Abstract
The insulin-like growth factor I (IGF-I) receptor (IGF-1R) is overexpressed in most human neoplasms tested so far. Many tumors in young patients produce high levels of the IGF-1R ligands, IGF-I and IGF-II. Given the complexity of the IGF signaling pathway, its complete inhibition may require combination therapies with antibodies targeting both IGF-1R and IGF-II.
Keywords: cancer target, chimeric antibody receptor, germline antibodies, therapeutic antibodies
The insulin-like growth factor (IGF) signaling pathway has been a major focus of study for monoclonal antibody (mAb)-based therapy throughout the past decade. More than ten mAbs targeting the IGF-I receptor (IGF-1R) have been tested in animal models and in humans. However, it has been challenging to translate the results from in vitro and animal studies into therapeutic efficacy. Results from clinical studies underscore the complexity of the IGF pathway. This system includes the ligands IGF-I and -II, both of which bind and activate the main receptor IGF-1R. IGF-II also has high affinity for the insulin receptor (IR) isoform A and the hybrid receptor (HR) IGF-1R/IR. The level of circulating IGF-I is regulated by the hypothalamus-pituitary gland-liver axis. IGF-I signaling is mostly mediated through the IGF-1R as IGF-I has relatively low affinity for the HR (Fig. 1). Most of the reported mAbs targeting IGF-1R do not inhibit signal transduction mediated by the hybrid receptor, although some such as figitumumab and SCH717454 do.1,2 It has been observed that a potent anti-IGF-1R and anti-HR mAb, SCH717454, inhibits both IGF-I and -II initiated phosphorylation of IGF-1R, but not the IGF-II-stimulated activity of the IR. Therefore, it is clear that anti-IGF-1R antibodies do not completely abolish IGF signaling because they do not entirely affect the activity of IGF-II on the IR and HR. A combination therapy with antibodies specific for the IGF-1R and for IGF-II may be superior in inhibiting the pathway, resulting in improved inhibition of IGF-driven tumorigenesis.
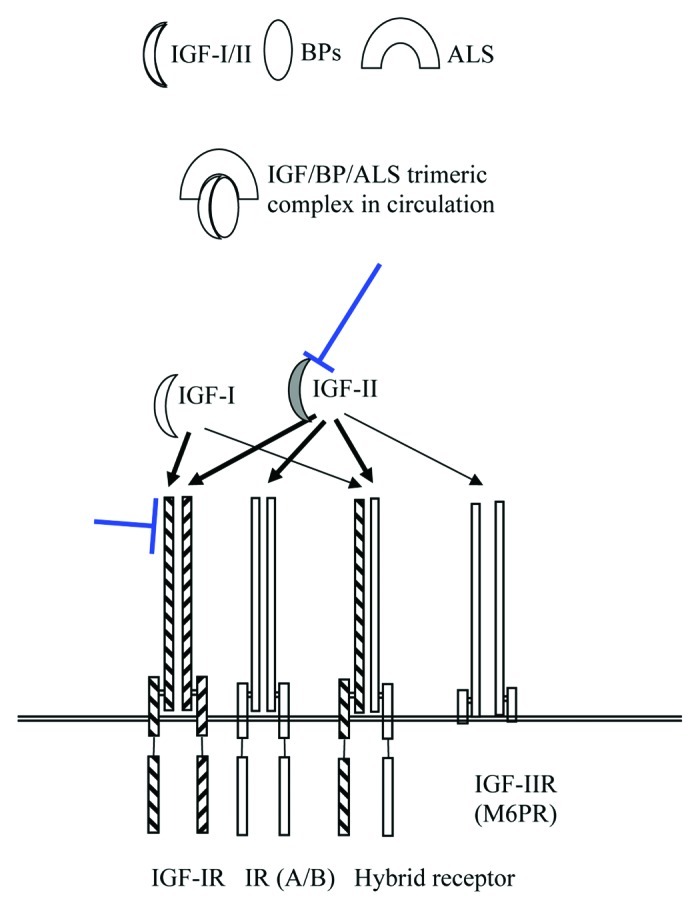
Figure 1. Schematic illustration of the main components of the IGF system. While both IGF-I and IGF-II bind to IGF-1R at high affinity, only IGF-II binds to the hybrid receptor at high affinity. IGF-II also binds to the insulin receptor and to IGF-2R (also known as mannose-6-phosphate receptor), which lack the cytoplasmic activation domain and functions as the regulator of circulating IGF-II levels. Because of a feedback mechanism, elevation of IGF-I levels has been observed following IGF-1R antibody therapy. IGF-II is produced by many childhood tumors such as neuroblastoma, Ewing’s sarcoma, rhabdomyosarcoma, and osteosarcoma. Thick arrows represent high affinity binding of IGFs to the receptors. Antibodies targeting IGF-1R and IGF-II (flat arrows) may block signaling from both ligands.
