Abstract
B cells are generally believed to operate as producers of high affinity antibodies to defend the body against microorganisms, whereas cellular cytotoxicity is considered as an exclusive prerogative of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). In conflict with this dogma, recent studies have demonstrated that the combination of interleukin-21 (IL-21) and B-cell receptor (BCR) stimulation enables B cells to produce and secrete the active form of the cytotoxic serine protease granzyme B (GrB). Although the production of GrB by B cells is not accompanied by that of perforin as in the case of many other GrB-secreting cells, recent findings suggest GrB secretion by B cells may play a significant role in early antiviral immune responses, in the regulation of autoimmune responses, and in cancer immunosurveillance. Here, we discuss in detail how GrB-secreting B cells may influence a variety of immune processes. A better understanding of the role that GrB-secreting B cells are playing in the immune system may allow for the development and improvement of novel immunotherapeutic approaches against infectious, autoimmune and malignant diseases.
Keywords: B cell receptor stimulation, CD40 ligand, cytotoxicity, IL-21, immune regulation
Introduction
B cells are central to the humoral immune response as they secrete high-affinity antibodies targeting invading microbial agents. B-cell activation is triggered by the crosslinking of the B-cell receptor (BCR) by specific antigens, which can then be processed and presented to T cells in an MHC-restricted manner.1,2 The most potent cytokine to induce the differentiation of human B cells into plasma cells is the interleukin (IL)-2 family cytokine IL-21.3 IL-21 is mainly provided by T follicular helper (TFH) cells, Th17 cells, and natural killer T (NKT) cells. IL-21 exerts pleiotropic autocrine and paracrine functions such as the induction of cytotoxicity and antitumor activity in CD8+ T, NK and NKT cells.4,5 In B cells, IL-21 stimulation can have at least three different outcomes. First, in the absence of both BCR engagement and T-cell help, IL-21 induces B-cell apoptosis.6 Second, in the presence of CD40 ligation, and either a BCR signal or a Toll-like receptor (TLR) signal, IL-21 fundamentally promotes the differentiation of B cells into long-lived memory and antibody-secreting plasma cells.7,8 Third, we observed that IL-21 can stimulate B cells to express and secrete the active form of the serine protease granzyme B (GrB). In contrast to plasma cell differentiation, however, this transcriptional program occurs only in the absence of CD40 ligation, and is therefore independent of classical T-cell help.9,10
Granzymes are closely related serine proteases that represent key components of the cytotoxic granules of NK cells and CTLs.11,12 Our understanding of the functions of granzymes in general is limited, perhaps with the exception of GrB, which is the most extensively studied member of the family. GrB is a 32 kDa protein that is released from cytotoxic cells via granule exocytosis and that initiates perforin-dependent death in target cells by cleaving caspase-3 at aspartic acid residues,13-15 as well as by activating additional cytotoxic pathways (Table 1).
Table 1. Currently known granzyme B functions.
| Functions resulting in cytotoxicity | Non-cytotoxic functions |
|---|---|
| Cleavage of aspartic acid residues80 |
Cleavage of extracellular matrix proteins17-19 |
| Target cell DNA fragmentation and caspase activation81-83 |
Cleavage of cell-surface receptors20-22 |
| BID cleavage84 and processing of caspase 385 |
Regulatory functions23,24,45 |
| AICD in T cells86-88 and NK cells41 |
Pro-inflammatory activity89 |
| |
Proteolysis of Von-Willebrandt factor25 and plasmin26 |
| |
Direct suppression of viral replication59,90 |
| |
Cleavage of autoantigens91 |
| |
Cleavage of IL-1α92 |
AICD, activation-induced cell death; IL-1α, interleukin-1α; NK, natural killer.
Apart from well-established cytotoxic functions, granzymes can mediate a variety of extracellular effects and hence participate, for instance, in inflammatory processes and tissue remodelling (Table 1).11,16 Various studies based on recombinant GrB revealed its ability to cleave extracellular matrix proteins such as fibronectin, laminin, vitronectin, and collagen IV.17-19 In this context, it was speculated that GrB may promote cell detachment, thereby inducing cell death via anoikis in primary endothelial cells. Furthermore, GrB has been associated with the cleavage of receptors on the surface of T cells, including (in selected circumstances) the TCR,20-22 which may explain—at least partially—the GrB-dependent immunosuppressive effects of regulatory T cells (Tregs) and plasmacytoid dendritic cells (pDCs).23,24 Other studies have described serum proteins involved in angiogenesis and blood clotting as GrB substrates.25,26 Moreover, elevated serum levels of GrB have been observed in several disease states including autoimmune disorders and allergic reactions.27
GrB is expressed not only by cytotoxic cells such as CTLs and NK cells, but also by a variety of normally non-cytotoxic cell types including CD34+ hematopoietic stem cells,28 keratinocytes,29 testicular Sertoli cells and placental syncytial trophoblasts,30 pDCs,31,32 basophils,33 mast cells,34 neutrophils,35 B cells9,10,36 and a variety of neoplastic cells such as breast carcinoma37 and urothelial carcinoma cells38 (Table 2). Furthermore, B-cell chronic lymphocytic leukemia (B-CLL) cells secrete low levels of GrB upon stimulation with IL-21, and are able to induce GrB-dependent apoptosis in bystander B-CLL cells.39 Not all GrB-expressing cells listed above co-express perforin, providing further support to the notion that GrB also exerts functions that are independent from its cytotoxic effects.
