Abstract
γδ T cells, including Vδ1 and Vδ2 T cells, can recognize tumor-associated ligands neglected by conventional αβ T cells in a MHC-independent manner. Little is known regarding the anticancer potential and the possibility to isolate and expand Vδ1 T cells to therapeutically relevant numbers. In this study, we have detected low frequencies of Vδ1 T cells among tumor-infiltrating lymphocyte (TIL) products for adoptive cell transfer generated from melanoma metastases. An increased frequency of Vδ1 T cells was found among the cell products from patients with an advanced disease stage. Vδ1 T cells displayed in vitro antitumor activities and sufficient proliferative potential to generate over 1 × 109 cells using current protocols for T cell transfer. Infusion of Vδ1 T cells together with high numbers of αβ TILs in a clinical trial was safe and well tolerated. These data suggest that Vδ1 T cells should be further scrutinized as a potentially useful tool for the treatment of patients with metastatic melanoma.
Keywords: Vδ1 T cells, adoptive T-cell therapy, melanoma, tumor-infiltrating lymphocytes, γδ T cells
Introduction
Two major subsets of T cells carrying the γδ T-cell receptor (TCR) have been described. T cells expressing the Vδ2 gene usually account for more than 90% of the circulating γδ T-cell pool (representing about 1–10% of human peripheral lymphocytes), while intraepithelial γδ T cells more commonly express the Vδ1 gene.1 Although no major differences exist relative to effector functions between T cells expressing the αβ TCR and γδ T cells, the latter are capable of recognizing tumor-associated ligands that are neglected by conventional αβ T cells in an MHC-independent manner.2 Most γδ T cells lack the surface expression of CD4 and CD8, in agreement with their non-MHC restricted recognition of unconventional antigens. Many preclinical studies and clinical trials have focused their attention on Vγ9Vδ2 T cells, as they can be easily isolated from the peripheral blood of most individuals and activated with conventional drugs such as synthetic phosphoantigens and aminobisphosphonates.2 However, little is known regarding the anticancer potential of γδ T cells expressing the Vδ1 gene.
It is currently believed that, due to their extremely limited diversity, Vδ1 T cells may not respond to a diversity of microbial antigens but rather to unique “stress antigens” that are markers of cell infection or transformation. Vδ1 T cells reside mainly within epithelial tissues such as the intestinal epithelium and epidermis, where they might provide a first line of immunosurveillance against malignancy1,3 by recognizing ligands such as MHC Class I-related molecules (i.e., MICA and MICB), whose expression can be induced in response to infection, injury or cellular transformation.4–6 These molecules have no role in the presentation of peptide antigens, but may function themselves as tumor-associated antigens.7 Of note, similarly to αβ T cells, Vδ1 T cells can inhibit tumor cell growth and recruit other immune cells by releasing a number of cytokines, including tumor necrosis factor α (TNFα) and interferon γ (IFNγ).8–9
Because of the intraepidermal location of normal melanocytes, we considered melanoma an appropriate model to study the antitumor properties of Vδ1 T cells. In addition, previous studies have reported a variable infiltration of melanoma lesions with γδ T cells.10–12 These observations prompted us to analyze clinical grade tumor-infiltrating lymphocyte (TIL) products from patients with metastatic melanoma. TILs were prepared according to current clinical protocols for adoptive T-cell transfer (ACT). Low frequencies of γδ T cells were found among TILs obtained from most patients, and Vδ1 T cells from patients with high γδ T-cell frequencies were further functionally characterized.
Results
γδ T cells in clinical grade TIL products from melanoma
Twenty-seven clinical grade TIL products generated from an equal number of patients with AJCC melanoma Stage III (n = 11) or Stage IV (n = 16) were available for analysis. All such products contained > 97% of CD3+ cells (data not shown). Patients and TIL characteristics are summarized in Table 1. Among TILs from 22 out of 27 patients a low frequency of CD4-CD8- (double negative, DN) γδ T cells was present. Vδ1 T cells were detected among TILs from 20 out of 27 patients (Table 1). The Vδ1+ subset represented the majority of γδ T cells detected, accounting for 60 ± 35%.
