Abstract
Arginase 1 (ARG1) is an important enzyme in amino acid metabolism that also exerts immunoregulatory function. High ARG1 expression, which is associated with cell cycle arrest and functional unresponsiveness in T cells, has been observed after trauma, infections and in cancer patients. We studied ARG1 expression in early-stage breast cancer patients (stage 1, n = 20; stage 2, n = 23) by multi-parametric flow cytometry and immunohistochemistry. Despite a low tumor burden, ARG1 expression was significantly increased in blood-derived myeloid cells of breast cancer patients compared with healthy controls. The ARG1hi myeloid population in the blood of cancer patients contained a high frequency of CD14+ cells and was, therefore, distinct from the granulocytic ARG1+ population observed in control individuals. Expression of ARG1 in patient blood cells correlated with tumor grade and was significantly reduced after surgical tumor removal. ARG1+ myeloid cells could also be detected in tumors and tumor-draining lymph nodes, where ARG1 expression levels exceeded those measured in the blood. We conclude that even patients with early-stage breast cancer exhibit tumor-related changes of ARG1 expression. The level of ARG1-mediated immunomodulation at this early stage remains to be determined. However, high ARG1 expression is likely to interfere with antitumor T-cell responses and immunotherapeutic interventions, making ARG1 or its downstream effector interesting therapeutic targets.
Keywords: Arginase, cancer, immunosuppression, mammary carcinoma, myeloid cells
Introduction
Arginase (ARG) is an enzyme catalyzing the conversion of the amino acid l-arginine (L-Arg) to ornithine and urea. There are two ARG isoforms: ARG1 is a cytosolic enzyme constitutively expressed in the liver that can be induced in cells of the myeloid lineage, while ARG2 is found in the mitochondria of cells in the brain, mammary glands, small intestine, kidney as well as in macrophages.1 Neutrophils represent the predominant ARG1-expressing population in the human blood.2 Human ARG1 is secreted into the microenvironment, in contrast to murine ARG1, which remains confined within the cells.2,3
In addition to its roles in amino acid metabolism, ARG1 can mediate immunoregulatory functions. T lymphocytes are unable to generate L-Arg and therefore rely on importing this conditionally essential amino acid from their microenvironment.4 T cells that are deprived of L-Arg undergo cell cycle arrest and lose the ability to proliferate in response to stimulation.5,6 Expression of ARG1 has frequently been described as an immunosuppressive mechanism employed by myeloid-derived suppressor cells (MDSC) as well as by M2-polarized and tumor-associated macrophages.7 Furthermore, high expression and/or activity of ARG1 as well as reduced levels of L-Arg are often observed in the blood of cancer patients.8-11
Even though the prognosis of breast cancer has significantly improved over the past decades, this malignancy still remains one of the most common cancers among women worldwide, killing more than 450,000 each year. Although breast cancer is sometimes considered a poorly immunogenic cancer, it has been shown to affect the immune system, for instance by inducing the accumulation of regulatory T cells.12 Most studies investigating immunity in breast cancer patients or patients suffering from other malignancies have focused on advanced disease stages, when immunosuppression is assumed to be profound. We have recently demonstrated that even patients with early-stage breast cancer exhibit alterations of the immune system, including pronounced T-cell differentiation and loss of molecules that are important for T-cell functions.13,14
Here, we further show that peripheral blood myeloid cells from patients with early-stage breast cancer express increased levels of ARG1, potentially exerting immunosuppressive effects. The ARG1-expressing myeloid population observed in breast cancer patients was distinct from that detected in healthy controls and decreased in size after surgical tumor removal. ARG1-producing cells could even be detected within the tumor, where ARG1 expression was even higher than in blood. These findings strengthen the notion that even early-stage tumors can modulate the immune system, with predominantly negative effects.