Table 2. Cells currently known to express granzyme B.
| Cell type | Perforin co-expression |
|---|---|
| Articular chondrocytes94 |
Yes |
| B cells9,10,36 |
No |
| Basophils33 |
No |
| B-CLL cells39 |
No |
| Breast carcinoma cells37 |
? |
| CD34+ hematopoietic stem cells28 |
Yes |
| CTL and NK cells93 |
Yes |
| Keratinocytes29 |
No/yes |
| Macrophages96 |
? |
| Mast cells34 |
No/yes |
| Mesenchymal stromal cells (unpublished observation) |
No |
| Monocyte-derived dendritic cells51 |
? |
| Neutrophils35 |
Yes |
| Plasmacytoid dendritic cells31,32 |
No |
| Platelets97 |
? |
| Regulatory T cells95 |
Yes |
| Testicular Sertoli cells and placental syncytial trophoblasts30 |
No |
| Urothelial carcinoma cells38 | No |
B-CLL, B-cell chronic lymphocytic leukemia; CTL, cytotoxic T lymphocyte; NK, natural killer.
Potential Roles of GrB-Secreting B Cells in Health and Disease
The primary objective of the present review is to discuss current knowledge on GrB secretion by B cells and its putative physiological and pathophysiological functions (Fig. 1). Several lines of evidence suggest that GrB-secreting B cells may be involved in early cell-mediated immune responses during inflammatory and neoplastic processes. First, the stimulus that drives the differentiation of B cells into GrB secretors, IL-21, is produced very early after viral infection.40 Since B cells recognize antigens in an MHC-independent manner, they are able to respond more rapidly than T cells, which require antigen presentation by professional antigen-presenting cells (APCs). Furthermore, the variety of antigens potentially recognized by the BCR is broader than that of antigens recognized by the TCR, being not limited to peptides, but also including carbohydrates, nucleic acids and other types of antigens.
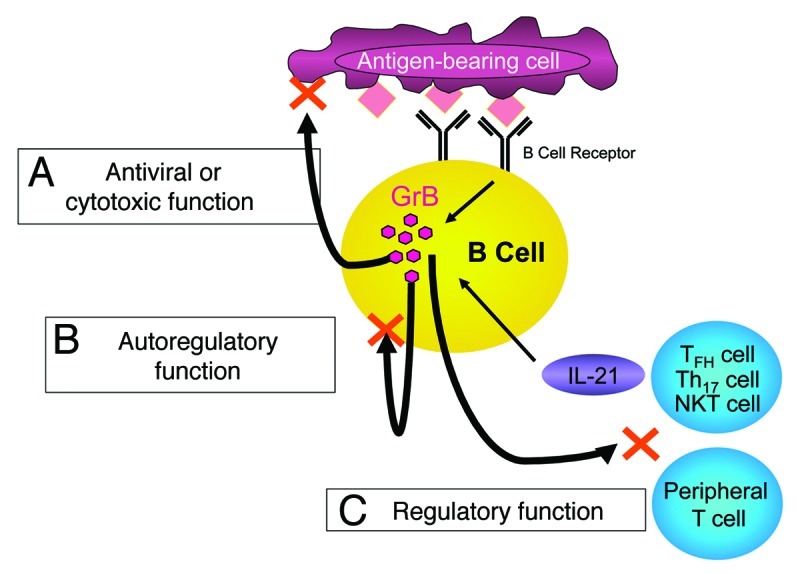
Figure 1. Possible immunological functions of granzyme B-secreting B cells. B cell-derived granzyme B (GrB) in the context of interleukin-21 (IL-21) stimulation and B-cell receptor (BCR)-engagement may mediate the following functions. (A) The direct, specific and MHC-independent recognition of antigens may stimulate cytotoxic or B cells with antiviral properties, particularly during the early phases of infections. (B) The leakage of GrB in the cytoplasm may result in the apoptotic demise of autoreactive B cells. (C) B cells may acquire a regulatory phenotype, involving GrB expression, that results in suppressive effects on other immune cells.
In line with these considerations, we demonstrated that viral antigens can induce significant GrB expression in B cells from individuals that had been previously vaccinated against the corresponding viral diseases, but not in B cells from unvaccinated donors.9 These findings and our very recent results showing that B cells can indeed exert cytotoxic effects toward other cells,10 suggest an early involvement of B cells in cytotoxic and/or antiviral immune responses (Fig. 1A). In a second scenario, B cell-produced GrB would serve autoregulatory purposes. Recent studies have reported that GrB can leak from the intracellular granules into the cytoplasm of both T cells24 and NK cells,41 upon incomplete activation, eventually leading to their apoptotic demise. In analogy with these observations, we found that CD5+ B cells from patients affected by systemic lupus erythematosus (SLE) exhibit a higher potential to express GrB as compared with healthy CD5- B cells, and that the circulating levels of IL-21 are significantly increased in SLE patients as compared with healthy individuals.36 In this setting, the viability of CD5+ B cells from SLE patients is strongly suppressed upon stimulation with IL-21 and anti-BCR antibodies. These observations and the fact that CD5+ B cells are thought to be involved in the pathogenesis of autoimmune diseases42-44 might be interpreted as an indication of the existence of a counter-regulatory loop that evolved to prevent the excessive activation of autoimmune B cells (Fig. 1B). Of note, the ability of IL-21 to induce GrB-dependent apoptosis in CD5+ B-CLL cells may involve similar mechanisms and may prove to be of therapeutic use in the future.39 Finally, a third possibility is that GrB-secreting B cells may exert regulatory functions on other immune cells similar to those mediated by Tregs or pDCs.23,24,45 In this context, B cell-derived GrB may not only target IL-21-producing cells, such as CD4+ T cells, TFH cells or NKT cells, but also bystander immune cells, via paracrine mechanisms (Fig. 1C). Supporting this hypothesis, we recently observed that GrB-expressing B cells potently suppress CD4+ T cell expansion, an effect that was associated with the GrB-dependent degradation of the TCR ζ chain (manuscript submitted).
