC1 and Pam3Cys induced a significant response at all timepoints tested (p = 0.002 at day 23 compared with vehicle-treated mice).
Figure 4. Cell-mediated immune response against MUC1 is stimulated in mice treated with MVA-MUC1 + Pam3Cys. ELISpot assays were performed using mononuclear cells purified from splenocytes pooled from tumor-bearing mice one week after each injection of MVA or vehicle. Cells were stimulated with MUC1 or irrelevant peptide. Data shown are means ± SD (n = 6) from two independent experiments. **p < 0.01, n.s. not significant, compared with the control, vehicle-treated mice.
These results demonstrate that the therapeutic effect observed in mice treated with MVA-MUC1 + Pam3Cys is associated with the stimulation of a MUC1-specific IFNγ response.
Pam3Cys protects Teffs from Treg-mediated immunosuppression
We investigated whether Pam3Cys affected Treg-mediated suppression in our cancer vaccine model. Pam3Cys moderately decreased splenic Treg/CD4+ T-cell ratios at day 23 (Fig. S2), while the combination of either MVA-MUC1 or MVA-N33 with Pam3Cys resulted in almost 2-fold reductions of the Treg/CD4+ T cell ratios at day 23 (respectively 13 and 15.5% compared with 22.8% in vehicle-treated mice), approaching the values observed in naïve mice (11%).
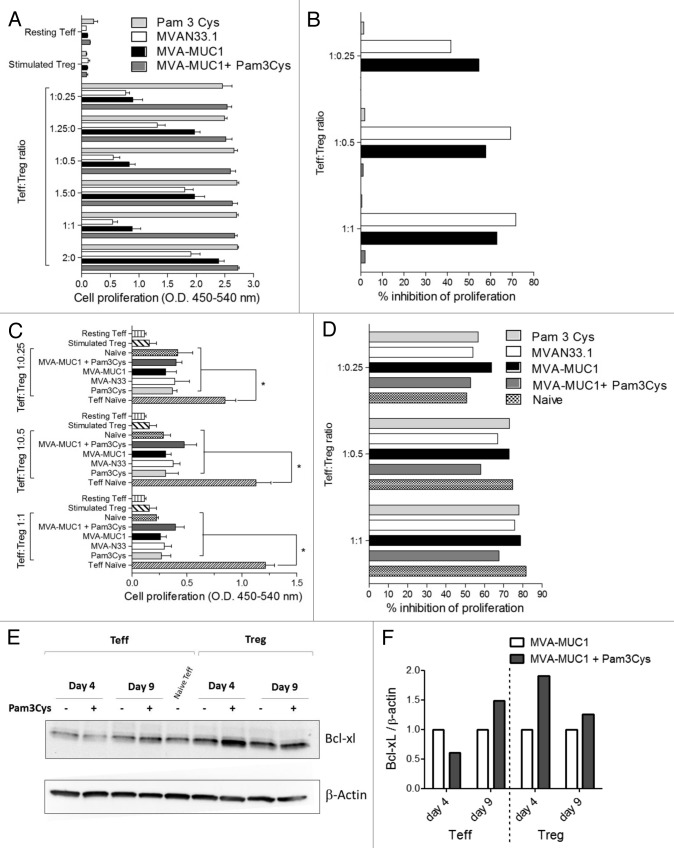
Teffs and Tregs purified from each group at day 9 were incubated either alone or in co-cultures with anti-CD3/anti-CD28 monoclonal antibodies. Proliferation of Teffs alone was increased when cells were isolated from mice treated with Pam3Cys, with or without MVA, compared with those that were recovered from mice injected with MVA vectors alone (Fig. 5A, p < 0.05). Tregs alone did not proliferate, whether or not mice had been treated with Pam3Cys. In Teff:Treg co-cultures, significant inhibition of proliferation was observed at all ratios tested for cells purified from mice treated with MVA vectors alone (p < 0.05). In stark contrast, the suppression of Teff proliferation was totally abolished when Teffs and Tregs were isolated from mice treated with Pam3Cys (Fig. 5B) .