Table 1. Summary of patient characteristics and clinical grade TILs.
| Patient n° | Sex | AJCC stage | Previous systemic treatments | Biopsy for TIL generation | γδ+ (%) | Vδ1+ (%) |
|---|---|---|---|---|---|---|
| 1 |
F |
IV |
None |
LN |
0.15 |
0.12 |
| 2 |
M |
IIIC |
None |
SC |
0.012 |
0.01 |
| 3 |
M |
IIIC |
None |
LN |
0.02 |
0.02 |
| 4 |
F |
IV |
IL-2/IFNα |
LN |
0.03 |
0.01 |
| 5 |
M |
IV |
IL-2/IFNα, DC vaccination |
LN |
0.84 |
0.37 |
| 6 |
M |
IV |
IL-2, DC vaccination |
SC |
0.12 |
0.11 |
| 7 |
F |
IV |
DC vaccination |
SC |
BLD |
BLD |
| 8 |
M |
IV |
IL-2/IFNα, anti-CD137, DC vaccination |
LN |
0.31 |
0.08 |
| 9 |
F |
IIIB |
None |
LN |
BLD |
BLD |
| 10 |
F |
IIIC |
None |
SC/LN |
0.02 |
BLD |
| 11 |
F |
IV |
IL-2/IFNα, temozolomide, anti-CTLA4 |
SC |
94.14 |
94 |
| 12 |
F |
IIIC |
None |
LN |
0.41 |
0.25 |
| 13 |
F |
IIIC |
None |
LN |
0.09 |
BLD |
| 14 |
M |
IIIC |
None |
LN |
BLD |
BLD |
| 15 |
M |
IV |
IL-2/IFNα, anti-CTLA4, DC vaccination |
LN |
0.01 |
0.01 |
| 16 |
M |
IV |
IL-2/IFNα, anti-CTLA4 |
SC |
3.08 |
2.98 |
| 17 |
F |
IV |
IL-2/IFNα, anti-CTLA4, DC vaccination |
LN |
0.25 |
0.15 |
| 18 |
F |
IV |
IL-2/IFNα |
LN |
10.67 |
7.8 |
| 19 |
M |
IV |
IL-2/IFNα, anti-CTLA4 |
SC |
0.17 |
0.1 |
| 20 |
M |
IV |
IL-2/IFNα, anti-CTLA4 |
LN |
2.47 |
2.03 |
| 21 |
M |
IV |
IL-2 |
LN |
0.172 |
0.002 |
| 22 |
M |
IV |
IL-2/IFNα, anti-CTLA4 |
SC |
3.57 |
2.86 |
| 23 |
F |
IIIC |
None |
LN |
BLD |
BLD |
| 24 |
F |
IIIC |
None |
LN |
0.11 |
0.03 |
| 25 |
M |
IIIC |
None |
SC |
BLD |
BLD |
| 26 |
M |
IIIC |
None |
LN |
2.37 |
0.48 |
| 27 | F | IV | IL-2/IFNα, anti-CTLA4 | SC | 1.37 | 1.29 |
AJCC, American Joint Comittee on Cancer; anti-CD137 (experimental treatment in protocol); BLD, below the limit of detection (< 0.001%); DC vaccination, dendritic cell vaccination (experimental protocol); IFNα, interferon α; IL-2, interlukin-2; LN, lymph node metastasis; SC, subcutaneous metastasis; TIL, tumor-infiltrating lymphocyte.
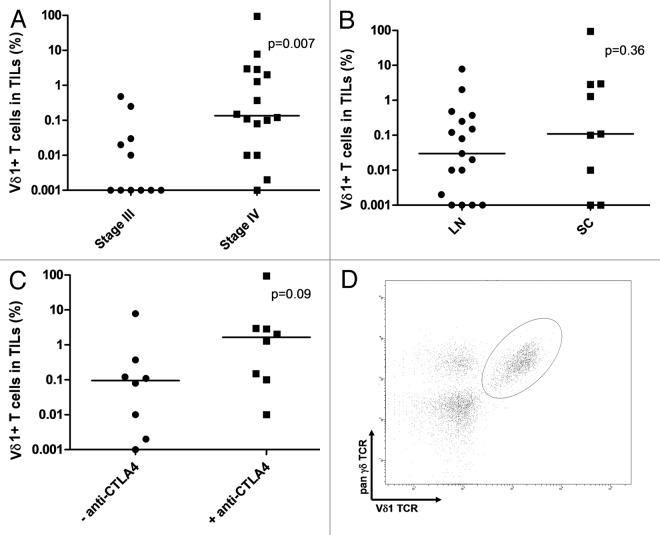
TILs from patients with AJCC Stage IV disease contained significantly more Vδ1 T cells than those from Stage III patients (Fig. 1A). However, 15 out of 16 Stage IV patients included in this study had received prior systemic immune-based therapies (Table 1), while none of the patients with Stage III disease had received these treatments. The most common therapy administered prior to tumor specimen collection was interleukin-2 (IL-2) (15/16 patients). Therefore, we cannot rule out a potential influence of immunotherapies on Vδ1 T cell infiltration.