These findings reveal yet unrecognized functions for IL-21-activated B cells involving GrB secretion and encompassing the regulation of antiviral immunity, the execution of cellular cytotoxicity and the control of other populations of immune cells. These novel functions of B cells will be extensively discussed in the following sections.
GrB+ B cells with cytotoxic potential
GrB-induced target cell death by CTLs and NK cells classically involves the secretion of perforin, which is considered to be critical for the killing process. Although the endosomal release of GrB needs an endosomolytic agent such as perforin, it is widely accepted that GrB enters the target cell in a perforin-independent way, namely, mannose-6-phosphate receptor-mediated or fluid phase endocytosis.46-48 Thus, the inability of B cells to produce perforin does not preclude a possible cytotoxic function. We therefore used normal B cells as well as several B-cell lines (which are either able or unable to secrete GrB upon IL-21 stimulation) in cytotoxicity assays together with different tumor cell lines including HeLa and G-361 cells. We found that B cells were able to induce significant cell death in these tumor cell lines when GrB was secreted, but not when GrB secretion was absent.10 Cytotoxicity was detected by Annexin V/PI staining, which allows for detection of slowly developing apoptosis, in contrast to the classical chromium release assay, which rather detects cell lysis instead of apoptosis. We also visualized the interactions of IL-21-activated B cells with HeLa cells by confocal microscopy using a fluorescent GrB substrate. By means of this approach, we demonstrated that B cell-derived GrB is transferred from B cells to HeLa cells in its active form, followed by the loss of HeLa cell size, which is compatible with an apoptotic shrinkage of the cells. An important unresolved question is how GrB reaches the target cell cytoplasm in the absence of perforin. Pore-forming bacterial lysins,14,48 viral delivery proteins,47,49 and heat shock proteins expressed by tumor cells50 are all known to mediate the uptake and endolysosomal release of GrB into the target cell. Therefore, the absence of perforin secretion by B cells may be compensated by the expression of proteins that can deliver extracellular GrB to the target cell cytoplasm. Such a mechanism may not only license, but also limit cytotoxic B cell responses to infected or (pre-)neoplastic cells.
Importantly, the effects of IL-21 on B-cell differentiation are crucially influenced by multiple T cell-derived factors. Previous studies indicate that the IL-21-induced differentiation of plasma cells is strongly supported by CD4+ T-cell help, which includes CD40 ligand (CD40L)- and IL-4-mediated signals.3 At the same time, CD40L and IL-4 strongly inhibit IL-21-induced GrB expression by B cells. Importantly, we recently found that fully activated CD4+ T cells (i.e., receiving TCR stimulation and co-stimulatory signals) express both IL-21 and CD40L, explaining their robust ability to favor the differentiation of plasma cells. In contrast, incompletely activated CD4+ T cells (receiving TCR stimulation only) express IL-21 but not CD40L, making them potent inducers of GrB production by B cells (Fig. 2).10
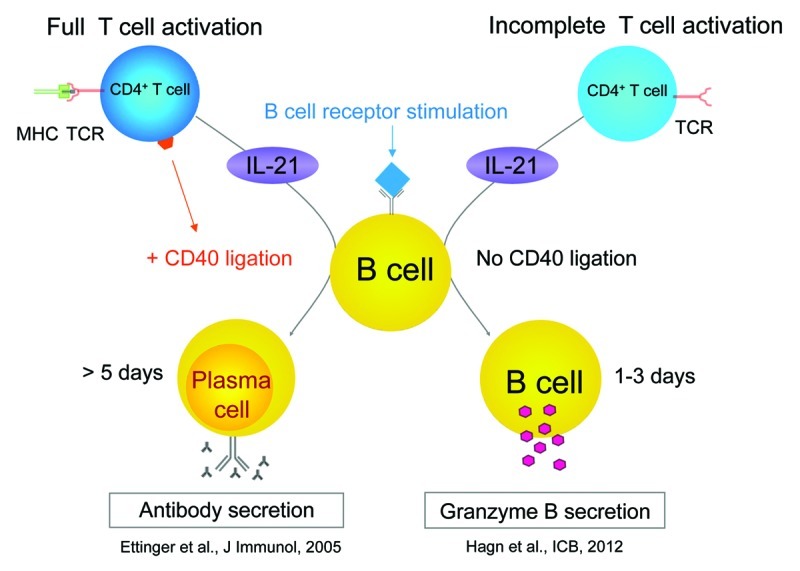
Figure 2. CD40 ligation regulates IL-21-induced differentiation of B cells into either plasma cells or granzyme B-secreting cells. Partial CD4+ T-cell activation. In the early phase of an immune response (0–3 d), a broad spectrum of low-affinity and partly self-reactive CD4+ T cells are present in a pre-activated state and secrete interleukin-21 (IL-21) but do not express CD40L. In this setting, B cells that are activated by specific B-cell receptor (BCR) crosslinking differentiate into granzyme B (GrB)-secreting B cells, owing to CD4+ T cell-derived IL-21 in the absence of CD40 ligation (right). Full CD4+ T-cell activation. In a later phase (> 5 d), upon additional co-stimulation by professional antigen-presenting cells (APCs) in the draining lymph nodes, antigen-specific T cells arrive at the site of inflammation in a fully activated state. Such CD4+ T cells express both IL-21 and CD40 ligand, supporting the differentiation of activated B cells into plasma cells (left).