Figure 5. Pam3Cys protects Teff from Treg-mediated suppression. (A) Teff and Treg purified at day 9 from the indicated mouse groups were stimulated in vitro with anti-CD3/anti-CD28 monoclonal antibodies, either alone or in Teff:Treg co-cultures at the indicated ratios. Cell proliferation measured by BrdU incorporation is shown as means ± SD (n = 4) from one out of two independent experiments with similar results. (B) Percentages of inhibition of cell proliferation were calculated from (A) as described in Materials and Methods. (C) Teffs were purified from naïve mice and co-cultured with Tregs from the indicated mouse groups at the indicated ratios and anti-CD3/anti-CD28 monoclonal antibodies. Cell proliferation is represented as means ± SD (n = 4) from one out of two independent experiments with similar results. *p < 0.05 between the indicated conditions. (D) Percentages of inhibition of cell proliferation were calculated from (C) as above. (E) Pam3Cys regulates BCL-XL levels in Tregs and Teffs with different kinetics. Teffs and Tregs were purified at days 4 and 9 from mice treated with MVA-MUC1 alone or combined with Pam3Cys. BCL-XL and β actin protein levels were analyzed by immunoblotting on the same membrane. (F) Band intensities were quantified. BCL-XL / β actin ratios were normalized for side-by-side comparison between Teffs or Tregs from Pam-3Cys-treated mice and the corresponding cells from mice treated with MVA-MUC1 only.
We then asked whether Pam3Cys may directly affect Treg functions. To this purpose, suppression assays were performed using Teffs isolated from naïve mice and co-cultured with Tregs that had been purified at day 9 from Pam3Cys- and/or MVA-treated mice. Control Tregs from naïve mice were included in the assay. Figure 5C shows that proliferation of naïve Teffs is significantly inhibited by Tregs purified from any of mouse groups, including mice treated with Pam3Cys (p < 0.05). Inhibition of proliferation was as strong as that observed with Tregs from naïve mice (Fig. 5D). These data indicate that 9 days after Pam3Cys treatment (6 days in vivo followed by 3 days ex vivo), Tregs are capable of suppressing Teffs from naïve mice. Thus, Teffs from mice treated with Pam3Cys can proliferate even in the presence of functionally immunosuppressive Tregs. Similar results were obtained when this analysis was performed at day 16, after the second administration of MVA and or Pam3Cys (data not shown).
As it had been reported that the anti-apoptotic Bcl-2 family member Bcl-xL can be upregulated by Pam3Cys in T-cell subsets, we analyzed Bcl-xL protein levels in Teffs and Tregs isolated from mice treated with Pam3Cys and/or MVA at days 4 and 9 (respectively 1 and 6 d after Pam3Cys treatment). Figure 5E and F shows that Bcl-xL was upregulated in Tregs one day after Pam3Cys treatment, whereas it was induced only after 6 d in Teffs. This late increase in Bcl-xL expression by Teffs may explain their resistance to Treg-mediated suppression.
