Abstract
The clinical success of immunomodulatory thalidomide derivatives has renewed the general interest in immunomodulatory anticancer compounds and prompted us to develop a high-throughput system to quantify immune effector-cell activity. We documented that the interaction between cancer cells, their stroma, anticancer agents and cells from the innate system are critical for determining the response of tumors to immunomodulatory strategies.
Keywords: cancer, high-throughput screening, immune effector cells, pharmacological immunomodulators, tumor heterogeneity
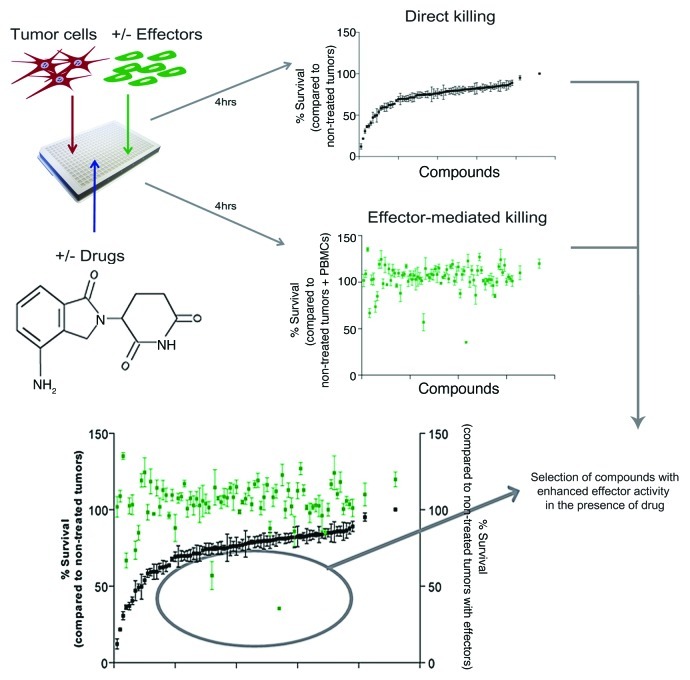
The enhancement of immune effector-cell activity is an appealing strategy for the treatment of various cancers. The development of clinically applicable therapies, however, requires preclinical assays that allow for the study of immunological interactions as well as novel agents that exert immunomodulatory effects. To date, the evaluation of chemical libraries of small molecules affecting antitumor immunity has been hampered by the lack of robust, sensitive and high-throughput scalable assays. The development of high-throughput screening strategies is needed for the analysis of tumors, stromal cells, anticancer compounds, immune effector-cell subsets and their mutual relationship. Expanding on our previous publication,1 we adapted our high-throughput approach to discover novel antitumor immunomodulatory mechanisms (Figure 1).2 We showed that pharmacological agents (i.e., lenalidomide, pomalidomide, bortezomib, and dexamethasone) as well as autologous bone marrow stromal cells can alter antitumor immunity.2 In addition, such a high-throughput screening approach allowed us to identify agents that enhance or inhibit innate antitumor cytotoxicity by indirect mechanisms. Interestingly, we discovered that a given compound can display different activity profiles depending on the tumor target, for instance, suppressing immunity against one tumor target but enhancing it against others. The immunomodulatory properties of a specific drug depend on a series of factors, including the interplay between the cancer cells, the immune effector cells, tumor microenvironment and the drug itself.2 Our approach allows for the analysis of multiple tumor targets, compounds and microenvironmental conditions, to fully evaluate the immunomodulatory properties of drugs.
Figure 1. Description of the compartment-specific bioluminescence (CS-BLI) platform for antitumor immunity screening. Luciferase-positive tumor cells are mixed in culture with interleukin-2 (IL-2)-stimulated peripheral blood mononuclear cells (PBMCs). High-throughput screening for specific antitumor activity in the presence and absence of immune-effector cells is shown for ~100 small molecule inhibitors. Direct antitumor activity (black) of a given compound was compared with its modulation of antitumor effector activity (green). Direct activity is normalized to the untreated controls, whereas the effector cell-mediated activity is normalized to control conditions including the drug but not effector cells. Effector cell-mediated data points (green) falling below the direct killing values (black) represent compounds that enhance antitumor immunity.