Figure 1. Vδ1 T cells in clinical grade tumor-infiltrating lymphocytes (TILs). (A–C) Frequency of detected Vδ1 T cells in clinical grade TIL products grouped for AJCC disease stage (A), biopsy origin (B) and prior treatment with anti-CTLA4 antibodies (only patients with stage IV disease are shown) (C). (D) FACS plot from a representative TIL product (from patient 17). An electonic gate was set on the CD3+CD4-CD8- live cell population. LN, lymph node metastatis; SC, subcutaneous metastasis.
No differences were observed between TIL products obtained from subcutaneous or lymph node metastases (Fig. 1B). However, stratification of patients with Stage IV disease based on their previous treatment with anti-CTLA4 antibodies showed a non-significant trend toward an increase of Vδ1 T cells in patients who had previously received this treatment (p = 0.09) (Fig. 1C). A typical plot showing the detection of Vδ1+ T cells in a representative TIL culture is depicted in Figure 1D.
Antitumor activity of Vδ1 T cells
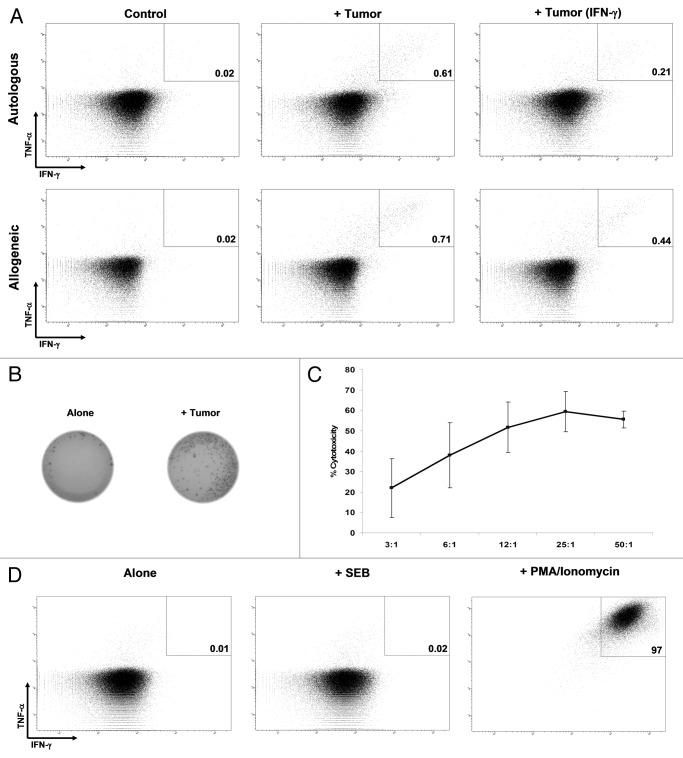
Vδ1 T cells from two patients selected for high frequency of Vδ1 T cells were stimulated with autologous (patient 11 and 18) or a panel of allogeneic HLA-A matched and HLA-A unmatched (patient 11) melanoma cell lines followed by intracellular cytokine staining (ICS) for type 1 cytokines, to assess whether they were able to recognize tumor cells and exert effector functions. Vδ1 T cells from both patients efficiently recognized and generated antitumor responses against autologous target cells with production of Type I cytokines (Fig. 2A), although the frequency of responding cells was low (below 1% in both cases). Additionally, Vδ1 T cells from patient 11 recognized both HLA-A matched and unmatched allogeneic melanoma cell lines (Fig. 2A). Concomitant antitumor responses of αβ T cells (CD8+ or CD4+ T cells) were detected in the same products stimulated with autologous tumors (Fig. 4).
Figure 2. Antitumor activity. (A) Responses of Vδ1 T cells (from patient 11, > 90% of Vδ1 T cells) evaluated with the production of tumor necrosis factor α (TNFα) and interferon γ (IFNγ) when unstimulated (control), or stimulated with autologous or an HLA-A-unmatched allogeneic melanoma cells. Both constitutive responses and responses upon stimulation of cancer cells with 100 IU/mL IFNγ for 72 h (+ IFNγ) are shown. All the plots are gated on Vδ1+ T cells. (B) IFNγ ELISPOT. Unstimulated tumor-infiltrating lymphocytes (TILs,control) or TILs stimulated with autologous tumor cells are shown. (C) Percentage of cytotoxicity at different effector:target ratios of unfractionated TILs vs. one HLA-A-unmatched allogeneic melanoma cell line. (D) Production of TNFα and IFNγ by unstimulated (control) TILs or TILs unspecifically stimulated with the Staphylococcal enterotoxin B (SEB) or PMA/Ionomycin.