Pam3Cys induces Th1, Th2 and Th17 cytokine responses
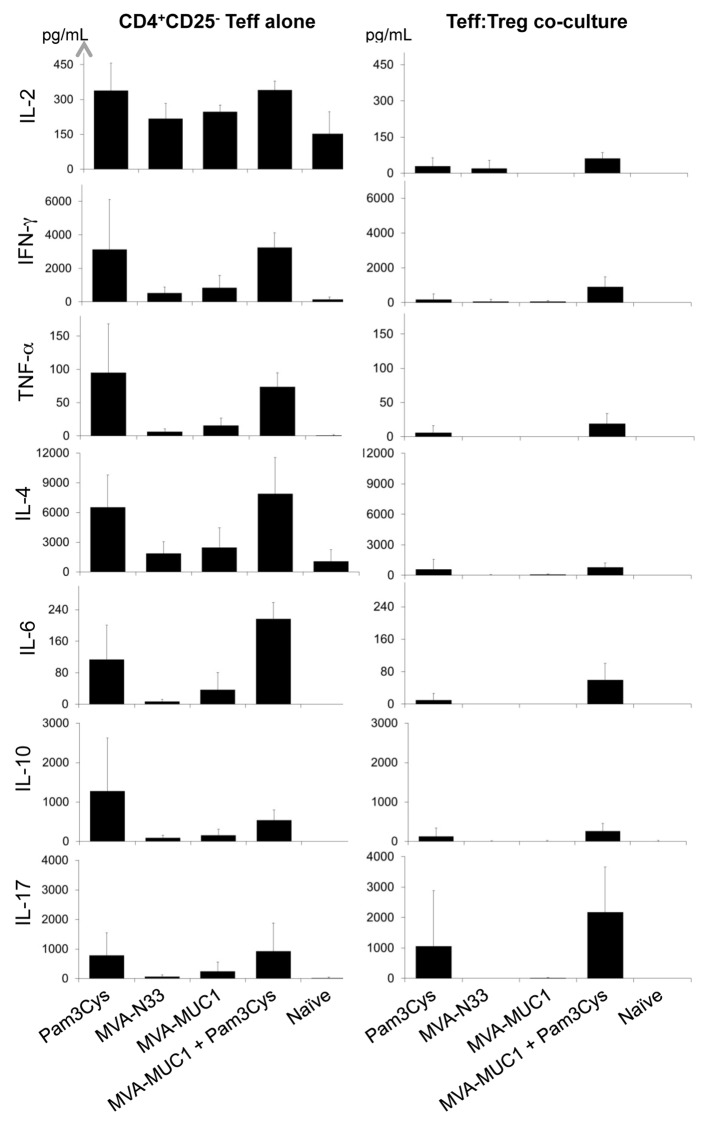
To examine how Pam3Cys and MVA treatments affect Th responses, cytokine levels were measured in culture supernatants from the ex vivo proliferation and suppression assays described above. Teffs isolated from naïve mice produced detectable amounts of interleukin (IL)-2, IL-4 and IFNγ upon stimulation but no IL-6, IL-10, IL-17 and tumor necrosis factor α (TNFα) (Fig. 6). None of these cytokines were detected in Teff:Treg co-cultures from the same mice. Likewise, Teffs from naïve mice co-cultured with Tregs from MVA- and/or Pam3Cys-treated mice did not produce any of these cytokines in detectable amounts (not shown). No cytokines were detected in the supernatants of stimulated Tregs alone (not shown).
Figure 6. Pam3Cys induces Th1- and Th2-type cytokine secretion by Teff and IL-17 production in Teff:Treg co-cultures ex vivo. The indicated cytokines were quantified in culture supernatants from Teffs (left panels) or Teff:Treg co-cultures at ratio 1:1 (right panels). Concentrations shown (pg/mL) are means ± SD (n = 4) calculated from two independent experiments.
In contrast, Teff isolated from mice treated with Pam3Cys produced a series of Th1- and Th2-type cytokines. IFNγ and TNFα as well as IL-6, IL-10 and IL-4 were strongly induced in Teffs from mice treated with Pam3Cys, compared with the same cells obtained from mice receiving MVA alone (Fig. 6). IL-2 was also detected in slightly increased quantities in the supernatants of Teffs when they were derived from Pam3Cys-treated mice. IL-2 levels were reduced in when Teffs and Tregs from Pam3Cys-treated mice were co-cultured. Both Th1-type (IFNα and TNFα) and Th2-type cytokines (IL-6, IL-10 and IL-4) were detected in significant amounts in the supernatant of Teff:Treg co-cultures when cells were purified from mice treated with the combination of Pam3Cys and MVA-MUC1. Only traces of these cytokines were produced when Teffs and Treg from mice treated with Pam3Cys alone were co-cultured. Of note, IL-17 was present at high levels in Teff:Treg co-cultures when these were obtained from mice treated with Pam3Cys combined with MVA-MUC1 (Fig. 6). IL-17 was also detected in the supernatant of Teffs from Pam3Cys-treated mice cultured alone, but at lower levels. Tregs alone did not produce any IL-17 (not shown).