In view of these findings, the expression of GrB and the accompanying cytotoxicity are obviously not restricted to T cells, but also present in B cells. GrB-secreting B cells may therefore play a so far unappreciated role as alternative cytotoxic cells, particularly when antigen-specific T cells are not yet fully activated, such as in the early phase of tumorigenesis or viral infection.
Another interesting possibility that we would like to discuss in this context is the involvement of GrB in antigen cross-presentation. A very recent report shows that, in mice, GrB is able to promote the exposure of “eat-me” signals on tumor cells succumbing from CTLs, thereby supporting phagocytosis and cross-presentation by specific APCs. Intriguingly, we noticed that three different types of human APCs share their ability to express GrB in the absence of perforin, namely B cells,9 pDCs31,32 and IFN-α-induced monocyte-derived DCs (moDCs),51 all of which are able to efficiently perform cross-presentation.52-54 The expression of GrB may therefore not only provide APCs with cytotoxic tools, as recently observed for interferon-producing killer DCs (IKDCs) in mice,55,56 but also could serve as a novel common effector molecule for APCs that are capable of cross-presenting antigens.
GrB+ B cells with antiviral activity
Many viruses have evolved strategies to evade recognition by the immune system and to modulate apoptosis for their own benefit.57 Granzymes (and particularly GrB) have been described to degrade viral proteins and factors that are required for viral replication or assembly via non-cytotoxic mechanisms.58,59 These property might therefore constitute an additional tool of the immune system for inactivating intracellular pathogens. In a recent study, we evaluated the potential of human B cells to secrete GrB shortly after vaccination of healthy donors against tick-borne encephalitis virus, hepatitis B and rabies. Of note, B-cell responses in vaccinated subjects showed a significant induction of GrB after in vitro re-exposure with the corresponding viral antigens, whereas a similar effect was not observed with B cells from unvaccinated donors.9 These data suggest that B cell-derived GrB may indeed counteract overwhelming viral replication at the beginning of viral infections. Activated antigen-specific B cells might recognize virus particles via their BCR and, in the presence of IL-21-secreting CD4+ T, TFH or NKT cells, begin to release GrB into the extracellular space. Secreted GrB might enter the cytosol of target cells along with the virus, where it may contribute to the intracellular control of the pathogen. Whether B cell-derived GrB in this context would operate by inducing the rapid apoptosis of infected cells, by cleaving viral proteins, or both, remains to be elucidated. Of note, we have recently found that high frequencies of GrB-expressing B cells can be detected in patients experiencing acute HIV infection, and that - in vitro - the expansion of the HI virus in CD4+ T cells is significantly suppressed by GrB+ B cells (unpublished results).
GrB as autoregulatory mediator in B cells
The paradigm that autoimmune diseases originate from defective T-cell regulation has recently been challenged by evidence that B cells also exhibit potent immunoregulatory properties. The multiple functions of B cells including antibody secretion, cytokine production, antigen presentation and formation of germinal centers endow these cells with characteristics that can either contribute to or counteract the development, re-activation or persistence of autoimmune disorders.42,60
Autoimmune diseases such as SLE have been linked to elevated serum levels of IL-21 und GrB.27,61-63 Since various autoimmune disorders have been associated with high frequencies of CD5+ B cells, we hypothesized the presence of a link between these three disease manifestations. Supporting this hypothesis, we observed a constitutive expression of GrB by CD5+ B cells from SLE patients, but not by CD5- B cells. Furthermore, we demonstrated a significant correlation between GrB and IL-21 serum levels in SLE patients, as well as a constistent expression of the IL-21 receptor on CD5+ B cells.36 Of note, IL-21 was able to induce significant apoptosis in activated, but not in resting, CD5+ B cells as obtained from SLE patients. This finding parallels results from a previous study with CD5+ malignant B cells from B-CLL patients, demonstrating that IL-21 and CpG oligodeoxynucleotides (CpG ODNs) can induce GrB-mediated apoptosis in CD5+ B-CLL cells but not in CD5- B cells.39 Altogether, these observations suggest that B cells may undergo activation-induced cell death (AICD), as previously described,64 a process that may involve the autologous expression of GrB, similar to what has been shown for NK and T cells.24,41
GrB+ B cells as regulatory B cells (Bregs)
Accumulating evidence from different laboratories suggests that B cells may fundamentally contribute to the regulation of immune responses. For example, by targeting auto-reactive T cells in the early phases of viral infection, they may counteract the potential development of autoimmunity. The pathways whereby regulatory B cells (Bregs) exert immunosuppressive functions may include the expression of CD25, the secretion of IL-10 and transforming growth factor β1 (TGF-β1), the production of antibodies that neutralize soluble factors, as well as direct interactions with other cell subsets, e.g., via cell surface-exposed CD1d or MHC class I molecules.65-69 A unique marker defining a Breg phenotype has not yet been described, and consistent differences have been reported between murine and human Bregs.65-67 Previous findings demonstrating the GrB-dependent regulation of T-cell responses by Tregs and pDCs23,32,70 prompted us to hypothesize that GrB may also operate as an immunoregulatory molecule in Breg. Apart from regulating auto-reactive or malignant B cells in an autocrine manner, B cell-derived GrB may exhibit non-cytotoxic immunoregulatory effects on various immune cell populations. In recent experiments, we found that B cells activated in the presence of IL-21 and BCR stimulation (but in the absence of CD40L) can indeed potently suppress the proliferation of co-cultured CD4+ T cells in a GrB-dependent manner. Of note, this effect was associated with the GrB-dependent cleavage of the TCR ζ chain, which has earlier been shown to be a GrB substrate.21 Further characterization of these GrB-producing Breg revealed a CD19+CD38+CD1d+CD147+ phenotype as well as the expression of additional immunoregulatory molecules including IL-10, indoleamine-2,3-dioxygenase (IDO) and CD25 (manuscript submitted).