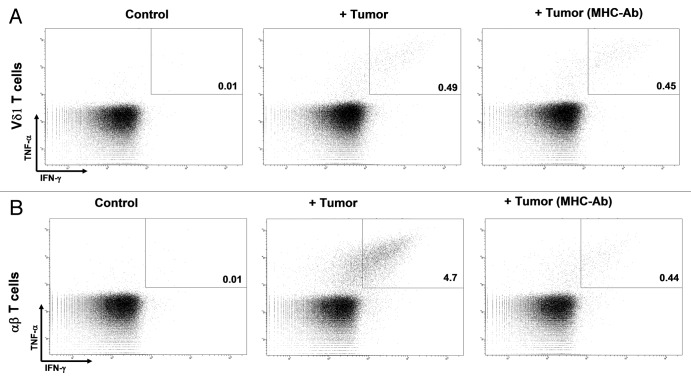
Figure 4. MHC involvement in target recognition. (A and B) Tumor-infiltrating lymphocytes (TILs) were stimulated with autologous tumor cells preincubated with isotype control or a combination of MHC Class I and II-blocking antibodies. While MHC Class I and Class II-blocking antibodies significantly affected tumor cell recognition by αβ T cells (B), they did not modify tumor cell recognition by Vδ1 T cells (A).
IFNγ production by cells from patient 11 (exhibiting > 90% of Vδ1+ cells, see Table 1) was further confirmed by ELISPOT (Fig. 2B). Furthermore the melanoma cell line (from patient 11) inducing the highest Vδ1 T-cell reactivity, as assessed by ICS, was chosen for testing Vδ1 T cell killing abilities. Data confirmed that Vδ1 T cells possess cytotoxic capacity (Fig. 2C). Recognition of healthy non-transformed cells by Vδ1 T cells was excluded, because of the absent or very low (< 0.1%) cytokine production when the TILs from patient 11 against autologous tumor cells were incubated with allogeneic irradiated peripheral blood mononuclear cells (PBMCs) from two different donors (data not shown).
Unspecific activation of Vδ1 T cells
Since the tumor-specific in vitro responses that we detected appeared to be quite low in frequency, we asked whether unspecific stimulating agents would induce a response in a larger fraction of Vδ1 cells. The Staphylococcal enterotoxin B (SEB) is a bacterial superantigen that is believed to stimulate T cells in an oligoclonal fashion depending on the expression of specific variable region gene elements in the β chain of the TCR (Vβ).15–17 However, it has been reported that also cells bearing the γδ TCR are capable to respond to a number of bacterial superantigens including SEB, and may therefore be involved in local immune responses to such antigens as they mimic bacterial infections.18 In addition, Vδ1 T cells isolated from patients with colorectal cancer or with multiple sclerosis have been proposed to efficiently respond to SEB stimulation.19–20
Based on these premises, we have tested the ability of SEB to induce Type 1 cytokine production in TIL products from the two patients displaying a high frequency of Vδ1+ T cells (patients 11 and 18). In this setting, SEB failed induce any response, whereas αβ T cells in the same TIL products responded strongly. In contrast, stimulation with the leukocyte activation cocktail (LAC) led to the production of Type 1 cytokines on > 95% of Vδ1 T cells (Fig. 2D), demonstrating the intrinsic capacity of the vast majority of this cell population to generate meaningful Th1-like responses.