When Pam3Cys was added in vitro to Teffs and Tregs purified from naive mice in suppression assays, similar cytokines were induced (Fig. S3). IFNγ, IL-4, IL-6, IL-10 and IL-17 were produced in increased amounts by Teff:Treg co-cultures stimulated with Pam3Cys. When IL-6 was blocked in the co-cultures, not only the production of the Th2 cytokines IL-4 and IL-10 but also that of IL-17 were inhibited. IL-17 secretion was again more consistent in Teff:Treg co-cultures than in Teffs cultured alone, suggesting that IL-17 in this setting is produced by Tregs. These results suggest that, in addition to inducing Th1- and Th2-type cytokine responses, Pam3Cys combined with a MVA-MUC1 vaccine may repolarize the T helper response toward a Th17 profile in an IL-6-dependent manner.
Discussion
In our in vitro system, the Treg-mediated suppression of Teff proliferation was cell-contact dependent, in line with earlier reports.7 Pam3Cys significantly augmented Teff proliferation in response to CD3/CD28 stimulation while Tregs proliferated only in the presence of Teffs. These results contrast with a previous study suggesting that Pam3Cys induces the proliferation of Tregs stimulated by CD3 crosslinking alone.23 Our results are more in agreement with other studies indicating that Tregs stimulated with Pam3Cys can proliferate in the presence of exogenous IL-2.22,24 Our results suggest that cytokines, notably IL-2, produced by stimulated Teffs allow for the proliferation of co-cultured Tregs. In our system, Pam3Cys inhibited the Treg-mediated suppression of Teff proliferation, as previously described.22,23 Pam3Cys has been shown to directly act on Tregs because its systemic administration inhibited the immunosuppressive activity of wild-type Tregs transferred into Tlr2−/− mice.22 However, Chen et al.24 found that highly purified FOXP3+ Tregs pre-treated with Pam3Cys remained fully suppressive when co-cultured with Teffs from Tlr2−/− mice. These authors also showed that TLR2 ligation on Tregs increase their survival through the upregulation of the anti-apoptotic protein Bcl-xL.24 Our results indicate that this upregulation is early and transient in vivo. Other authors described that Pam3Cys treatment transiently downregulates FOXP3 at the mRNA level and the Treg immunosuppressive activity within 8 to 15 h after TCR stimulation.23 In this latter study, Pam3Cys-treated Tregs regained their suppressive function after 7 days. In our ex vivo experiments, Teffs and Tregs were purified 6 days after treatment with Pam3Cys before being tested in 3 days proliferation and suppression assays. At this late timepoint, Tregs were functionally suppressive, while the proliferation of Teffs was augmented. The resistance of Teff to Treg-mediated suppression correlated with increased BCL-XL expression. Thus, Pam3Cys in vivo upregulates BCL-XL levels in both Teffs and Tregs, but with different kinetics. These results are in line with previous studies showing that TLR2 stimulation modulates the immune response by affecting both Teffs and Tregs.23,26
Pam3Cys is known to stimulate TLR1/2 and MVA to activate TLR2 and TLR6, among other receptors involved in innate immunity.18 Pam3Cys has also been reported to upregulate TLR2 at the mRNA levels, particularly in Tregs, which might facilitate the TLR2-mediated sensing of MVA by Tregs.24 TCR activation has been found to induce TLR2 expression in other T-cell subsets,28 which may in turn increase the stimulatory effects of Pam3Cys. TLR2 ligation is known to enhance both the proliferation and survival of antigen-specific CD8+ T cells.27 Increased TLR-mediated signaling may thus overcome tumor-induced immunosuppression and break tolerance to the tumor antigen encoded by MVA.