Importantly, we found such GrB+ B cells to infiltrate the microenvironment of various solid tumors including breast, ovarian, cervical and colon carcinomas. While the infiltration of solid tumors with B cells has been described before, it has been associated with either tumor progression or regression, depending on tumor type and stage.71-74 These observations coupled to our recent results describing GrB+ B cells as either Bregs or cytotoxic cells raise the controversial question of whether tumor-infiltrating GrB+ B cells rather contribute to or impede antitumor immune responses. Most likely, additional parameters including the tumor phenotype and the activation status of other immune cells determine the overall effects of GrB+ B cells on antitumor immunity. Nevertheless, GrB appears to represent a novel immunomodulatory molecule expressed not only by Tregs and pDCs, but also by human B cells. Since B cells are abundantly present in the peripheral circulation, GrB+ Bregs may thus play a regulatory role not only in antitumor immunity, but also in systemic inflammatory processes such as autoimmune disorders and graft-vs.-host diseases.
Using Mouse Models to Dissect the Function of GrB in Human B Cells
Establishing a reliable mouse model would obviously constitute a critical step toward the in-depth characterization of the biological role of GrB secretion by B cells. Experimental mouse models (above all, gene knockout strains) provide researchers with tools that are not available in the human system, and have been indispensable for our understanding of granzyme biology. This said, despite an extensive study based on multiple distinct mouse models, we were unable to detect GrB expression in murine B cells. This study involved various in vitro experiments using different classical B-cell stimuli in the context of IL-21 co-stimulation, in vivo infection and vaccination models, as well as a transgenic BCR mouse model that allowed us to specifically restimulate B cells in vivo and in vitro.75 Albeit disappointing, our study was not the first study to point out inter-species differences regarding the biology of granzymes. Indeed, human, mouse and rat GrB have previously been shown to exhibit distinct phenotypic and functional characteristics, which probably originated to address species-specific evolutionary challenges.76-79 These observations indicate that mouse models will not bring light into the mystery of granzyme biology in human B cells, and that findings on murine granzymes should be extended very carefully to the human immune system and vice versa.
Concluding Remarks
In contrast to T cells, B lymphocytes have the capacity to recognize antigens in a MHC-independent manner, i.e., they do not require the presence of professional APCs for BCR activation. Therefore, B lymphocytes might be the first cells to recognize antigens in an rapid and specific manner, for example during incipient inflammatory processes or early during malignant transformation. B cells may hence be critical in bridging the temporal gap between the first appearance of novel antigens and the completion of antigen-specific T-cell priming in draining lymph nodes. In this setting, the existence of GrB+ B cells with cytotoxic properties may allow for an early induction of antigen-specific, cytotoxic immune responses. On the other hand, the immunoregulatory function of GrB+ B cells may prevent local T cells, some of which exhibit minor specificities for self-antigens, from becoming activated in situ during inflammatory processes and to promote autoimmune responses. Overall, GrB expression appears to place B cells at the boundary of the adaptive and innate immune systems, combining the specificity of adaptive immune responses with the speed of their innate counterparts. Novel immunotherapeutic approaches may therefore involve the adoptive transfer of GrB-expressing B cells or their induction in vivo using recombinant IL-21. On the other hand, depletion of extracellular GrB derived from B cells and other immune cells during chronic inflammatory processes or along with vaccinations may represent a novel approach to induce more effective and broader cellular immune responses.
In summary, a growing body of evidence suggests that B cells are far more than mere antibody secretors and that their role in the immune system extends beyond mounting humoral immune responses. In the present review, we have discussed for the first time a novel role of B cells that involves the expression and secretion of GrB. The findings discussed above suggests that B cells may deeply contribute to immunosurveillance, early antiviral immune responses as well as to the modulation of inflammatory processes.
Acknowledgments
This work received grant support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) to M.H. and B.J.
