Abstract
We have recently developed a protein vaccine against breast cancer in which HER2 is delivered to dendritic cells (DCs) in vivo through receptors expressed on their surface. Our results indicate that this is a promising approach to induce durable, broad and integrated immunity against breast cancer.
Keywords: DEC205, HER2, breast cancer, dendritic cell targeting, protein vaccine
Great advances have been made to improve remission rates in breast cancer patients. However, it is necessary to search for new treatment strategies to reduce the risk of relapse, which today remains high. HER2 is a protein that is overexpressed in a sizeable fraction of breast cancers, hence constituting an attractive target for immunotherapy. For instance, Herceptin (trastuzumab) is a licensed monoclonal antibody that binds to the extracellular domain of HER2.1 Although HER2 is expressed by malignant cells as a non-mutated self-antigen, immune responses can be elicited by vaccination. Compared with passive immunotherapy with trastuzumab, active vaccination has several advantages. Thus, unlike antibody therapy, vaccination induces more durable protection, particularly through vaccine-induced T cells that require relatively low levels of HER2 expression for recognizing cancer cells. Only small amounts of peptide-MHC complexes are required for T-cell recognition, and there is evidence that a large proportion of breast cancers, while not sensitive to trastuzumab, still express HER2 levels that are sufficient for T-cell recognition. Therefore, active vaccination has the potential to address a larger population of women than anti-HER2 antibodies, which target patients with high HER2 expression levels. In addition, because vaccine-induced immunity is durable, strong and distinct in its mechanism, vaccines are a logical way to improve resistance to the development of (micro)metastasis.
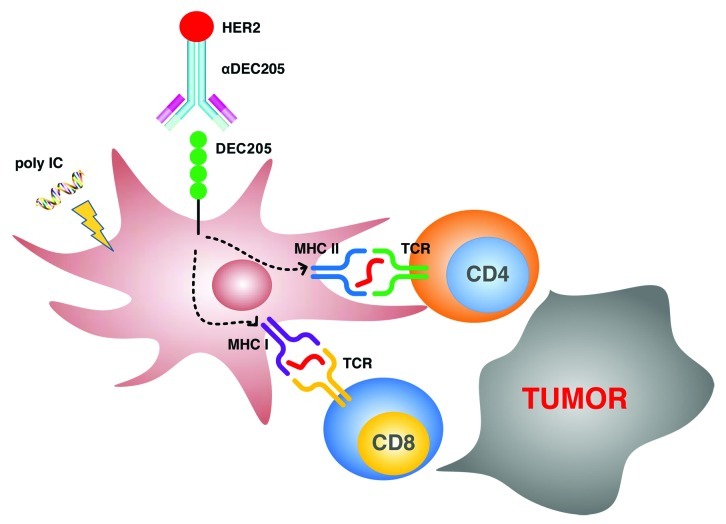
Protein vaccines are an attractive platform for cancer vaccines because they can easily be manufactured and administrated repeatedly. However, the soluble HER2 protein as a vaccine only shows weak immunogenicity, and has usually failed to confer protection against HER2-expression tumors. Our focus was hence to directly deliver HER2 protein to dendritic cells (DC) in vivo. DCs are potent antigen-presenting cells and are capable of processing the HER2 protein to liberate peptides for presentation on MHC class I and II complexes to CD8+ and CD4+ T cells, respectively (Fig. 1). In addition, DCs are immune-initiating cells that are able to find in vivo rare clones of HER2-specific T cells, expanding them and inducing critical helper and killer anticancer T cell functions. Discovery of antigen uptake receptors on DCs has enabled the engineering of monoclonal anti-receptor antibodies that are efficiently and specifically delivered to DCs in situ, with no need for DC isolation as in previous DC-based vaccines.2 One of the receptors that were targeted in our recent study is DEC205, a Type I C-type lectin that is abundant on CD8α+ DCs.3 Ralph Steinman and his collaborators have developed an efficient DC-delivery system via DEC205, which after extensive preclinical studies in mice is now being tested in proof of concept studies in volunteers.4 Studies in mouse models have demonstrated that a foreign antigen, such as the HIV gag p24 protein, when targeted to DCs by an anti-DEC205 antibody, is efficiently processed and presented to T cells. In fact, antigen delivery through DEC205 increases the efficiency of antigen presentation relative to non-targeted antigens over 100-fold, and it induces both CD4+/CD8+ T-cell and antibody responses. An ongoing study in healthy volunteers has demonstrated that an anti-DEC205-p24 vaccine administered together with polyriboinosinic:polyribocytidylic acid (polyI:C), a synthetic double-stranded RNA as adjuvant, elicits an integrated B- and T-cell immunity.