Results
We investigated the role of the immunomodulatory enzyme ARG1 in patients with stage 1 (n = 20) or stage 2 (n = 23) breast cancer. All patients were scheduled to undergo surgical tumor resection and sentinel lymph node biopsy. None had received preoperative therapy. Ten healthy women undergoing similar surgery for non-neoplastic lesions were enrolled as controls. Blood samples from cancer patients and healthy donors (HDs) were drawn before and 3–4 weeks after surgery. Patient characteristics have been previously published.13
The expression of ARG1 is increased in the peripheral blood mononuclear cells from breast cancer patients
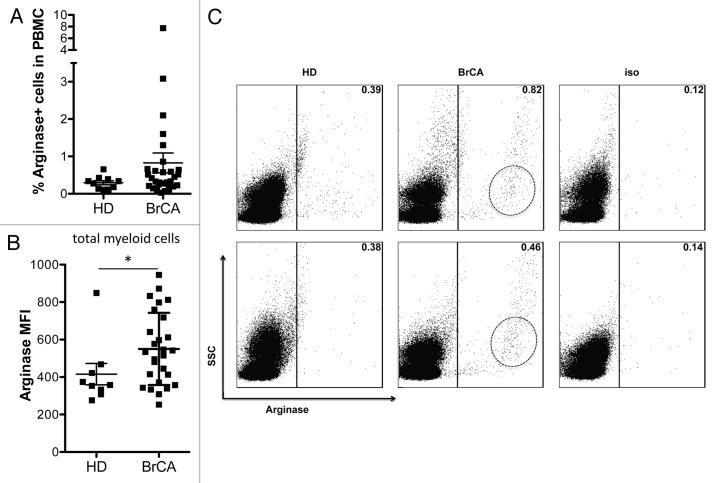
We observed a trend towards a higher frequency of ARG1-expressing cells in peripheral blood mononuclear cells (PBMCs) from cancer patients compared with those from HDs, though this did not reach statistical significance (Fig. 1A). However, we did detect significantly higher levels of ARG1 expression in myeloid cells of breast cancer patients compared with those from HDs (Fig. 1B). As illustrated in Figure 1C, the predominant ARG1-expressing cell population in HDs exhibited a rather elevated side-scatter (SSC), indicating that it was probably composed of granulocytes. These cells were positive for ARG1, but most of them expressed only low levels of the enzyme. Instead, myeloid cells from breast cancer patients expressed high levels of ARG1, reflected in their high ARG1 mean fluorescence intensity (MFI), particularly among cells exhibiting a SSC compatible with that of monocytes. Granulocytic cells in breast cancer patients expressed high ARG1 levels, too. However, they were unlikely to account for the significant difference between patients and controls, as the former had a significantly reduced frequency of cells with granulocyte characteristics (Fig. S1A), making the granulocyte ARG1 expression a minor contributor to the total ARG1 mean fluorescence intensity (MFI).
Figure 1. Arginase 1 expression is increased in peripheral blood of breast cancer patients. (A) Frequency of ARG1+ myeloid cells in the peripheral blood of healthy donors (HD, n = 10) and breast cancer (BrCA) patients (n = 30). (B) Mean fluorescence intensity (MFI) of ARG1 in myeloid cells from the peripheral blood of HD (n = 9) and BrCA patients (n = 27). (C) Representative examples of ARG1 positivity and scatter characteristics of ARG1+ cells in 2 HD (left) and 2 BrCA patients (middle); the right panel shows the isotype controls.
ARG1-expressing cells in the blood of breast cancer patients and healthy controls are distinct from each other
Since the ARG1hi cells in breast cancer patients did not appear to be granulocytes, we set out to characterize them further. In accordance with their myeloid cell FSC/SSC profile (not shown), most of these cells expressed CD11b. The difference in ARG1 expression between cells obtained from breast cancer patients and HDs became highly significant when the comparison was limited to CD11b+ cells (Fig. 2A). A similar difference was detectable upon gating on the CD14+ population (Fig. 2C). Of note, all CD14+ cells express CD11b. On the other hand, no difference in ARG1 expression was observed when comparing CD11b+CD14- cells from cancer patients to those drawn from HDs (data not shown), suggesting that the difference in ARG1 expression resides in the CD14+ myeloid population. This was further substantiated by the fact that there was no difference in ARG1 expression between CD14- myeloid cells obtained from breast cancer patients and HDs (Fig. 2D). Of note, the frequency of CD14+ cells obtained from breast cancer patients and HDs was similar (Fig. S1B). Figure 2B shows a representative example of CD14 expression by ARG1+ cells derived from breast cancer patients and HDs.
