Abstract
Solid tumors are frequently resistant to immunotherapy. We demonstrated that low-dose tumor necrosis factorα (TNFα), when directly targeted to the tumor environment, exerts dual effects by improving vessel functionality and activating immune cells. This vascular remodeling in an inflammatory context enhances active immunotherapy and promotes tumor regression.
Keywords: immune therapy, stroma reprogramming, vessel remodeling
Tumor growth relies on interactions with stromal cells, which can also contribute to immune evasion and limit the efficacy of immunotherapy. For instance, solid tumors often develop abnormal and leaky blood vessels, which facilitate hypoxia and increase interstitial fluid pressure, two parameters known to interfere with anticancer therapy.1 However, the tumor stroma is highly dynamic in nature and recent publications have highlighted that reversing abnormal features of stromal cells can largely improve the outcome of immunotherapy.2,3 In this context, we became interested in tumor necrosis factor α (TNFα) as it is highly upregulated in tumors exhibiting normalized blood vessels and succumbing to an immune responses, suggesting a local immunomodulatory function.4 TNFα is a pleiotropic inflammatory cytokine best known for its capacity to induce tumor and endothelial cell death. However, high-dose TNFα is toxic for normal tissue, which consistently restricts its clinical applications. Tumor-targeting strategies such as conjugating TNFα with vessel homing peptides have been shown to prevent systemic toxicity, and low-dose TNFα also improves the efficacy of chemotherapy.5 Synergism between intratumoral TNFα and chemotherapy has been attributed to increased vascular permeability, but analyses of stromal effects in vivo have so far been limited.6 Moreover, the role of TNFα as an adjuvant to immunotherapy has not been explored until recently.7
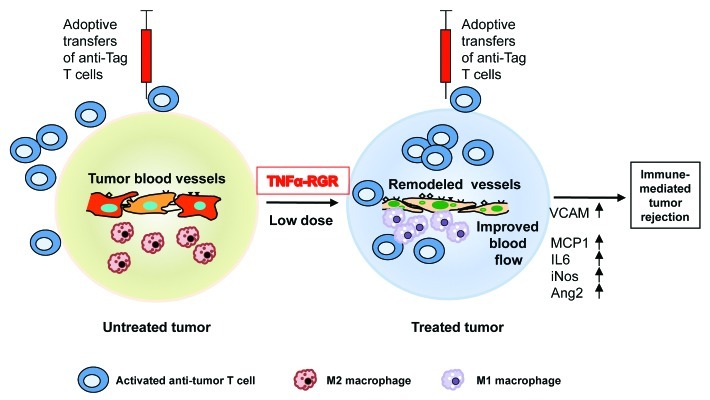
Our work demonstrated that tumor-targeted TNFα has profound effects on the tumor microenvironment by stabilizing blood vessels and potentiating immunotherapy.8 TNFα was conjugated to a vascular homing peptide which specifically binds to angiogenic tumor vessels and used in a murine model of pancreatic endocrine tumors (RIPTag, expression of the SV40 Large T antigen by the rat insulin gene promoter). Peptide-coupled TNFα accumulates around tumor vessels, attracts T cells into the tumor microenvironment and primes an endogenous antitumor CD8+ T cell-dependent immune response, ultimately enhancing overall survival.8 Considering the immunostimulatory properties of tumor-targeted TNFα monotherapy, we hypothesized that it could also function as adjuvant in conjunction with active immunotherapy. Indeed, intratumoral TNFα “opens” tumors to the influx of adoptively transferred, pre-activated effector cells (Fig. 1). This is remarkable since fully activated effector cells are per se to penetrate into insulinomas in RIPTag mice. Under TNFα therapy, however, transgenic T cells specific for the model tumor antigen Tag accumulate and proliferate in the tumor, leading to very significant improvements in survival.
Figure 1. Intratumoral low-dose tumor necrosis factor α (TNFα-RGR, TNFα conjugated with vascular homing peptide, injected i.v.) increases tumor vessel stability and vascular perfusion. Remodeled vessels are highly activated and express VCAM, vascular cell adhesion molecule (VCAM). Tumor resident macrophages switch from a M2 to a M1 phenotype, express high levels of VCAM, monocyte chemotactic protein 1 (MCP1), interleukin 6 (IL-6), inducible nitric oxide synthetase (iNOS) and angiopoietin 2 (Ang2), and cluster around tumor vessels. Adoptively transferred antitumor T cells are unable to penetrate into untreated tumors, but infiltrate tumors after “pre-conditioning” with TNFα, which leads to tumor regression.