Phenotype and proliferative capacity of Vδ1 T cells
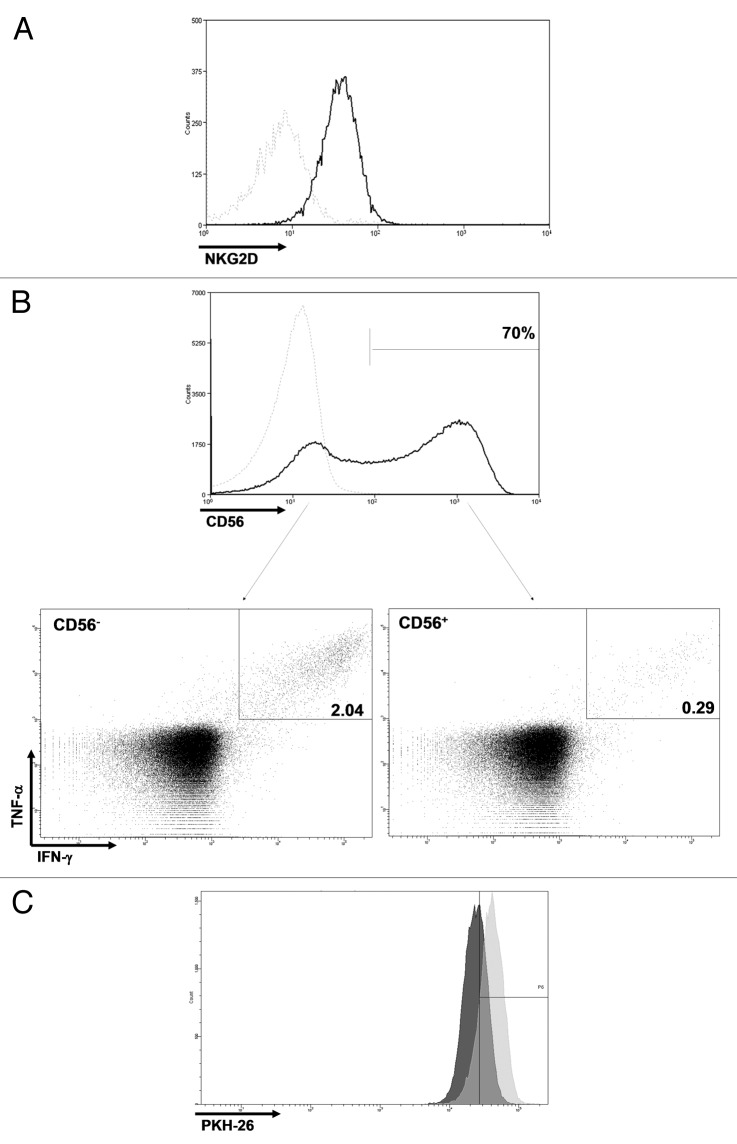
Clinical grade Vδ1 TILs from both patients analyzed (patients 11 and 18) displayed an activated (CD44+CD69+) phenotype (data not shown). In addition, they expressed the activating receptor NKG2D (Fig. 3A) and (about 70 and 100% of all Vδ1 T cells from patient 11 and 18, respectively) the neural cell adhesion molecule 1 CD56 (Fig. 3B). CD56 expression has previously been suggested as a specific signature of highly efficient antitumor Vδ2 T cells.21 Therefore, we examined whether this was also the case for Vδ1 T cells, testing TILs from patient 11. Surprisingly, cells endowed with antitumor effector capacities were much enriched in the CD56- population (Fig. 3B). Moreover, we compared the proliferative capacity of Vδ1 T cells to that of αβ T cells during the rapid expansion protocol (REP) by means of the PKH dilution assay. Data indicate that Vδ1+ T cells proliferate significantly more than αβ T cells in the same culture, with 45% undivided cells vs. 85% (Fig. 3C).
Figure 3. Phenotypic and proliferative characteristics. (A) Vδ1 T cells expressed NKG2D. (B) CD56 was expressed by a large fraction of Vδ1 T cells, and cells with in vitro anticancer activity were enriched in the CD56- compartment. Dotted light gray line: isotype control. (C) From day 8 to day 10 of the rapid expansion protocol (REP), undivided cells were 85% of αβ T cells vs. 45% of Vδ1 T cells. Light gray, αβ T cells; dark gray, Vδ1 T cells.
Involvement of MHC, IFNγ treatment, NKG2D or MICA/B in tumor recognition
Additional experiments confirmed that both autologous and allogeneic tumor recognition of Vδ1 T cells from patient 11 were not dependent on MHC, as cancer cell preincubation with a combination of antibodies blocking MHC Class I and Class II molecules did not influence Vδ1 T cell recognition (Fig. 4A). As a positive control, these antibodies were shown to almost completely abrogate αβ T-cell responses (Fig. 4B). In line with these observations, the pretreatment of cancer cells with a known inducer of MHC expression as well as of the antigen-processing machinery such as IFNγ (for 72 h at 100 IU/mL), which according to our recent data are able to increase the frequency of tumor-reactive αβ T cells in clinical grade TIL products,28 was not able to foster anticancer responses by Vδ1 T cells (Fig. 2A).