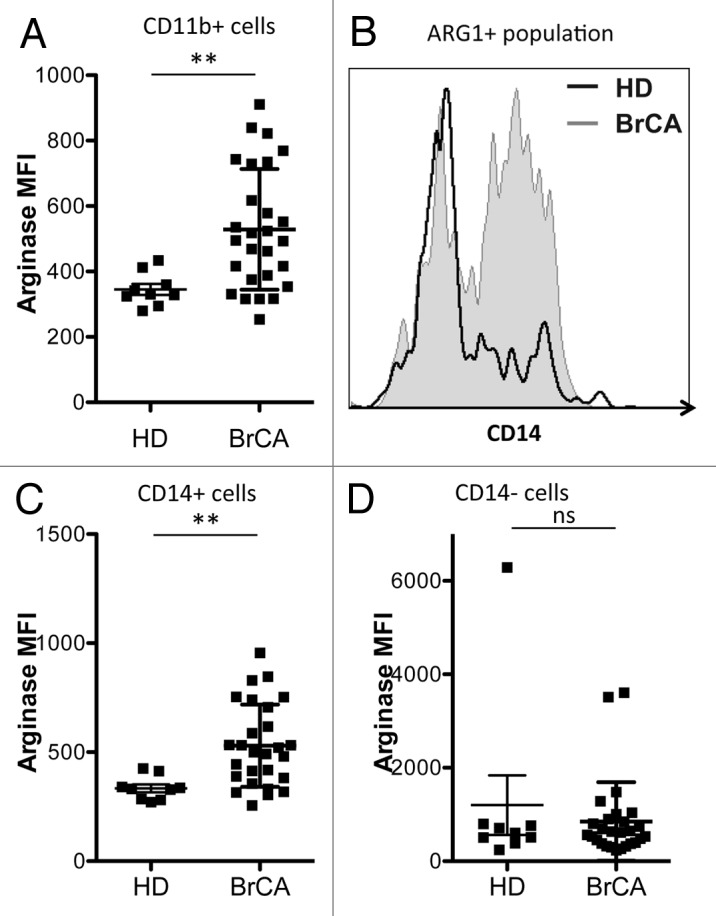
Figure 2. Arginase 1-positive cells in breast cancer patients are distinct from those in healthy controls. (A) Mean fluorescence intensity (MFI) of ARG1+ among CD11b+ myeloid cells from the peripheral blood of healthy donors (HD, n = 9) and breast cancer (BrCA) patients (n = 27). (B) representative histogram of CD14 expression within the gated ARG1+ population in HD and BrCA patients. (C and D) MFI of ARG1 among CD14+ (C) and CD14- (D) myeloid cells from the peripheral blood of HD (n = 9) and BrCA patients (n = 27).
ARG1 expression correlates with tumor grades
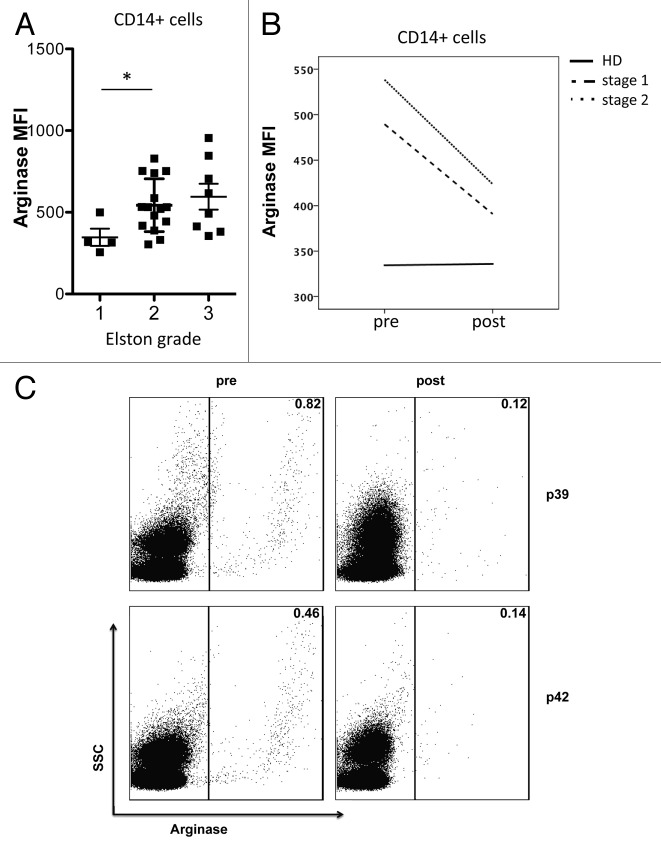
Breast carcinomas are classified into Elston grade 1 to 3 based on their tubuli formation, nuclear pleomorphism, and mitotic activity, with grade 3 corresponding to more aggressive tumor characteristics. Interestingly, patients with a higher Elston grade had a higher expression of ARG1 in their circulating CD14+ myeloid population (Fig. 3A). This difference was significant between patients with Elston grades 1 and 2. We further investigated ARG1 expression in relation to other clinical parameters, such as presence of lymph node metastasis, tumor size, and type of tumor, but did not detect other significant correlations.