Several clinical reports have shown that elevated levels of IGF-II are present in young patients with Ewing sarcoma, osteosarcoma, neuroblastoma and rhabdomyosarcoma. These cancer cells exhibit IGF-II-dependent growth patterns through autocrine and paracrine mechanisms. Thus, these young patients may benefit from therapies capable of completely abolishing the IGF signaling. There are encouraging results from clinical studies on these tumors. In Phase I study, cixutumumab (a fully human anti-IGF-1R IgG1) in combination with an mTOR inhibitor led to tumor regression in 5/17 (29%) Ewing sarcoma patients.3 A Phase II study with R1507 (a fully human IgG1 specific to IGF-1R by Roche) in patients with refractory Ewing sarcoma showed 10% complete /partial responses.4 However, IGF-II could decrease the inhibitory effect of IGF-1R antibodies.2 Results from these studies suggest that IGF-1R mAbs alone are probably not sufficient for complete tumor inhibition, calling for combination therapyies. Clearly, even among Ewing sarcoma patients, there is a subgroup of patients who are sensitive to such monotherapy. Thus, identifying predictive biomarkers would help achieve better benefits. Monitoring plasma IGF-II levels may help in the selection of patients who may be sensitive to therapy and in the prediction of treatment outcome.
Unlike IGF-I, circulating IGF-II levels are not under the growth hormone regulation. Since we first reported the anti-IGF-II human mAb m610 in 2006,5 at least three additional mAbs of the same type have been described.6-8 These three mAbs are all cross-reactive, recognizing both IGF-I and -II, with higher affinity (2–60 pM) for IGF-II than for IGF-I. An ideal anti-ligand antibody binds to the target and promotes its catabolism or removal rather than retaining it in the circulation. For antibodies targeting cell surface proteins, an excessive affinity may hinder tissue penetration. However, for soluble ligands such as IGFs, a very high affinity is desirable, because it allows for the continuous sequestration of the ligand from its receptor, and prevents slow dissociation of the ligand. Presumably, a reduction of circulating IGF-II may change the equilibrium between the IGF-II levels in tumor tissues and in the circulation, thus promoting the efflux of IGF-II from tumors. In the case of IGF-I and -II, antibodies should also compete for the binding of these ligands with binding proteins. We have developed a IGF-I/IGF-II cross-reactive mAb, m708.5, recognizing both IGFs with a high pM affinity.8
It is reported that antigen/antibody complexes including multiple antibodies have higher clearance efficiency from the circulation than those with one single type of antibodies.9 Antibodies targeting non-overlapping epitopes on a ligand are hence capable of cross-linking multiple molecules of the ligand and promote the formation of large immune complexes. We engineered a bi-specific antibody (m660) that combines an improved version of m610 (m610.27) and a VH domain antibody which binds to a distinct epitope on IGF-II as compared with m610.27.10 We observed that m660 is able to form large immune complexes involving IGF-II and more than two molecules of m660, which are recognized by the low affinity Fcγ receptors II (FcγRII) and III. These Fc receptors normally have low (μM) affinity to single IgG1 molecules, and are not efficient in promoting the internalization of IgG/antigen complexes. However, when an antigen is covered by multiple IgG1, as seen with IGF-II and m660, FcγRII and III on macrophages, natural killer cells, and neutrophils bind to the complex and internalize it efficiently. We found that cells with macrophage-like activity take up the IGF-II/m660 complex, whereas IGF-II/m610.27 was not internalized. Therefore, we hypothesized that m660 might have the potential to irreversibly deplete IGF-II from tumor environment by generating immune complexes that are recognized by FcγRII/III and hence disrupting the equilibrium of IGF-II between the tumor tissue and circulation..
Although the removal of the IGF-II/m660 immune complex from the circulation has not yet been observed in animal models, mAbs targeting non-overlapping epitopes on soluble ligands represent a newer generation of anti-ligand antibodies. Combining such mAbs with an IGF-1R antibody might constitute a promising therapy for IGF-driven tumors.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20925
References
- 1.Schmitz S, Kaminsky-Forrett MC, Henry S, Zanetta S, Geoffrois L, Bompas E, et al. Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02) Ann Oncol. 2012 doi: 10.1093/annonc/mdr574. [DOI] [PubMed] [Google Scholar]
- 2.Bid HK, Zhan J, Phelps DA, Kurmasheva RT, Houghton PJ. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol Cancer Ther. 2012;11:649–59. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naing A, Lorusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin Growth Factor-Receptor (IGF-1R) Antibody Cixutumumab Combined with the mTOR Inhibitor Temsirolimus in Patients with Refractory Ewing’s Sarcoma Family Tumors. Clin Cancer Res. 2012;18:2625–31. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–7. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitrov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–20. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- 6.Dransfield DT, Cohen EH, Chang Q, Sparrow LG, Bentley JD, Dolezal O, et al. A human monoclonal antibody against insulin-like growth factor-II blocks the growth of human hepatocellular carcinoma cell lines in vitro and in vivo. Mol Cancer Ther. 2010;9:1809–19. doi: 10.1158/1535-7163.MCT-09-1134. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–40. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Feng Y, Zhu Z, Dimitrov DS. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol Cancer Ther. 2011;10:1677–85. doi: 10.1158/1535-7163.MCT-11-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–48. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Feng Y, Zhao Q, Zhu Z, Dimitrov DS. Human monoclonal antibodies targeting nonoverlapping epitopes on insulin-like growth factor II as a novel type of candidate cancer therapeutics. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]