Pam3Cys has previously been shown to potentiate cytotoxic Teff functions.29 In line with this observation, we observed highly significant MUC1-specific IFNγ responses using splenocytes from mice treated with the combination of MVA-MUC1 with Pam3Cys. Th1-type cytokines such as IFNγ, IL-2 and TNFα and also Th2 cytokines like IL-6, IL-10 and IL-4 were produced when Teffs from Pam3Cys-treated mice were activated ex vivo. It has been described that Teffs can develop resistance to Treg-mediated immunosuppression through IL-6 signaling34 or through the IL-4-induced activation of STAT6.35 We detected a high production of these two cytokines by Teffs from mice treated with MVA-MUC1 and Pam3Cys, further supporting the evidence that Teffs had become resistant to Treg-mediated suppression in this setting. Since IL-6 is known to induce Bcl-xL expression through STAT3, Bcl-xL upregulation and the resistance to Treg-mediated suppression observed in Teffs ex vivo may be direct consequences of IL-6 signaling.36
In addition to the Th1/Th2 cytokines produced by Teffs purified from Pam3Cys-treated mice stimulated ex vivo, we found IL-17 in the supernatant of Teff:Treg co-cultures. The secretion of these cytokines was concomitant with the abrogation of Treg-mediated suppression in vitro as well as in vivo. IL-17 is secreted by Th17 cells, which have been reported to differentiate either from naïve Th cells37 or from Tregs.9,10 The association of IL-6 and transforming growth factor β (TGFβ) induces Th17 differentiation.38 In vitro, we found that IL-6, as induced in TLR2-stimulated Teff:Treg co-cultures, stimulates the production of IL-17. Our results suggest that Tregs contribute to IL-17 production in an IL-6-dependent manner. The presence of Th17 cells in cancer patients often correlates with better prognosis39,40 and Th17-polarized cells may be more powerful than Th1-type cells in driving anticancer cytotoxicity.41 In this latter study, IFNγ was shown to be required for the therapeutic effect of Th17 cells adoptively transferred to tumor-bearing mice. We found this cytokine to be induced in Teff:Treg co-cultures when these cells were purified from Pam3Cys-treated mice.
While this manuscript was in preparation, Nyirenda et al. published that human Tregs cultured in the presence of Pam3Cys exhibit reduced immunosuppressive function and increased secretion of IL-6 and IL-17.11 These results demonstrate that Treg themselves are converted into Th17 cells when stimulated through TLR2 and are in agreement with our observations. Moreover, these authors have shown that both IL-6 and IL-17 are necessary for the TLR2-mediated reversal of Treg functions.
TLR2 activation during Th17 cell differentiation has previously been shown to enhance cell proliferation and IL-17 secretion.42 A shift from Treg to Th17 cells in vivo might explain the reduced Treg/CD4+ T cell ratios observed following Pam3Cys treatment and be associated with a better control of tumor progression in vaccinated mice. Our results indicate that therapeutic benefits are observed when Th1, Th2 and IL-17-producing cells differentiate upon TLR2 stimulation and specific activation is provided by the MVA-based tumor antigen vaccine. This suggests that Pam3Cys treatment should be combined with a virus-based vaccine to elicit therapeutic responses in tumor-bearing hosts. Pam3Cys could be a potent adjuvant for improving cancer immunotherapy using MVA-based tumor antigen vaccines.
Materials and Methods
T-cell suppression and proliferation assay
CD4+CD25+ Tregs and CD4+CD25- Teffs were purified from C57BL/6 mouse splenocytes using a mouse CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Teff (3 × 104 cells) were mixed with Tregs at the indicated Teff:Treg ratios in complete DMEM (Sigma-Aldrich) containing 10% fetal calf serum (FCS) (PAA Laboratories). Cells were stimulated with soluble anti-CD3 (5 µg/mL) and anti-CD28 (1 µg/mL) monoclonal antibodies (mAb) (BD PharMingen). Four replicate wells were used for each experimental condition, except for negative controls in duplicates. These consisted in either CD3- and CD28-stimulated Tregs (3 × 104 cells) or resting Teffs (3 × 104 cells). Proliferation of a Teff:Treg co-culture was compared with that of the same total number of Teff, in order to limit cytokine or nutrient deprivation effects. Cell proliferation was assessed in 72 h cultures using ELISA bromodeoxyuridine (BrdU) kit (Roche Diagnostics). Absorbance was measured on an ELISA plate reader (Berthold Technologies). Results are presented as means of optical densities (O.D.) or as percentages of inhibition of cell proliferation calculated as 100 × [1 - (O.D. of Teff:Treg co-culture / O.D. of Teff culture)].