Figure 3. Arginase 1 MFI is correlated with tumor grade and decreases after surgical tumor removal. (A) Mean fluorescence intensity (MFI) of ARG1 among CD14+ myeloid cells from the peripheral blood of patients with Elston grade 1 (n = 4), 2 (n = 15) or 3 (n = 8) tumors. (B) MFI of ARG1 among CD14+ myeloid cells from the peripheral blood of healthy controls (n = 9/11) or breast cancer patients (n = 27/34) before and after surgery
ARG1 expression in peripheral blood cells is reduced after tumor removal
Three to four weeks after surgery, a second blood sample was collected from all patients and controls. While ARG1 expression in CD14+ myeloid cells from HDs remained constant over time, ARG1 levels in CD14+ cells from breast cancer patients significantly decreased after tumor removal (Fig. 3B and C).
High levels of ARG1 expression can be detected in tumors and tumor-draining lymph nodes from breast cancer patients
In addition to peripheral blood, we analyzed ARG1 expression in tumors and sentinel lymph nodes from cancer patients. ARG1 expression was significantly higher in the myeloid cells found within tumor and lymph nodes than in those obtained from blood samples (Fig. 4A). Tumor-associated CD14+ cells expressed higher ARG1 than those found in the blood and lymph nodes (Fig. 4B). Analysis of myeloid marker expression on tissue-resident ARG1+ cells revealed that the majority of ARG1+ cells in the tumor expressed little or no CD14, though they did express CD11b and CD33 to varying extents, indicating that they were of myeloid lineage. ARG1+ cells in tumor-draining lymph nodes expressed intermediate levels of CD11b, CD33, and CD14 (data not shown). The intensity of ARG1 expression in the tumor was not correlated with tumor grade, tumor type, metastatic status, or tumor size. No difference was detected between ARG1 expression in myeloid cells from tumor-free vs. metastatic sentinel lymph nodes.
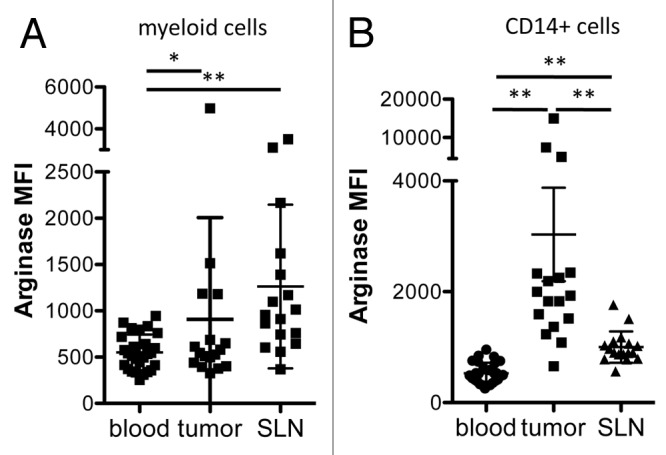
Figure 4. Arginase 1 MFI is higher in tumor-associated than blood-derived myeloid cells. Mean fluorescence intensity (MFI) of ARG1 among total myeloid cells (A) or CD14+ myeloid cells (B) from the peripheral blood (n = 27), tumors (n = 17) or sentinel lymph nodes (SLNs, n = 17) of breast cancer patients.
