Abstract
We have investigated the role of the Ca2+-binding protein S100A9 on tumor growth in prostate cancer and T-cell lymphoma models. We found that the expression of, S100A9 and its interaction with Toll-like receptor 4 (TLR4) is critical for tumor growth in these settings.
Keywords: S100A9, TGF, TLR4, cancer, myeloid cells
Our interest in the Ca2+-binding protein S100A9 was prompted by the observation that a family of small molecules in development for the treatment of autoimmune diseases and cancer, namely quinolone-3-carboxamides (Q compounds), bind to S100A9, thus inhibiting its interaction with innate immunity receptors such as Toll-like receptor 4 (TLR4) and receptor for advanced glycation end products (RAGE).1 Such initial findings were made in 2005, and since then several articles have been published that link S100A9 to tumor growth and metastasis.2,3
We decided to investigate whether the absence of S100A9, RAGE or TLR4 influence tumor cell growth. We initially wanted to use a spontaneous tumor model in immunocompetent animals, since we suspected that part of the antitumor activity exerted by Q compounds4 would be mediated by the immune system. We had a special interest in prostate cancer as one Q compound is in clinical development for this specific indication.5 Hence, we started to breed S100A9−/− and TLR4−/− mice with TRAMP mice. The TRAMP model is a transgenic murine model of prostate cancer in which the SV40 large T antigen is expressed under the control of the probasin promoter.6 In our hands, TRAMP mice developed palpable tumors between weeks 20 and 30 of life, which was significantly delayed in both the S100A9−/− and TLR4−/− background.7 When we investigated S100A9 expression in prostate tumors, we noted that while S100A9 was barely absent in nonmalignant prostate tissue, it was readily detectable in prostate tumors and, interestingly, often associated with myeloid rather than tumor cells.
We then switched tumor model in favor of the EL4 lymphoma, in particular for being able to perform more controlled experiments with regard to cell populations and gene expression.7 In this model, we observed reduced tumor growth in S100A9−/− and TLR4−/− mice, but not in RAGE−/− (lacking the gene encoding RAGE) animals. Interestingly, we have recently found that S100A9 can stimulate cytokine secretion in monocytes upon interaction with TLR4, while S100A9/RAGE interactions appear to be less important for this effect.10 We believe that these data may, at least partially, explain the differential impact of TLR4 and RAGE on EL4 tumor growth.
In order to understand the mechanisms that underlie sich a differential tumor growth, we analyzed the myeloid cell compartment in our mouse strains and observed a reduced ratio of Ly6G+C+/Ly6C2+ cells in both S100A9−/− and TLR4−/− animals compared with wild type C57BL/6 mice and RAGE−/− mice. We then proceeded to investigate the expression profile of various genes that are known to be involved in the regulation of myeloid cell function. We looked for a marker whose expression pattern would be modified in S100A9−/− and TLR4−/− animals relative to their wild type counterparts. We found that transforming growth factor β (TGFβ) expression in splenic myeloid cells was lower in both S100A9−/− and TLR4−/− animals than in wild type mice. Circulating TGFβ did not differ in wild type C57BL/6, S100A9−/−, TLR4−/− and RAGE−/− animals. However, when EL4 lymphoma cells were inoculated in wild type C57BL/6 or RAGE−/− mice the circulating levels of TGFβ increased. This did not occur in S100A9−/− and TLR4−/− animals, which rather maintained circulating TGFβ to the levels of tumor-free animals). This was an interesting observation since TGFβ has been involved in cancer progression in other models.8
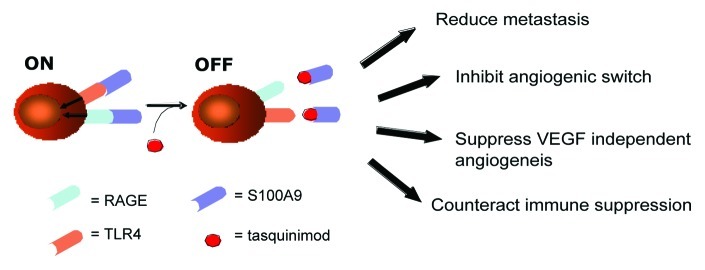
Our hypothesis based on the work discussed above was that an interaction between S100A9 and TLR4 leads to increased TGFβ production, in turn stimulating tumor growth. To investigate this further we took advantage of a small molecule, tasquinimod, as a probe. Tasquinimod has been shown to bind to S100A9 hence inhibit its interaction with TLR4. Treating C57BL/6 mice bearing EL4 lymphomas with tasquinimod inhibited tumor growth, in parallel with a significant downregulation of serum TGFβ levels.
Taken together, these observations suggest that, in the TRAMP and EL4 models, the interaction between S100A9 and TLR4 promotes tumor growth, with a negligible role for RAGE. This is interesting as RAGE expression has been shown to be critical for tumor growth in other models.9 In the future, it will be important to investigate the role of S100A9 in such RAGE-dependent tumor models. Should an effect be observed, targeting the mixed TLR4 and RAGE ligand S100A9 might constitute a new appraoch to anticancer therapy (Fig. 1).
Figure 1. Mechanism of action of tasquinimod. The Ca2+-binding protein S100A9 on myeloid cells is a putative molecular target of tasquinimod.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21027
References
- 1.Björk P, Björk A, Vogl T, Stenström M, Liberg D, Olsson A, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–71. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 3.Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, et al. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11:5146–52. doi: 10.1158/1078-0432.CCR-05-0352. [DOI] [PubMed] [Google Scholar]
- 4.Olsson A, Björk A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9:107. doi: 10.1186/1476-4598-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pili R, Häggman M, Stadler WM, Gingrich J, Assikis V, Björk A, et al. Phase II Randomized Double Blind Placebo-Controlled Study to Determine the Efficacy of Tasquinimod in Asymptomatic Patients with Metastatic Castrate-Resistant Prostate Cancer. J Clin Oncol. 2011;29:4022–8. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Källberg E, Vogl T, Liberg D, Olsson A, Björk P, Wikström P, et al. S100A9 interaction with TLR4 promotes tumor growth. PLoS One. 2012;7:e34207. doi: 10.1371/journal.pone.0034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–43. doi: 10.1093/carcin/bgn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riva M, Källberg E, Björk P, Hancz D, Vogl T, Roth J, et al. Induction of nuclear factor-κB responses by the S100A9 protein is Toll-like receptor-4-dependent. Immunology. 2012;137:172–82. doi: 10.1111/j.1365-2567.2012.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]