Glossary
Abbreviations:
- AICD
activation-induced cell death
- APC
antigen-presenting cell
- B-CLL
B-cell chronic lymphocytic leukaemia
- BCR
B-cell receptor
- Breg
regulatory B cell
- CD40L
CD40 ligand CpG ODN, CpG oligodeoxynucleotide
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- EBV
Epstein-Barr virus
- GrB
granzyme B
- IDO
indoleamine-2,3-dioxygenase
- IKDC
interferon-producing killer dendritic cell
- IL-21
interleukin-21
- moDC
monocyte-derived DC
- NK
natural killer
- NKT
natural killer T
- pDC
plasmacytoid DC
- PI-9
proteinase-inhibitor 9
- SLE
systemic lupus erythematosus
- TCR
T-cell receptor
- TFH
T follicular helper
- TGF-β1
transforming growth factor β1
- Treg
regulatory T cell
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22354
References
- 1.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–79. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 4.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 5.Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol. 2008;84:348–56. doi: 10.1189/jlb.0308149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–65. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 7.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 8.Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagn M, Schwesinger E, Ebel V, Sontheimer K, Maier J, Beyer T, et al. Human B cells secrete granzyme B when recognizing viral antigens in the context of the acute phase cytokine IL-21. J Immunol. 2009;183:1838–45. doi: 10.4049/jimmunol.0901066. [DOI] [PubMed] [Google Scholar]
- 10.Hagn M, Sontheimer K, Dahlke K, Brueggemann S, Kaltenmeier C, Beyer T, et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol Cell Biol. 2012;90:457–67. doi: 10.1038/icb.2011.64. [DOI] [PubMed] [Google Scholar]
- 11.Hoves S, Trapani JA, Voskoboinik I. The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol. 2009 doi: 10.1189/jlb.0909608. [DOI] [PubMed] [Google Scholar]
- 12.Ewen CL, Kane KP, Bleackley RC. A quarter century of granzymes. Cell Death Differ. 2012;19:28–35. doi: 10.1038/cdd.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters PJ, Borst J, Oorschot V, Fukuda M, Krähenbühl O, Tschopp J, et al. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browne KA, Blink E, Sutton VR, Froelich CJ, Jans DA, Trapani JA. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol Cell Biol. 1999;19:8604–15. doi: 10.1128/mcb.19.12.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19:339–47. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Froelich CJ, Pardo J, Simon MM. Granule-associated serine proteases: granzymes might not just be killer proteases. Trends Immunol. 2009;30:117–23. doi: 10.1016/j.it.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Simon MM, Kramer MD, Prester M, Gay S. Mouse T-cell associated serine proteinase 1 degrades collagen type IV: a structural basis for the migration of lymphocytes through vascular basement membranes. Immunology. 1991;73:117–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Choy JC, Hung VH, Hunter AL, Cheung PK, Motyka B, Goping IS, et al. Granzyme B induces smooth muscle cell apoptosis in the absence of perforin: involvement of extracellular matrix degradation. Arterioscler Thromb Vasc Biol. 2004;24:2245–50. doi: 10.1161/01.ATV.0000147162.51930.b7. [DOI] [PubMed] [Google Scholar]
- 19.Buzza MS, Zamurs L, Sun J, Bird CH, Smith AI, Trapani JA, et al. Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem. 2005;280:23549–58. doi: 10.1074/jbc.M412001200. [DOI] [PubMed] [Google Scholar]
- 20.Loeb CR, Harris JL, Craik CS. Granzyme B proteolyzes receptors important to proliferation and survival, tipping the balance toward apoptosis. J Biol Chem. 2006;281:28326–35. doi: 10.1074/jbc.M604544200. [DOI] [PubMed] [Google Scholar]
- 21.Wieckowski E, Wang GQ, Gastman BR, Goldstein LA, Rabinowich H. Granzyme B-mediated degradation of T-cell receptor zeta chain. Cancer Res. 2002;62:4884–9. [PubMed] [Google Scholar]
- 22.Ganor Y, Teichberg VI, Levite M. TCR activation eliminates glutamate receptor GluR3 from the cell surface of normal human T cells, via an autocrine/paracrine granzyme B-mediated proteolytic cleavage. J Immunol. 2007;178:683–92. doi: 10.4049/jimmunol.178.2.683. [DOI] [PubMed] [Google Scholar]
- 23.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–6. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 24.Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–47. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Buzza MS, Dyson JM, Choi H, Gardiner EE, Andrews RK, Kaiserman D, et al. Antihemostatic activity of human granzyme B mediated by cleavage of von Willebrand factor. J Biol Chem. 2008;283:22498–504. doi: 10.1074/jbc.M709080200. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan-Kehoe MJ, Drinane MC, Mollmark J, Casciola-Rosen L, Hummers LK, Hall A, et al. Antiangiogenic plasma activity in patients with systemic sclerosis. Arthritis Rheum. 2007;56:3448–58. doi: 10.1002/art.22861. [DOI] [PubMed] [Google Scholar]
- 27.Buzza MS, Bird PI. Extracellular granzymes: current perspectives. Biol Chem. 2006;387:827–37. doi: 10.1515/BC.2006.106. [DOI] [PubMed] [Google Scholar]
- 28.Berthou C, Marolleau JP, Lafaurie C, Soulié A, Dal Cortivo L, Bourge JF, et al. Granzyme B and perforin lytic proteins are expressed in CD34+ peripheral blood progenitor cells mobilized by chemotherapy and granulocyte colony-stimulating factor. Blood. 1995;86:3500–6. [PubMed] [Google Scholar]
- 29.Berthou C, Michel L, Soulié A, Jean-Louis F, Flageul B, Dubertret L, et al. Acquisition of granzyme B and Fas ligand proteins by human keratinocytes contributes to epidermal cell defense. J Immunol. 1997;159:5293–300. [PubMed] [Google Scholar]