ARG1-expressing cells can infiltrate the tumor
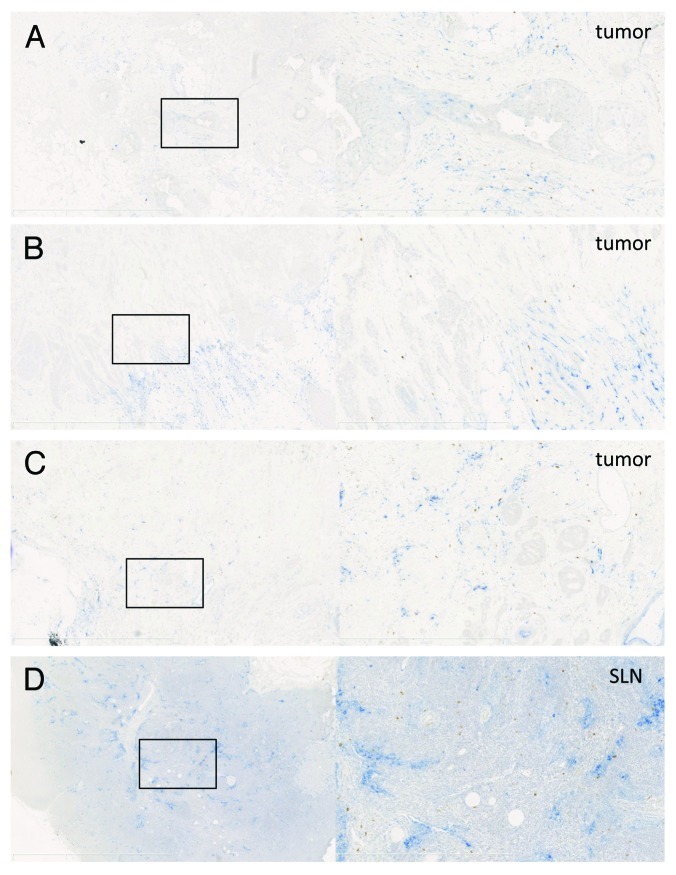
To better evaluate the spatial distribution of ARG1-expressing cells, immunohistochemical double staining of CD14 and ARG1 was performed. Figures 5A-C show representative examples of ARG1-positive cells in the tumor and their location relative to CD14+ myeloid cells. CD14+ cells could be detected in the majority of analyzed tumors. These cells were typically localized in the tumor periphery, but sometimes, CD14+ areas could also be detected within the tumor. ARG1-expressing cells were mostly localized in areas containing CD14+ cells. Consequently, most ARG1+ cells were located in the tumor periphery. Due to the diffuse nature of the CD14 staining, it was not possible to count CD14+ARG1+ double-positive cells, though occasionally, cells that appeared to be truly double-positive were observed.
Figure 5. Arginase 1 staining in tumors and tumor-draining lymph nodes. (A–D) Examples of CD14 (blue, nuclear hematoxilin is light blue) and ARG1 (brown) staining in breast tumors (A–C) and a tumor-draining lymph node (D). Left panels = 2.5× magnification, right panels = 10× magnification of the selection on the left side.
Overall, 43% (13/30) of tumors analyzed by immunohistochemistry (IHC) contained ARG1+ cells. This correlates reasonably well with the number of tumors (10/17) in which the MFI of ARG1 in myeloid cells was > 550, the level used to define ARG1+ cells by flow cytometry. However, even though differences in staining intensity could be observed by IHC, it was difficult to correlate such intensity scores to MFI values as measured by flow cytometry (data not shown). Based on their morphology, no ARG1-producing tumor cells were detected. We also analyzed CD14 and ARG1 expression in tumor-draining lymph nodes. In 44% (14/32) of sentinel lymph nodes, ARG1+ cells were found. While CD14+ cells were mostly localized in the paracortex and sinuses, ARG1+ cells could be observed anywhere within the lymph node and did not show a tendency to concentrate in areas containing CD14+ cells. A representative example of this distribution is shown in Figure 5D.
Discussion
We investigated the expression of ARG1 in blood, lymph nodes, and tumors from patients with early-stage breast cancer. Previous studies have defined ARG1 as a potent immunomodulatory factor mediating T-cell suppression as exerted by MDSCs and tumor-associated macrophages. Increased ARG1 activity has been detected in the serum of patients with advanced cancer, including renal cell carcinoma, colorectal cancer, glioblastoma, and breast cancer.8,9,11,15 As a result of high ARG1 expression, decreased levels of arginine have been detected in the serum of cancer patients, and this has been shown to correlate with impaired T-cell function. A similar change in arginine metabolism has been observed in patients suffering from traumatic injuries,16-18 active infection,19,20 and sepsis.21 As a consequence, arginine-supplemented nutrition has been given to surgical and critically ill patients, and in some studies this was shown to reduce infectious complications in surgical patients, including patients undergoing tumor resection.22,23
To our knowledge, we are among the first to report a similar role for ARG1 in early-stage cancer patients. Strikingly, the presence of minimal tumors resulted in the upregulation of ARG1 expression by peripheral blood myeloid cells. Interestingly, the ARG1-expressing population in breast cancer patient blood contained a significant population of CD14+ myeloid cells and was therefore distinct from the mainly granulocytic ARG1+ cells observed in the blood of healthy donors.
The systemic changes observed here are somewhat puzzling with regard to the limited tumor burden in our patient population, but nonetheless are likely to reflect the immunomodulatory capacity of these early tumors. Of note, no clear association of ARG1 expression and T-cell dysfunction, including decreased proliferation or altered ability to produce cytokines in response to stimulation (as described in ref. 13), could be observed (data not shown). Furthermore, ARG1-producing cells did not exhibit any of the phenotypes classically associated with MDSCs, and we did not observe increased frequencies of potential MDSCs, including commonly described monocytic and granuloytic populations, in our cancer patients (I.P. unpublished observations). The functional consequences of the high ARG1 expression that we observed remain to be determined.