To measure the proliferation of Teffs and Tregs in co-cultures, each of these cell populations was labeled with 10 µM CFSE (Invitrogen) and 72 h co-cultures were analyzed by flow cytometry on a FACSCanto (BD Biosciences).
In transwell assays, 3 × 104 Teff were incubated in the bottom chambers of Millicel-96 plates (Millipore) in medium containing anti-CD3 and anti-CD28 monoclonal antibodies with or without 10 µg/mL anti-IL-6 mAb (MP5–20F3 rat IgG1, BioXCell). Upper chambers were filled with medium containing either 3 × 104 Tregs, Teffs, or Teffs+Tregs (3 × 104 cells each). Teff proliferation in the bottom chamber was measured after 72 h by BrdU incorporation.
MVA vectors
MVA-MUC1 was generated by introducing a modified sequence of the human MUC1 cDNA (NCBI Nucleotide database# NM_002456.5) under the control of the pH5R promoter into the natural deletion II of the parental virus MVA-TGN33.1.43 The MVA-TGN33.1 vector without any inserted transgene (“empty” vector) is designated MVA-N33.
Mouse model of cancer immunotherapy
Female five-week old C57BL/6 mice (Charles River Laboratories) were housed in a specific-pathogen free animal facility and acclimated before the experiments. All in vivo experiments were performed in compliance with the CEE directive 86/609 and in compliance with the French laws. The RMA-MUC1 lymphoma cell line was a gift from Joyce Taylor-Papadimitriou.44 MUC1 cell surface expression was verified by immunostaining using the anti-MUC1 antibody H23.43 At day 0.5 × 105 RMA-MUC1 cells were injected subcutaneously into the right flanks of mice. Two, eight and 15 d later, mice were injected subcutaneously with 5 × 107 plaque-forming units (p.f.u.) of MVA vectors or vehicle near the site of tumor implantation. Twenty-four hours after each injection of MVA or vehicle (at days 3, 9 and 16), 100 µg of Pam3Cys (Pam3CysSK4, InvivoGen) were injected intraperitoneally to the indicated mouse groups. Tumor volumes were monitored every other day by caliper measurements of each dimension and calculated using following formula: V = 4/3 π (length/2)(width/2)(depth/2). Mice were euthanized when tumor volumes were above 2 cm3.
Flow cytometry
Staining for cell surface markers was done by incubating 1 × 106 splenocytes (pooled from 5 mice per group except for day 23, 1 to 5 mice per group depending on survival) or 0.5 to 1 × 106 cultured Teffs or Tregs with anti-CD4 and anti-CD25 mAbs (BD PharMingen) for 30 min at 4°C. For the detection of FoxP3, cells were fixed and permeabilized using FOXP3 Fix/Perm solution (BioLegend). Cells were incubated with anti-FoxP3 mAb or isotype control IgG1 (BioLegend) for 1 h at room temperature. Cells were washed with permeabilization buffer and analyzed on a FACSAria III using the FACSDiva software (BD Biosciences).
ELISpot assay
Mononuclear cells were obtained from splenocytes by density gradient centrifugation on Lympholyte-M (Cedarlane). One million mononuclear cells in triplicate wells were seeded in 96-well MSIP plates (Millipore) coated with anti-IFNγ mAb (Mabtech AB) and cultured in complete RPMI-1640 medium (Sigma) with 10% FCS, in the presence of 1 µg/mL of MUC1 peptide (LSYTNPAVAATSANL, 15mer identified as a T cell epitope in C57BL/6 mice, unpublished data) or irrelevant HPV16-E2 peptide (HIDYWKHMRLECAIY). After 18 h culture, immunospots were revealed using biotinylated anti-IFNγ detection mAb (Mabtech), Extravidin (Sigma) and BCIP/NBT solution (Sigma). Spots were counted using Bioreader 4000 PRO-S (BIO-SYS).