- 30.Hirst CE, Buzza MS, Sutton VR, Trapani JA, Loveland KL, Bird PI. Perforin-independent expression of granzyme B and proteinase inhibitor 9 in human testis and placenta suggests a role for granzyme B-mediated proteolysis in reproduction. Mol Hum Reprod. 2001;7:1133–42. doi: 10.1093/molehr/7.12.1133. [DOI] [PubMed] [Google Scholar]
- 31.Rissoan MC, Duhen T, Bridon JM, Bendriss-Vermare N, Péronne C, de Saint Vis B, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 32.Jahrsdorfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, et al. Granzyme B produced by human plasmacytoid dendritic cells suppresses T cell expansion. Blood. 2009 doi: 10.1182/blood-2009-07-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschopp CM, Spiegl N, Didichenko S, Lutmann W, Julius P, Virchow JC, et al. Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood. 2006;108:2290–9. doi: 10.1182/blood-2006-03-010348. [DOI] [PubMed] [Google Scholar]
- 34.Strik MC, de Koning PJ, Kleijmeer MJ, Bladergroen BA, Wolbink AM, Griffith JM, et al. Human mast cells produce and release the cytotoxic lymphocyte associated protease granzyme B upon activation. Mol Immunol. 2007;44:3462–72. doi: 10.1016/j.molimm.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Wagner C, Iking-Konert C, Denefleh B, Stegmaier S, Hug F, Hänsch GM. Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood. 2004;103:1099–104. doi: 10.1182/blood-2003-04-1069. [DOI] [PubMed] [Google Scholar]
- 36.Hagn M, Ebel V, Sontheimer K, Schwesinger E, Lunov O, Beyer T, et al. CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol. 2010;40:2060–9. doi: 10.1002/eji.200940113. [DOI] [PubMed] [Google Scholar]
- 37.Hu SX, Wang S, Wang JP, Mills GB, Zhou Y, Xu HJ. Expression of endogenous granzyme B in a subset of human primary breast carcinomas. Br J Cancer. 2003;89:135–9. doi: 10.1038/sj.bjc.6601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Eliseo D, Pisu P, Romano C, Tubaro A, De Nunzio C, Morrone S, et al. Granzyme B is expressed in urothelial carcinoma and promotes cancer cell invasion. Int J Cancer. 2010;127:1283–94. doi: 10.1002/ijc.25135. [DOI] [PubMed] [Google Scholar]
- 39.Jahrsdörfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712–9. doi: 10.1182/blood-2006-03-014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holm C, Nyvold CG, Paludan SR, Thomsen AR, Hokland M. Interleukin-21 mRNA expression during virus infections. Cytokine. 2006;33:41–5. doi: 10.1016/j.cyto.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Ida H, Nakashima T, Kedersha NL, Yamasaki S, Huang M, Izumi Y, et al. Granzyme B leakage-induced cell death: a new type of activation-induced natural killer cell death. Eur J Immunol. 2003;33:3284–92. doi: 10.1002/eji.200324376. [DOI] [PubMed] [Google Scholar]
- 42.Renaudineau Y, Pers JO, Bendaoud B, Jamin C, Youinou P. Dysfunctional B cells in systemic lupus erythematosus. Autoimmun Rev. 2004;3:516–23. doi: 10.1016/j.autrev.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Hayakawa K, Hardy RR. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 44.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 45.Salti SM, Hammelev EM, Grewal JL, Reddy ST, Zemple SJ, Grossman WJ, et al. Granzyme B regulates antiviral CD8+ T cell responses. J Immunol. 2011;187:6301–9. doi: 10.4049/jimmunol.1100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinkoski MJ, Hobman M, Heibein JA, Tomaselli K, Li F, Seth P, et al. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998;92:1044–54. [PubMed] [Google Scholar]
- 47.Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, et al. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271:29073–9. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 48.Kurschus FC, Kleinschmidt M, Fellows E, Dornmair K, Rudolph R, Lilie H, et al. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]
- 49.Seth P. Mechanism of adenovirus-mediated endosome lysis: role of the intact adenovirus capsid structure. Biochem Biophys Res Commun. 1994;205:1318–24. doi: 10.1006/bbrc.1994.2809. [DOI] [PubMed] [Google Scholar]
- 50.Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278:41173–81. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 51.Korthals M, Safaian N, Kronenwett R, Maihöfer D, Schott M, Papewalis C, et al. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi: 10.1186/1479-5876-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mariño E, Tan B, Binge L, Mackay CR, Grey ST. B-Cell Cross-Presentation of Autologous Antigen Precipitates Diabetes. Diabetes. 2012 doi: 10.2337/db12-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, et al. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–17. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 54.Di Pucchio T, Chatterjee B, Smed-Sörensen A, Clayton S, Palazzo A, Montes M, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–7. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–13. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 56.Taieb J, Chaput N, Ménard C, Apetoh L, Ullrich E, Bonmort M, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–9. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 57.Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta. 2004;1659:178–89. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Romero V, Andrade F. Non-apoptotic functions of granzymes. Tissue Antigens. 2008;71:409–16. doi: 10.1111/j.1399-0039.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 59.Andrade F. Non-cytotoxic antiviral activities of granzymes in the context of the immune antiviral state. Immunol Rev. 2010;235:128–46. doi: 10.1111/j.0105-2896.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 60.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–8. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Spaeny-Dekking EH, Hanna WL, Wolbink AM, Wever PC, Kummer JA, Swaak AJ, et al. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–6. [PubMed] [Google Scholar]