To get further insights into the elements underlying the recognition of cancer cells by Vδ1 T cells, experiments with antibodies blocking NKG2D on TILs or MICA/B on autologous and allogeneic target cells before stimulation were performed (on TILs from patient 11). Neither NKG2D nor MICA/B blockade did appear to significantly influence Vδ1 T-cell tumor recognition and responses (data not shown).
Association of Vδ1 T cells infusion and clinical response to ACT
Eleven samples analyzed in this study derived from cell products infused into patients with stage IV melanoma in the context of the ACT trial NCT00937625. The average number of infused Vδ1 T cells was 1.4 ± 2.5 × 109 (average of total T-cells infused > 50 × 109). No major cell infusion-related side effects were observed.29
To date, ten patients were evaluated for clinical response. Three patients experienced an objective response (three complete responses, all ongoing to date). There were no differences between responding vs. non-responding patients for the frequency of Vδ1 T cells in infusion products, but the sample size was small (n = 10, p = 0.83). Notably, one patient achieving a complete response (patient no 18, see Table 1) was infused with 7.8% Vδ1 T cells (approximately 6.5 x 109 cells in total). However, the cell product contained also high numbers of tumor-specific CD8+ T cells (data not shown).
Discussion
The large-scale application of novel immunotherapies such as the adoptive transfer of ex vivo expanded TILs after host lymphodepletion has the potential to significantly improve the prognosis of patients with metastatic melanoma.22 In this report, we showed that the products for infusion generated with current techniques are composed of a heterogeneous population of T cells, which contain not only CD4+ and CD8+ T lymphocytes (as previously reported) but also a small and in some cases significant fraction of γδ T cells, with a prevalence of the Vδ1 subset. Similar to a previous report,10 we demonstrated that Vδ1 T cells are present in most infiltrates of human melanoma. In addition, we found that these cells exerted non MHC-restricted antitumor effector functions toward both autologous and allogeneic melanoma and that, importantly, they could be efficiently expanded with current clinical scale methods for ACT. Indeed, detectable amounts of Vδ1 T cells in TILs after REP were found in 20 out of 27 patients analyzed, and ACT infusion products from ten patients contained on average more than 1 × 109 of these cells. On the contrary, other melanoma-infiltrating immune cells such as natural killer (NK) cells do not efficiently proliferate using these expansion methods (namely REP) and are not commonly detected among clinical grade TILs.13,23
We detected an increased frequency of Vδ1 T cells in TIL products from patients with AJCC stage IV compared with Stage III disease. This difference is in apparent contrast with the findings obtained by Bachelez et al.,10 who reported a higher frequency of Vδ1 T cells in primary than in metastatic melanoma specimens. However, the settings considered are certainly different as the samples used in our study were all represented by regional or distant metastatic tumor sites. Thus, different expression of surface molecules on malignant melanocytes at subsequent stages of tumor progression, leading to the recruitment of different lymphocyte subsets, may underlie the observed discrepancy. Moreover, over 90% of patients with AJCC stage IV disease in our study were treated with immunotherapies before specimen collection, namely, about 85% received IL-2 and 50% were also treated with anti-CTLA4 antibodies. Therefore, the possibility that immunotherapies would induce a relative increase of Vδ1 T-cell over αβ T-cell infiltration cannot be ruled out, in particular for IL-2, which represented the most common treatment administered to our group of patients.
Several reports pointed out that CD56 is an important effector marker of NK, αβ and Vγ9Vδ2 T cells.21,24–27 Our data indicated that this may not apply to Vδ1 T cells, as tumor-reactive Vδ1 T cells were enriched in the CD56- compartment. This assumption needs to be validated as these results were obtained with TILs from a single patient.
In conclusion, the demonstration that Vδ1 T cells can exert antitumor effector functions in vitro coupled with data showing that large numbers (up to 6.5x109 cells) of these cells can be safely transferred into patients with metastatic melanoma suggest that Vδ1 T cells may represent a potentially useful therapeutic tool that should be further scrutinized. In addition, given their theoretical role in immunosurveillance, strategies for the stimulation of Vδ1 T-cell function may be translated into adjuvant treatments for patients at high risk of relapse.