We also observed cells with high ARG1 expression within the tumors, though they appeared to be phenotypically distinct from their circulating counterparts. Though they mostly co-localized with CD14+ cells, a few of them were double-positive when analyzed by flow cytometry. Interestingly, even within the tumor, CD14+ARG1+ cells expressed high levels of ARG1, exceeding those observed in the total myeloid population. Of note, ARG1+ cells, like CD14+ cells, were mostly located in the tumor periphery and rarely infiltrated the tumor mass. There was great variation in the number of ARG1+ cells observed in the tumor, yet we did not observe any correlation between the frequency of ARG1+ cells or staining intensity and clinical characteristics.
In conclusion, we report the modulation of ARG1 expression in early-stage cancer patients. The increase in ARG1 expression seemed to be clearly tumor-dependent, as it decreased after tumor resection and was associated with tumor grade. However, no obvious correlation with other clinical features could be detected, and the biological significance of the high ARG1 expression in this patient population remains to be determined. It is likely that the observed differences reflect the early stages of tumor-mediated immune modulation. At a later stage, ARG1-dependent starvation of lymphocytes or production of reactive oxygen species might contribute to immunosuppression and favor tumor progression.
Due to its universal role in amino acid metabolism, ARG1 is a suboptimal therapeutic target. However, several drugs have been shown to act preferentially on cells that can exert immunosuppression via ARG1-dependent mechanisms24,25 and these could therefore be applied to improve antitumor immunity in cancers that promote ARG1 upregulation.
Materials and Methods
Patients
This study included 43 patients with primary, unilateral, invasive breast cancer. Details on this patient population have previously been published26 and are summarized in Table 1. As control individuals, we included 10 subjects scheduled for the surgical removal of benign breast lesions, such as fibroadenomas or papillomas. All participants provided informed written consent before inclusion. This study was approved by the Regional Ethical Review Board at Karolinska Institutet, Stockholm.
Table 1. Patient and tumor characteristics*.
| |
Median (range) |
N (%) |
|---|---|---|
| Age | 61 (39–91) | |
|
Tumor diameter (mm) |
15 (6–100) |
|
|
Histological type |
|
|
| ductal |
|
36 (83.7) |
| lobular |
|
5 (11.6) |
| mixed |
|
1 (2.3) |
| medullar |
|
1 (2.3) |
|
Estrogen receptor status |
|
|
| positive |
|
35 (81.4) |
| negative |
|
8 (18.6) |
|
Progesterone receptor status |
|
|
| positive |
|
30 (69.8) |
| negative |
|
13 (30.2) |
|
HER2 status (FISHa) |
|
|
| positive |
|
3 (7) |
| negative |
|
40 (93) |
|
HER2-neu status (IHCb) |
|
|
| 0 |
|
30 (69.8) |
| 1+ |
|
5 (11.6) |
| 2+ |
|
5 (11.6) |
| 3+ |
|
3 (7) |
|
LVIc |
|
|
| yes |
|
10 (23.3) |
| no |
|
33 (76.7) |
|
Elston histological grading |
|
|
| 1 |
|
9 (20.9) |
| 2 |
|
21 (48.8) |
| 3 |
|
13 (30.2) |
|
Ki67 in tumor cells (%)d |
23.4 (3.0) |
|
|
SN result |
|
|
|
N0 |
|
32 (74.4) |
| negative |
|
28 (65.1) |
| submicrometastasis |
|
4 (9.3) |
|
N1 |
|
11 (25.6) |
| micrometastasis |
|
2 (4.6) |
| macrometastasis |
|
9 (20.9) |
|
Tumor stage |
|
|
| 1a and b |
|
20 (46.5) |
| 2a and b | 23 (53.5) |
a Fluorescence in vitro hybridization; bimmunohistochemistry; clymphovascular invasion; dKi67, mean (SD). *This information has previously been published.
Surgical procedure and handling of specimens
All patients were surgerized under general anesthesia. Sentinel lymph nodes were surgically removed as previously described. Samples for flow cytometric analysis were collected by scraping the surface of fresh tissues repeatedly with a scalpel and suspending the collected cells in cold PBS + 1% human serum albumin (HSA, Octopharma, 001693) as previously described. Paraffin-embedded tissue sections from the same cases were obtained from the local biobank. Blood samples were taken before and 2–4 weeks after surgery. Isolation of peripheral blood mononuclear cells was performed using density gradient centrifugation over Ficoll (VWR, 17–1440–03).