- 62.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 63.Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Ann Rheum Dis. 2008;67(Suppl 3):iii83–6. doi: 10.1136/ard.2008.098400. [DOI] [PubMed] [Google Scholar]
- 64.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 66.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–14. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 67.Lampropoulou V, Calderon-Gomez E, Roch T, Neves P, Shen P, Stervbo U, et al. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol Rev. 2010;233:146–61. doi: 10.1111/j.0105-2896.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 68.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Loebbermann J, Thornton H, Durant L, Sparwasser T, Webster KE, Sprent J, et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 2012;5:161–72. doi: 10.1038/mi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 72.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milne K, Barnes RO, Girardin A, Mawer MA, Nesslinger NJ, Ng A, et al. Tumor-infiltrating T cells correlate with NY-ESO-1-specific autoantibodies in ovarian cancer. PLoS One. 2008;3:e3409. doi: 10.1371/journal.pone.0003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–8. [PubMed] [Google Scholar]
- 75.Hagn M, Belz GT, Kallies A, Sutton VR, Thia KY, Tarlinton DM, et al. Activated mouse B cells lack expression of granzyme B. J Immunol. 2012;188:3886–92. doi: 10.4049/jimmunol.1103285. [DOI] [PubMed] [Google Scholar]
- 76.Casciola-Rosen L, Garcia-Calvo M, Bull HG, Becker JW, Hines T, Thornberry NA, et al. Mouse and human granzyme B have distinct tetrapeptide specificities and abilities to recruit the bid pathway. J Biol Chem. 2007;282:4545–52. doi: 10.1074/jbc.M606564200. [DOI] [PubMed] [Google Scholar]
- 77.Cullen SP, Adrain C, Lüthi AU, Duriez PJ, Martin SJ. Human and murine granzyme B exhibit divergent substrate preferences. J Cell Biol. 2007;176:435–44. doi: 10.1083/jcb.200612025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaiserman D, Bird CH, Sun J, Matthews A, Ung K, Whisstock JC, et al. The major human and mouse granzymes are structurally and functionally divergent. J Cell Biol. 2006;175:619–30. doi: 10.1083/jcb.200606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thia KY, Trapani JA. The granzyme B gene is highly polymorphic in wild mice but essentially invariant in common inbred laboratory strains. Tissue Antigens. 2007;70:198–204. doi: 10.1111/j.1399-0039.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 80.Odake S, Kam CM, Narasimhan L, Poe M, Blake JT, Krahenbuhl O, et al. Human and murine cytotoxic T lymphocyte serine proteases: subsite mapping with peptide thioester substrates and inhibition of enzyme activity and cytolysis by isocoumarins. Biochemistry. 1991;30:2217–27. doi: 10.1021/bi00222a027. [DOI] [PubMed] [Google Scholar]
- 81.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553–66. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–87. doi: 10.1016/0092-8674(94)90376-X. [DOI] [PubMed] [Google Scholar]
- 83.Pardo J, Bosque A, Brehm R, Wallich R, Naval J, Müllbacher A, et al. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J Cell Biol. 2004;167:457–68. doi: 10.1083/jcb.200406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, et al. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192:1403–14. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sutton VR, Wowk ME, Cancilla M, Trapani JA. Caspase activation by granzyme B is indirect, and caspase autoprocessing requires the release of proapoptotic mitochondrial factors. Immunity. 2003;18:319–29. doi: 10.1016/S1074-7613(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 86.Sharma V, Delgado M, Ganea D. Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J Immunol. 2006;176:97–110. doi: 10.4049/jimmunol.176.1.97. [DOI] [PubMed] [Google Scholar]
- 87.Mateo V, Ménager M, de Saint-Basile G, Stolzenberg MC, Roquelaure B, André N, et al. Perforin-dependent apoptosis functionally compensates Fas deficiency in activation-induced cell death of human T lymphocytes. Blood. 2007;110:4285–92. doi: 10.1182/blood-2007-05-088286. [DOI] [PubMed] [Google Scholar]
- 88.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M, et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29:720–33. doi: 10.1016/j.immuni.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–71. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Lüthi AU, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol Cell. 2011;44:265–78. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masson D, Nabholz M, Estrade C, Tschopp J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. 1986;5:1595–600. doi: 10.1002/j.1460-2075.1986.tb04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horiuchi K, Saito S, Sasaki R, Tomatsu T, Toyama Y. Expression of granzyme B in human articular chondrocytes. J Rheumatol. 2003;30:1799–810. [PubMed] [Google Scholar]
- 95.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–8. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 96.Kim WJ, Kim H, Suk K, Lee WH. Macrophages express granzyme B in the lesion areas of atherosclerosis and rheumatoid arthritis. Immunol Lett. 2007;111:57–65. doi: 10.1016/j.imlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Freishtat RJ, Natale J, Benton AS, Cohen J, Sharron M, Wiles AA, et al. Sepsis alters the megakaryocyte-platelet transcriptional axis resulting in granzyme B-mediated lymphotoxicity. Am J Respir Crit Care Med. 2009;179:467–73. doi: 10.1164/rccm.200807-1085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


