Abstract
Oncolytic virotherapy represents a recent approach to anticancer therapy. Rodent autonomous parvoviruses (PVs) represent naturally oncolytic viruses that are non-pathogenic for humans but possess and extended tropism, being capable of infecting transformed cells of both rodent and human origin. Recent work from our group demonstrate that PVs can act as direct lytic agents and adjuvants, stimulating antitumor immune responses against glioma and pancreatic ductal adenocarcinoma (PDAC).
Keywords: immune response, oncolytic viruses, parvoviruses
Oncolytic viruses (OVs) have attracted increasing attention as novel weapons in the fight against cancer. Nowadays, more than 20 viruses have been recognized as potential OVs, with new candidates continuing to emerge while established viruses enter clinical trials. Among OVs, non-modified rodent parvoviruses (PVs) are especially interesting, as they are non-pathogenic for humans and possess oncotropic and oncolytic features.1 Recent work from our group focused on the ability of the parvoviruses Minute Virus of Mice (MVMp) and rat parvovirus H-1 (H-1PV) to trigger, direct and indirectly, the activation of the immune system against tumor cells.
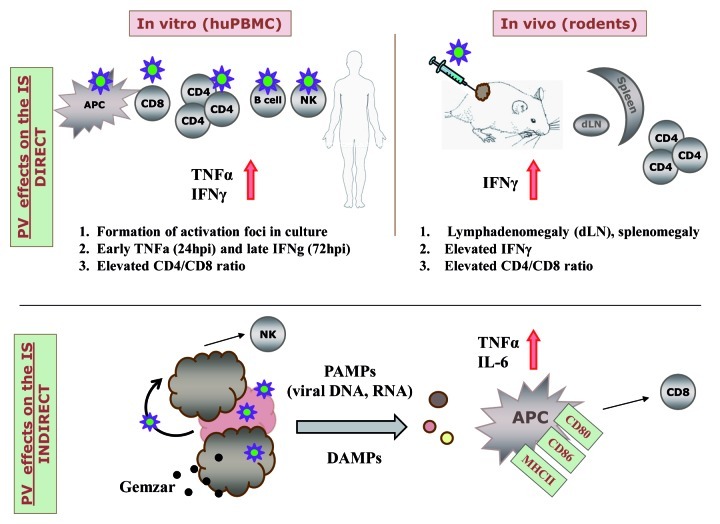
In terms of direct effects on the immune system, our work showed that in vitro infection of immune cells including human peripheral blood mononuclear cell (PBMCs) and mouse dendritic cells (DCs) by PVs is abortive, since virus can enter cells, but fails to replicate and produce new virions.2,3 However, infection of human immune cells does not remain completely silent. H-1PV treatment leads indeed to PBMC foci formation as a result of T cell proliferation, with the early release of tumor necrosis factor α (TNFα) and the late secreion of interferon γ (IFNγ) (Fig. 1).2 Analysis of MVMp-treated hemangiosarcoma in mice as well as of H-1PV-treated pancreatic carcinoma in rats and other animal models revealed that in the first days following intratumoral inoculation, PVs are expressed most strongly in the tumor, but can also be detected in lymphatic tissues, notably in tumor draining lymph nodes (DLNs).4-6 In both glioma and pancreas cancer models, immunocompetent animals treated with PVs exhibit spleno- and lymphadenomegaly, with an increase of the T-cell compartment and production of IFNγ in DLNs and splenocytes.2,3 Increased CD4+/CD8+ cell ratios were observed in both human and rodent systems.
Figure 1. Schematic presentation of parvoviruses effects on the immune system (IS). The direct effects were observed upon in vitro infection of human total peripheral blood mononuclear cells (PBMCs) and PBMC subpopulations or in vivo treatment of tumors in rodent models. Indirect effects were observed in co-culture systems upon viro- or combined chemovirotherapy of tumor cell lines in vitro. Antigen-presenting cells (APCs) and natural killer (NK) cells were activated directly upon oncolysis of tumor cells, while CTL killing was stimulated by dendritic cells (DCs) conditioned with tumor H-1PV oncolysates.