Conventional cytotoxic agents may exert part of their antitumor effects by activating the immune system against cancer cells.3-5 There has been an increasing interest in the discovery of novel immunomodulatory agents, and these compounds have become key therapeutics for the management of multiple myeloma (MM).6-8 Our findings can have major implications for the development of therapeutics targeting many neoplasms. Indeed, we demonstrated that our high-throughput scalable system is also appropriate for the study of immunomodulation in lymphoma and leukemia cell lines.2 We were able to identify novel immunomodulatory agents and evaluate their effects across a panel of MM.1S, RPMI8226, KMS34 and Dox40 myeloma, as well as HT, Oci-Ly1 and Farage lymphoma and KU812F leukemia cells. This broad approach allows for the understanding of the mechanisms shared by these tumor cell lines, each with its unique genetic background, oncogenic drivers and response to immunomodulation.
The role of the tumor microenvironment in antitumor immunity is also well recognized,9,10 however, the ability to evaluate the interactions between tumor cells and their stroma at a preclinical level has been limited. Screening autologous stromal cells with immune effector cells, in the context of matched MHC antigens from cells isolated from the same patient, we observed that stromal cells inhibit antitumor immunity as provided by interleukin-2 (IL-2)-stimulated peripheral blood mononuclear cells (PBMCs).2 Some drugs could counteract this effect, but not completely abrogate it. For example, stromal cells dramatically limited immune responses against MM.1S cells, yet lenalidomide or pomalidomide blocked such a stromal protection, indicating part of the efficacy of clinically active therapies may result from the inhibition of stromal mechanisms.
Arguably, the most important finding in our studies was that anticancer compounds do not necessarily exert immunostimulatory or immunosuppressive effects consistently across all tumor targets. We screened an 800 compound library and compared the consistency of immunomodulatory effects against MM.1S myeloma vs. HT lymphoma cells. Only 2.1% of the library enhanced antitumor immunity against both MM.1S and HT cells, while 7.5% had contrasting effects. This suggests that selection of the specific tumor model is a critical element for the development of immunomodulatory antitumor strategies. Indeed, immunomodulatory drugs act not only on the host immune system but also on tumor and stromal cells, resulting in distinct outcomes depending on the precise interacting factors. The complexity of these interactions is daunting, but its understanding opens the door for the discovery of agents that can modulate host-tumor interactions at multiple levels.
The development of first-in-class agents often requires a paradigm shift in thinking, along with enabling models and technologies. Historically, immune-based models have relied on a single cell line, often being associated with difficulties in the translation of in vitro results to patients. This translation might also have been complicated by the largely neglected role of the tumor microenvironment and its influence on tumors targeted by immunomodulatory agents The discovery of first-in-class anticancer agents with immunomodulatory properties, irrespective on whether they exert direct anticancer activity on not, will be facilitated by our methodology and other novel approaches. A comprehensive evaluation of these parameters is relevant not only for innate immune responses, but also for adaptive responses by T and dendritic cells. More broadly, immunologic therapies enhancing graft-vs.-tumor (GVT) but not graft-vs.-host (GVH) mechanisms will be important to oncology patients eligible to transplantation and will enable a more effective translation of preclinical discoveries to the bedside.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21058
References
- 1.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–9. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillin DW, Delmore J, Negri JM, Vanneman M, Koyama S, Schlossman RL, et al. Compartment-Specific Bioluminescence Imaging platform for the high-throughput evaluation of antitumor immune function. Blood. 2012;119:e131–8. doi: 10.1182/blood-2011-04-348490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Kroemer G. The dilemma of anticancer therapy: tumor-specific versus immune effects. Blood. 2008;112:4364–5. doi: 10.1182/blood-2008-09-176693. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Kroemer G. Anticancer immunochemotherapy using adjuvants with direct cytotoxic effects. J Clin Invest. 2009;119:2127–30. doi: 10.1172/JCI39991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Lenalidomide in multiple myeloma. Expert Rev Anticancer Ther. 2006;6:1165–73. doi: 10.1586/14737140.6.8.1165. [DOI] [PubMed] [Google Scholar]
- 7.Hideshima T, Richardson PG, Anderson KC. Current therapeutic uses of lenalidomide in multiple myeloma. Expert Opin Investig Drugs. 2006;15:171–9. doi: 10.1517/13543784.15.2.171. [DOI] [PubMed] [Google Scholar]
- 8.De Raeve H, Vanderkerken K. Immunomodulatory drugs as a therapy for multiple myeloma. Curr Pharm Biotechnol. 2006;7:415–21. doi: 10.2174/138920106779116847. [DOI] [PubMed] [Google Scholar]
- 9.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]