Figure 1. Induction of T-cell immunity against breast cancer through a HER2 protein vaccine targeted to dendritic cells (DCs) in vivo. Maturation of DCs is induced by the Toll-like receptor 3 (TLR3 = ligand polyI:C. HER2 protein is delivered to DCs via an anti-DEC205 hybrid antibody. DCs are able to process the HER2 protein to liberate peptides for presentation on MHC Class I and II complexes to CD8+ and CD4+ T cells, respectively.
Recently, we provided proof of concept evidence - in a preclinical setting - on a way to load DCs with the HER2 protein and to stimulate the immune system to enhance the antitumor immunity initiated by DCs.5 One of the major challenges in DC-based cancer vaccination is to include in the vaccine protocol an adjuvant to optimize DC maturation. Emphasis is now being placed on microbial mimics as adjuvants, for example agonists of innate microbial recognition receptors such as Toll-like receptors (TLRs).6 We found that the TLR3 ligand polyI:C is a superior adjuvant for inducing T-cell immunity in mice, as within 4 h of injection DCs mature to become immunogenic.7 We showed that TLR agonists are required to elicit tumor antigen-specific T-cell responses when mice were injected with DEC205-HER2 hybrid monoclonal antibody. We demonstrated that the HER2 protein - when delivered through DEC205 - induces broad, potent, multifunctional and durable Th1 responses, as well as CD8+ T-cell and B-cell responses. Such integrated immunity is desirable for tumor immunotherapy. Furthermore, using a mouse transplantable tumor model, we demonstrated that the DEC205-HER2 vaccination provides significant long-term survival benefits to mice challenged with neu-expressing tumors. This protection is mediated by both CD4+ and CD8+ T cells, with CD8+ T cells playing a more dominant role.
Discovery of new antigen uptake receptors on DCs expands the spectrum of DC-targeting strategies. For example, a recently identified member of the triggering (Trem) family of receptors expressed on myeloid cells, Trem-like 4 (Treml4), has also been tested for targeting DCs.8,9 High expression levels of the Treml4 receptor (which belongs to the Ig superfamily) can be found on CD8α+ DCs and, unlike DEC205, on subsets of splenic macrophages. However, targeting of HER2 to mouse DCs through the Treml4 receptor also can induce integrated and protective CD4+ and CD8+ T-cell immunity against breast cancer similar to what we observed with the anti-DEC205-HER2 vaccination.
Although targeting DCs in vivo is a promising new approach for cancer immunotherapy, more studies are required to design an effective vaccine for cancer patients. For example, we need to explore the intrinsic properties of different antigen uptake receptors expressed on DCs and evaluate different immunological outcomes when targeting different subsets of mouse/human DCs. Despite limited knowledge, preclinical studies have now provided proof of principle for in vivo DC-targeting vaccination approaches. Alone or in combination with other therapeutic approaches, this vaccine strategy may have an impact on all stages of breast cancer, to prevent disease, contain micrometastases, and resist existing lesions.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20982
References
- 1.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 2.Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 4.Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. 2012;271:183–92. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, et al. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res. 2012;14:R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–93. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, et al. A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. 2009;182:1278–86. doi: 10.4049/jimmunol.182.3.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi H, Zaidi N, Wang B, Matos I, Fiorese C, Lubkin A, et al. Treml4, an Ig superfamily member, mediates presentation of several antigens to T cells in vivo, including protective immunity to HER2 protein. J Immunol. 2012;188:1147–55. doi: 10.4049/jimmunol.1102541. [DOI] [PMC free article] [PubMed] [Google Scholar]