Western-blot
Thirty-six µg proteins from each whole-cell extract were analyzed by Western-blot with primary antibodies specific for Bcl-xL (Cell Signaling) or β actin (Sigma). After incubation with secondary antibodies (Dako), signal was detected using the Immun-Star WesternC Chemiluminescence kit (Bio-Rad), quantified using the ChemiDoc XRS Imager and analyzed with the software Image Lab from Bio-Rad.
Cytokine titration
IL-2, IL-6, IL-10, IFNγ, TNFα, IL-4 and IL-17 were titrated in the culture supernatant from in vitro suppression assays using the mouse Th1 / Th2 FlowCytomix Pro kit (Bender MedSystems) following the manufacturer’s instructions. Samples were acquired on a FACSCanto (BD Biosciences) and analyzed using FlowCytomix Pro software (Bender MedSystems).
Statistical analysis
Mann-Whitney’s test was performed using Prism software (GraphPad) for pairwise comparisons between experimental groups in all models, except for the analysis of mouse survival where a Log-rank test was performed using Statistica software (StatSoft).
Supplementary Material
Acknowledgments
PMII project supported by OSEO. Laurent Amiset and Laetitia Fend are recipients of CIFRE doctoral fellowships.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21479
References
- 1.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065X.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–26. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkord E, Alcantar-Orozco EM, Dovedi SJ, Tran DQ, Hawkins RE, Gilham DE. T regulatory cells in cancer: recent advances and therapeutic potential. Expert Opin Biol Ther. 2010;10:1573–86. doi: 10.1517/14712598.2010.529126. [DOI] [PubMed] [Google Scholar]
- 5.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–71. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res. 2010;46:79–93. doi: 10.1007/s12026-009-8124-7. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–5. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 10.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–81. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyirenda MH, Sanvito L, Darlington PJ, O’Brien K, Zhang GX, Constantinescu CS, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–90. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 13.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acres B, Bonnefoy JY. Clinical development of MVA-based therapeutic cancer vaccines. Expert Rev Vaccines. 2008;7:889–93. doi: 10.1586/14760584.7.7.889. [DOI] [PubMed] [Google Scholar]
- 16.McCurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004;38:1749–53. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481–6. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- 18.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 20.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 21.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–94. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–53. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J Immunol. 2009;183:4458–66. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J Immunol. 2010;184:4733–40. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Luo F, Cai Y, Liu N, Wang L, Xu D, et al. TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol. 2011;186:1963–9. doi: 10.4049/jimmunol.1002320. [DOI] [PubMed] [Google Scholar]
- 27.Cottalorda A, Verschelde C, Marçais A, Tomkowiak M, Musette P, Uematsu S, et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–93. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 28.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–34. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008;22:3628–37. doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Golovina TN, Vonderheide RH. Regulatory T cells: overcoming suppression of T-cell immunity. Cancer J. 2010;16:342–7. doi: 10.1097/PPO.0b013e3181eb336d. [DOI] [PubMed] [Google Scholar]
- 32.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 34.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183:155–63. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–95. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 40.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185:4063–71. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wreschner DH, Hareuveni M, Tsarfaty I, Smorodinsky N, Horev J, Zaretsky J, et al. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur J Biochem. 1990;189:463–73. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 44.Graham RA, Burchell JM, Beverley P, Taylor-Papadimitriou J. Intramuscular immunisation with MUC1 cDNA can protect C57 mice challenged with MUC1-expressing syngeneic mouse tumour cells. Int J Cancer. 1996;65:664–70. doi: 10.1002/(SICI)1097-0215(19960301)65:5<664::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.