Materials and methods
TIL products and melanoma cell lines
All the procedures were approved by the Scientific Ethics Committee for the Capital Region of Denmark. Written informed consent was obtained from patients before any procedure according to the Declaration of Helsinki. Tumor specimens of at least 1cm3 were obtained from patients with melanoma AJCC Stage III or IV undergoing standard-of-care surgical procedures or specimen collection for enrolment in a clinical trial (clinicaltrials.gov identifier: NCT 00937625). Clinical grade TIL products were generated from tumor fragments obtained from patients with metastatic melanoma with a 2 step protocol including a slow expansion IL-2 containing media (pre-REP phase), and a REP, as described previously.13 Melanoma cell lines were generated from tumor fragments, as described.13
Flow cytometry and antitumor activity of TILs
The following fluorochrome-conjugated antibodies were used for flow cytometry: FITC-conjugated TCR δ TCS1 (TCR1055, Thermo-Fisher), CD4 (345768), CD44, CD69 (347823) ; PE-conjugated CD56 (345812), pan-γδ TCR (333141), ; PECy7-conjugated IFN-γ (557643); PerCP-conjugated CD8 (345774), NKG2D (FAB139C, R&D Systems); APC-conjugated TNF-α (554514). Unless otherwise specified, antibodies used for flow cytometry were from BD. Fixation/Permeabilization Buffer (00–5223–56 and 00–5123–43), Permeabilization Buffer (00–8333–56) and Fixable Viability Dye eFluor® 450 (65–0863–14) were from Ebiosciences, GolgiPlug (555029) from BD and SEB (S4881) from Sigma-Aldrich. Leukocyte Activation Cocktail with GolgiPlug (550583, LAC; containing phorbol 12-myristate 13-acetate/ionomycin and brefeldin A) was from BD.
Evaluation of antitumor activity by ICS was performed as previously described.13 For blocking experiments, target or effector (for NKG2D blockade) cells were incubated for 30 min at 37°C with blocking antibodies. Antibodies used were anti-HLA-ABC (M0736), anti-HLA-DR, DP, DQ (M0775, both from Dako), anti-NKG2D (MAB139, clone 149810, R&D Systems), anti-MICA/MICB (320909, clone 6D4, Biolegend). The number of IFNγ-secreting TILs was quantified by IFN γ ELISPOT, as previously described.13 To assess the cytotoxic abilities of Vδ1 T cells, a flow-cytometry based assay was applied as previously described.14 A small modification of the assay was introduced by replacement of carboxyfluorescein succinimidyl ester with PKH-26 (PKH26GL-1KT, Sigma-Aldrich) and propidium iodide with 7-actinomycin D (559925, BD).
Analysis of cell proliferation
To assess the proliferation of Vδ1 T cells, TILs were stained with PKH26 Red Fluorescent Cell Linker at day 8 of REP according to the manufacturer’s instructions. Red fluorescence from the CD4+ or CD8+ cell populations and from the CD4-CD8- cells was assessed after 48 h. As negative control (no proliferation) and to set the gate of the undivided cells, a PKH-stained cell sample was stored at 4°C for the entire period of incubation. Samples were analyzed using a BD FACSCanto II flow cytometer. Analysis was performed with BD FacsDiva Software. Dead cells were excluded from the analysis based on 7-actinomycin D positivity.
Assessment of clinical responses
Responses to ACT regimens were assessed with standard RECIST criteria in the context of a pilot clinical trial of T-cell therapy for patients with advanced melanoma (clinicaltrials.gov identifier NCT00937625).29
Statistical analyses
Data from different groups were compared with the non parametric two-tailed Mann-Whitney test. All the analyses were performed with Graph Pad Prism 5 software (Graph Pad Software Inc.).
Acknowledgments
Kirsten Nikolajsen is acknowledged for laboratory assistance. The studies were supported by grants from Aase and Ejnar Danielsens Foundation, The Danish Cancer Society, the Lundbeck Foundation and the Capital Region of Denmark Research Foundation.