Flow cytometry
Six to nine-parameter flow cytometry was used to analyze the phenotype and function of immune cells in peripheral blood, lymph nodes, and tumor. This method allows the characterization of multiple cellular characteristics even in small sample amounts. Antibodies: CD68-Fitc (Biolegends, 333806), HLA-DR-PE (BD, 555812), CD33-APC (Caltag, MHCD3305), CD11b-APC-Cy7 (BD, 560914), CD14-Pacific Blue (Biolegends, 301828), and ARG1 (HyCult Biotech, HM2162). Cells were analyzed using an LSRII flow cytometer (BD Biosciences) and FlowJo® analysis software (Treestar).
Phenotyping
Antibody incubations and washes occurred in the dark in PBS + 1% HSA at 4°C unless otherwise indicated. Stainings were performed in 96-well v-bottom plates for 20 min (extracellular staining), followed by 45 min (if intracellular staining was necessary). BD Fix/Perm kit (BD, 554722) was used according to the manufacturer’s instructions to permeabilize cells for intracellular staining and PermWash buffer from the same kit was used for subsequent washing and staining of the intracellular markers. Titration of antibody concentrations was performed prior to study initiation to yield optimal signal-to-noise ratios on control cells. In order to prevent unspecific binding, Fc receptors were blocked before the addition of antibodies by 10 min incubation with 10 μg/mL human IgG (Baxter, LE12L370AH). The intracellular staining of ARG1 was preceded by conjugation of the ARG1 antibody with Zenon-Pacific Orange reagent (Invitrogen, Z25257) according to the manufacturer’s instructions. Granulocytes were used to establish the ARG1 staining procedure. PBMCs, granulocytic and myeloid cells were defined based on their forward- and side-scatter characteristics. Expression of typical myeloid markers, such as CD11b, were confirmed in the gated myeloid population (not shown).
Immunohistochemistry
Paraffin-embedded tissues were cut into 5 μm sections. Glass-mounted tissue sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol, then washed in water. Slides were brought to boil in citrate buffer (pH 6), then boiled at lower effect for 20 min and cooled in water for 20 min. Endogenous peroxidase activity was quenched in 0.5% H2O2 for 30 min, and slides were then rinsed and placed in TBS. To reduce unspecific antibody binding, tissue sections were incubated in 2.5% normal horse serum (Impress, Vector Laboratories, S-2012) for 30 min and then incubated with a mix of mouse anti-CD14 (Novocastra, CD14–223) 1:75 diluted in 1% BSA in TBS and rabbit anti-ARG1 antibody (ATLAS ab:s,HPA024006) 3000-fold dilution in 1% BSA in TBS overnight. After rinsing three times, the secondary biotinylated horse anti-mouse (1:200) and ImPress anti-rabbit peroxidase (PO) coupled antibodies (Vector Laboratories, BA2000 and MP-7401) were mixed together, added and incubated for 30 min. During incubation, the alkaline phosphatase (AP) Avidin-Biotin-Complex (ABC) (Vector Laboratories, PK-4000) was prepared. After washing the slides three times in TBS, ABC-AP was added and incubated for 30 min, and slides were then again washed three times in TBS. Staining was visualized by 3 min incubation with diaminobenzidine (DAB, Vector Laboratories, SK-4100) for ARG1 detection, followed by washing and 20 min incubation with Vector Blue (Vector Laboratories,SK-5300) for CD14 detection. Nuclei were counterstained by a quick dip in a 1% hematoxylin solution followed by a rinse in lukewarm water. Slides were mounted using a water-based mounting reagent (Kaisers Glyceringelantine, MERCK Millipore, 109242).
Statistical methods
Prior to analysis, the normal distribution of all variables was tested using the Shapiro-Wilk test. Based on data normality, the unpaired Student’s t-test or the Mann-Whitney test were employed to compare breast cancer patients and healthy controls. Since the median age of the control population was significantly lower than that of the breast cancer patients, all variables were further tested for correlation with age. If a parameter was found to significantly correlate with age, additional linear regression was performed. For the analysis of parameters with a continuous distribution, the unpaired Student’s t-test, the Mann-Whitney test, a one-way ANOVA or the Kruskal-Wallis test were used. To study the differences in the expression of an immunological parameter in two separate tissue locations at the same time point, the paired Student’s t-test, or the Wilcoxon signed rank test were performed. PASW Statistics 18.0® software was used for all analyses, and statistical significance was set at the 0.05 level for all tests.