Besides these direct effects on the immune system, PVs appear to modulate it indirectly, by killing tumor cells. It has been shown that the antitumor cellular immune response plays an important role in the oncosuppressive effect of PV in rodent tumor models.2,7 This suggests that PV-mediated oncolysis might result in the release of tumor-associated antigens and possibly also of pathogen- and damage- associated molecular patterns. These signals may account for the activation and maturation of antigen-presenting cells (APCs), as reported for human DCs in a melanoma model8 and mouse DCs and microglia in a glioma model,3 correlating with higher expression of activation markers (CD80, CD83, CD86) and release of pro-inflammatory cytokines (TNFα, IL-6). APCs provide the initial cue for innate and adaptive immune responses. The in vivo analysis of immunodeficient and immunocompetent mice bearing glioma revealed a clear difference in their susceptibility to MVMp-mediated tumor suppression. While immunocompetent animals were fully protected from tumor outgrowth by infected glioma cells, immunodeficient mice were less competent for MVMp-dependent tumor inhibition, with only 20% of the recipients being protected. Thus, the infection of glioma cells by MVMp enhanced their capacity to stimulate a tumor-specific immune response. This response was mediated by T cells, as inferred from the induction of IFNγ-producing T cells detected by ELISpot assays, as well as by its abolition in Rag2−/− mice, which lack T cells but not natural killer (NK) cells. In the human system, the co-culture of DCs with H-1PV-infected melanoma cells led to the generation of effective tumor-specific cytotoxic T lymphocytes.8 NK cells, which constitute an important effector of innate immunity, also seem to sense PV-mediated tumor cell killing, reacting with IFNγ production, most probably at the early stages of the immune response.9
Recent work from our lab suggests that the synergistic combination of gemcitabine with parvovirotherapy can induce a panel of immunogenic cell death (ICD) determinants in pancreatic cancer cells, offering a tempting strategy for allogenic vaccination (manuscript in preparation). In line with this possibility, we previously reported that infection of hepatoma cells with CpG-armed PVs (allowing for the cancer-specific PV-mediated amplification of the immunostimulatory CpG motifs) enhances the capacity of these cells to serve as autologous tumor vaccines in a lung metastasis rat model. These effects correlated with the expression of IFNγ in the mediastinal lymph nodes draining the metastases and occurred in the absence of a direct infection of metastatic deposits with the CpG-modified PVs, the latter serving simply as an adjuvant and potential ICD-inducer in the vaccine preparation.10
Altogether, these findings provide first evidence that the interplay between PVs and both cancer and immune cells is detrimental for the former and stimulating for the latter, leading not only to direct oncolysis, but also to the break of immune tolerance to tumors with a Th1 bias. The mechanisms of direct and indirect immunostimulation by PVs are interrelated and difficult to discriminate, resulting in synergistic effects between virus infection and the immune reactions of the host against tumor growth. In general, rodent PVs (being non-pathogenic for humans) seem not to trigger overt immune/inflammatory reactions by the human immune system, compared with pathogenic viruses engineered to become oncolytic. Nevertheless, as silent fine-tuning agents, PVs may allow the exposure of cryptic tumor antigens, hence promoting their immunogeniccity, without strongly “attracting the attention” of the immune system toward viral infection itself. Nonetheless, recent clinical applications of PVs in glioma multiforme patients should give the ultimate proof whether PVs are completely safe oncolytic tools that can stably become part of immunotherapeutic strategies.
Glossary
Abbreviations:
- DC
dendritic cell
- DLN
draining lymph nodes
- hpi
hours post infection
- IS
immune system
- ICD
immunogenic cell death
- MVMp
Minute Virus of Mice
- OVs
oncolytic viruses
- PDAC
pancreatic ductal adenocarcinoma
- PBMC
peripheral blood mononuclear cell
- PVs
parvoviruses
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21097
References
- 1.Rommelaere J, Geletneky K, Angelova AL, Daeffler L, Dinsart C, Kiprianova I, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine Growth Factor Rev. 2010;21:185–95. doi: 10.1016/j.cytogfr.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Grekova S, Aprahamian M, Giese N, Schmitt S, Giese T, Falk CS, et al. Immune cells participate in the oncosuppressive activity of parvovirus H-1PV and are activated as a result of their abortive infection with this agent. Cancer Biol Ther. 2011;10:1280–9. doi: 10.4161/cbt.10.12.13455. [DOI] [PubMed] [Google Scholar]
- 3.Grekova SP, Raykov Z, Zawatzky R, Rommelaere J, Koch U. Activation of a glioma-specific immune response by oncolytic parvovirus Minute Virus of Mice infection. Cancer Gene Ther. 2012 doi: 10.1038/cgt.2012.20. [DOI] [PubMed] [Google Scholar]
- 4.Giese NA, Raykov Z, DeMartino L, Vecchi A, Sozzani S, Dinsart C, et al. Suppression of metastatic hemangiosarcoma by a parvovirus MVMp vector transducing the IP-10 chemokine into immunocompetent mice. Cancer Gene Ther. 2002;9:432–42. doi: 10.1038/sj.cgt.7700457. [DOI] [PubMed] [Google Scholar]
- 5.Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15:511–9. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 6.Raykov Z, Savelyeva L, Balboni G, Giese T, Rommelaere J, Giese NA. B1 lymphocytes and myeloid dendritic cells in lymphoid organs are preferential extratumoral sites of parvovirus minute virus of mice prototype strain expression. J Virol. 2005;79:3517–24. doi: 10.1128/JVI.79.6.3517-3524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raykov Z, Grekova S, Galabov AS, Balboni G, Koch U, Aprahamian M, et al. Combined oncolytic and vaccination activities of parvovirus H-1 in a metastatic tumor model. Oncol Rep. 2007;17:1493–9. [PubMed] [Google Scholar]
- 8.Moehler MH, Zeidler M, Wilsberg V, Cornelis JJ, Woelfel T, Rommelaere J, et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum Gene Ther. 2005;16:996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- 9.Bhat R, Dempe S, Dinsart C, Rommelaere J. Enhancement of NK cell antitumor responses using an oncolytic parvovirus. Int J Cancer. 2011;128:908–19. doi: 10.1002/ijc.25415. [DOI] [PubMed] [Google Scholar]
- 10.Raykov Z, Grekova S, Leuchs B, Aprahamian M, Rommelaere J. Arming parvoviruses with CpG motifs to improve their oncosuppressive capacity. Int J Cancer. 2008;122:2880–4. doi: 10.1002/ijc.23472. [DOI] [PubMed] [Google Scholar]