Glossary
Abbreviations:
- ACT
adoptive T-cell therapy
- AJCC
American Joint Committee on Cancer
- CTLA-4
cytotoxic T lymphocyte antigen 4
- ICS
intracellular cytokine staining
- LAC
leukocyte activation cocktail
- RECIST
response evaluation criteria in solid tumors
- REP
rapid expansion protocol
- SEB
Staphylococcal enterotoxin B
- TCR
T-cell receptor
- TIL
tumor-infiltrating lymphocyte
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21659
References
- 1.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 3.Shimura E, Hozumi N, Kanagawa O, Metzger D, Chambon P, Radtke F, et al. Epidermal gammadelta T cells sense precancerous cellular dysregulation and initiate immune responses. Int Immunol. 2010;22:329–40. doi: 10.1093/intimm/dxq014. [DOI] [PubMed] [Google Scholar]
- 4.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–40. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Huang J, Chen H, Cui L, He W. Vdelta1 T cell receptor binds specifically to MHC I chain related A: molecular and biochemical evidences. Biochem Biophys Res Commun. 2006;339:232–40. doi: 10.1016/j.bbrc.2005.10.198. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Cui L, He W. Distinct pattern of human Vdelta1 gammadelta T cells recognizing MICA. Cell Mol Immunol. 2005;2:253–8. [PubMed] [Google Scholar]
- 9.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 10.Bachelez H, Flageul B, Degos L, Boumsell L, Bensussan A. TCR gamma delta bearing T lymphocytes infiltrating human primary cutaneous melanomas. J Invest Dermatol. 1992;98:369–74. doi: 10.1111/1523-1747.ep12499808. [DOI] [PubMed] [Google Scholar]
- 11.Yazdi AS, Morstedt K, Puchta U, Ghoreschi K, Flaig MJ, Rocken M, et al. Heterogeneity of T-cell clones infiltrating primary malignant melanomas. J Invest Dermatol. 2006;126:393–8. doi: 10.1038/sj.jid.5700082. [DOI] [PubMed] [Google Scholar]
- 12.Petrini I, Scatena C, Naccarato AG, Petrini M. CD57 and γδ T-cell receptor expression in nodal metastatic spread of melanoma. Eur J Clin Invest. 2012;42:575–6. doi: 10.1111/j.1365-2362.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 13.Donia M, Junker N, Ellebaek E, Andersen MH, Straten PT, Svane IM. Characterization and comparison of “Standard” and “Young” tumor infiltrating lymphocytes for adoptive cell therapy at a Danish Translational Research Institution. Scand J Immunol. 2011 doi: 10.1111/j.1365-3083.2011.02640.x. [DOI] [PubMed] [Google Scholar]
- 14.Godoy-Ramirez K, Mäkitalo B, Thorstensson R, Sandström E, Biberfeld G, Gaines H. A novel assay for assessment of HIV-specific cytotoxicity by multiparameter flow cytometry. Cytometry A. 2005;68:71–80. doi: 10.1002/cyto.a.20189. [DOI] [PubMed] [Google Scholar]
- 15.Roberts AI, Blumberg RS, Christ AD, Brolin RE, Ebert EC. Staphylococcal enterotoxin B induces potent cytotoxic activity by intraepithelial lymphocytes. Immunology. 2000;101:185–90. doi: 10.1046/j.1365-2567.2000.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayball JD, Robinson JH, O’Hehir RE, Verhoef A, Lamb JR, Lake RA. Identification of two binding sites in staphylococcal enterotoxin B that confer specificity for TCR V beta gene products. Int Immunol. 1994;6:199–211. doi: 10.1093/intimm/6.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- 18.Rust CJ, Koning F. Gamma delta T cell reactivity towards bacterial superantigens. Semin Immunol. 1993;5:41–6. doi: 10.1006/smim.1993.1006. [DOI] [PubMed] [Google Scholar]
- 19.Maeurer M, Zitvogel L, Elder E, Storkus WJ, Lotze MT. Human intestinal V delta 1+ T cells obtained from patients with colon cancer respond exclusively to SEB but not to SEA. Nat Immun. 1995;14:188–97. [PubMed] [Google Scholar]
- 20.Stinissen P, Vandevyver C, Raus J, Zhang J. Superantigen reactivity of gamma delta T cell clones isolated from patients with multiple sclerosis and controls. Cell Immunol. 1995;166:227–35. doi: 10.1006/cimm.1995.9975. [DOI] [PubMed] [Google Scholar]
- 21.Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, et al. Isopentenyl pyrophosphate-activated CD56+ gammadelta T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 2010;33:547–56. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vgamma2Vdelta2 T cells. BMC Immunol. 2009;10:50. doi: 10.1186/1471-2172-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohavy O, Targan SR. CD56 marks an effector T cell subset in the human intestine. J Immunol. 2007;178:5524–32. doi: 10.4049/jimmunol.178.9.5524. [DOI] [PubMed] [Google Scholar]
- 26.Casado JG, Soto R, DelaRosa O, Peralbo E, del Carmen Muñoz-Villanueva M, Rioja L, et al. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–71. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 28.Donia M, Hansen M, Sendrup SL, Zeeberg Iversen T, Ellebaek E, Andersen MH. Methods to improve adoptive T-cell therapy for melanoma: IFN-gammma enhances anticancer responses of cell products for infusion. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.336. [DOI] [PubMed] [Google Scholar]
- 29.Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met O, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]