Supplementary Material
Acknowledgments
The authors wish to sincerely thank senior pathologist Anders Höög and biobank manager Lisa Viberg for their help in establishing the methodological and logistical procedures necessary for this study. We also highly appreciate the help of all staff at the Breast Center, the surgical ward A23a, and the Department of Pathology, in the enrolment of patients and the collection of research samples. We would further like to thank Inger Bodin for excellent technical assistance and the establishment and performance of the immunohistochemical stainings. I.P. is supported by the Robert Lundgrens Foundation, the Lars Hiertas Memorial Foundation, the Sigurd and Elsa Goljes Memorial foundation and a KID grant from Karolinska Institutet; J.B. is supported by a grant from the Swedish Breast Cancer Association (BRO); R.K. is supported by the Swedish Cancer Society, the Swedish Medical Research Council, the Cancer Society of Stockholm, the European Union (EUCAAD), the Karolinska Institutet and an “ALF project” grant from the Stockholm City Council.
Glossary
Abbreviations:
- ARG1
arginase 1
- L-Arg
L-Arginine
- DAB
diaminobenzidine
- FSC
forward scatter
- HD
healthy donors
- MDSC
myeloid-derived suppressor cells
- SSC
side-scatter
- SLN
sentinel lymph node
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental material may be found here: www.landesbioscience.com/journals/oncoimmunology/article/21678
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21678
References
- 1.Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsen LC, Theilgaard-Mönch K, Christensen EI, Borregaard N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood. 2007;109:3084–7. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 3.Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–56. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Graboń W, Mielczarek-Puta M, Chrzanowska A, Barańczyk-Kuźma A. L-arginine as a factor increasing arginase significance in diagnosis of primary and metastatic colorectal cancer. Clin Biochem. 2009;42:353–7. doi: 10.1016/j.clinbiochem.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Polat MF, Taysi S, Polat S, Böyük A, Bakan E. Elevated serum arginase activity levels in patients with breast cancer. Surg Today. 2003;33:655–61. doi: 10.1007/s00595-002-2563-2. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 13.Boniface JD, Poschke I, Mao Y, Kiessling R. Tumor-dependent down-regulation of the ζ-chain in T-cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer. 2012;131:129–39. doi: 10.1002/ijc.26355. [DOI] [PubMed] [Google Scholar]
- 14.Poschke I, De Boniface J, Mao Y, Kiessling R. Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int J Cancer. 2012;131:1611–20. doi: 10.1002/ijc.27410. [DOI] [PubMed] [Google Scholar]
- 15.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryk JA, Popovic PJ, Zenati MS, Munera V, Pribis JP, Ochoa JB. Nature of myeloid cells expressing arginase 1 in peripheral blood after trauma. J Trauma. 2010;68:843–52. doi: 10.1097/TA.0b013e3181b026e4. [DOI] [PubMed] [Google Scholar]
- 17.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–94. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 18.Ochoa JB, Bernard AC, Mistry SK, Morris SM, Jr., Figert PL, Maley ME, et al. Trauma increases extrahepatic arginase activity. Surgery. 2000;127:419–26. doi: 10.1067/msy.2000.104745. [DOI] [PubMed] [Google Scholar]
- 19.Brys L, Beschin A, Raes G, Ghassabeh GH, Noël W, Brandt J, et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol. 2005;174:6095–104. doi: 10.4049/jimmunol.174.10.6095. [DOI] [PubMed] [Google Scholar]
- 20.Zea AH, Culotta KS, Ali J, Mason C, Park HJ, Zabaleta J, et al. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–93. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 21.Davis JS, Anstey NM. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Crit Care Med. 2011;39:380–5. doi: 10.1097/CCM.0b013e3181ffd9f7. [DOI] [PubMed] [Google Scholar]
- 22.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137(Suppl 2):1681S–6S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 23.Moskovitz DN, Kim YI. Does perioperative immunonutrition reduce postoperative complications in patients with gastrointestinal cancer undergoing operations? Nutr Rev. 2004;62:443–7. doi: 10.1111/j.1753-4887.2004.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 24.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–48. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boniface JD, Poschke I, Mao Y, Kiessling R. Tumor-dependent down-regulation of the ζ-chain in T-cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer. 2012;131:129–39. doi: 10.1002/ijc.26355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.