These results raised the question of how local TNFα renders the tumor microenvironment permissive for antitumor immune responses. Our results clearly show that low-dose TNFα does not compromise barrier function or destroy vessels. Instead, it induces a regular vascular network with small vessel calibers surrounded by stabilizing mural cells. Overall, vessels are less leaky and tumor perfusion is improved. This is an important finding in the field of tumor immunology as it demonstrates that a functional vasculature and an improved tumor perfusion greatly enhance tumor-specific immune responses. This is further supported by the observation that targeting to the tumor another inflammatory cytokine, interferon γ (IFNγ, which predominantly induces endothelial cell death) fails to support immune cell infiltration.8 Also, repetitive, low-dose TNFα infusion into tumors ultimately induces endothelial cell death and hence limits the influx of effector cells. Interestingly, reduction of vascular leakiness by pharmacological or genetic normalization of the tumor vasculature also enhances adoptive T-cell therapy.2,9 In contrast, destruction of tumor blood vessels, for instance by vasculature-disrupting agents that stimulate production of high TNFα levels does not support active T-cell immunotherapy.10
Besides vascular remodeling, intratumoral TNFα elicits widespread stromal activation and elevated expression of the vascular cell adhesion molecule (VCAM) on endothelial cells, fibroblasts and macrophages. We demonstrated that macrophages play an important role in amplifying vessel activation by secreting angiopoietin 2 (Ang2), a tyrosine kinase receptor ligand that—in conjunction with TNFα—upregulates the expression of endothelial adhesion molecules, hence facilitating leukocyte extravasation. Once tumor-specific effector cells have reached the tumor site, they encounter a favorable inflammatory environment since low-dose TNFα also relieves iummunosuppression by tumor-resident macrophages. Thus, TNFα acts on multiple stromal cells to improve tumor perfusion, leukocyte extravasation and immune stimulation. Along similar lines, the histidine-rich glycoprotein (HRG) has recently been shown to polarize macrophages to create an immunostimulatory tumor environment that also normalizes blood vessels.3
Collectively, our study reveals that low-dose TNFα targeted into solid tumors is a promising adjuvant that improves vessel function and antitumor immunity. which can be exploited in the context of active and passive immunotherapy. Our findings also encourage further development of combination therapies that simultaneously alter tumor-associated stroma and activate antitumor immune responses.
Glossary
Abbreviations:
- Ang2
angiopoietin 2
- HGR
histidine-rich glycoprotein
- IFNγ
interferon γ
- IL-6
interleukin 6
- iNOS
inducible nitric oxide
- MCP1
monocyte chemotactic protein 1
- Tag
SV40 Large T antigen
- RIP
rat insulin gene promoter
- TNFα
tumor necrosis factor α
- VCAM
vascular cell adhesion molecule
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20981
References
- 1.Johansson A, Ganss R. Remodeling of tumor stroma and response to therapy. Remodeling of tumor stroma and response to therapy. Cancers. 2012;4:340–53. doi: 10.3390/cancers4020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–4. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 3.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–70. [PubMed] [Google Scholar]
- 5.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475–82. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Laarhoven HW, Gambarota G, Heerschap A, Lok J, Verhagen I, Corti A, et al. Effects of the tumor vasculature targeting agent NGR-TNF on the tumor microenvironment in murine lymphomas. Invest New Drugs. 2006;24:27–36. doi: 10.1007/s10637-005-4540-2. [DOI] [PubMed] [Google Scholar]
- 7.Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, et al. Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687–94. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- 8.Johansson A, Hamzah J, Payne CJ, Ganss RT. Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A. 2012;109:7841–6. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews KE, Hermans IF, Roberts JM, Ching LM, Ronchese F. 5,6-Dimethylxanthenone-4-acetic acid treatment of a non-immunogenic tumour does not synergize with active or passive CD8+ T-cell immunotherapy. Immunol Cell Biol. 2006;84:383–9. doi: 10.1111/j.1440-1711.2006.01448.x. [DOI] [PubMed] [Google Scholar]