Abstract
Constitutively stabilized HSP90 client proteins are crucial for cancer cell survival and proliferation. Thus, despite—or perhaps because of—their pleiotropic effects on variety of critical oncoproteins, HSP90 inhibitors represent a promising new class of anticancer drugs. We identified MIF as an essential HSP90 client protein in a murine model of Her2-overexpressing breast cancer.
Keywords: CHIP, HSP90 inhibitors, MIF, breast cancer, client
One major trend in modern cancer therapy is to develop drugs that target a specific signaling pathway, aimed at achieving the selective killing of cancer cells with reduced side effects for normal tissues. Examples of this trend include inhibitors of the oncogenic protein kinases, ERBB2 (HER2) and BCR-ABL, which interfere with hyperactivated survival signaling. Such selective (“clean”) inhibitors are initially quite effective. However, the long-term use of these compounds is often limited by the acquisition of resistance, either upon the mutation of drug targets or by other bypass mechanisms that cancer cells develop in response to this selective pressure. In contrast, pleiotropic (“dirty”) drugs simultaneously affect several regulatory pathways that are requied for the survival of cancer cells, thus being less prone to generate resistance. This notion has led to the search for new pleiotropic agents that efficiency and specifically eliminate cancer cells. Such novel drugs are exemplified by inhibitors of protein chaperons and by compounds that influence the chromatin status.
Hsp90 Chaperone Alterations in Cancer: Adaptive Response to a Malignant Lifestyle
The heat shock 90 KDa protein (HSP90) is the core ATPase of a stress-induced multi-component machinery. The HSP90 complex drives the correct folding of nascent client proteins, normally protecting them from aggregation or assisting their proteasome-mediated degradation if they become irreversibly damaged.1 Cancer cells are in a constant state of proteotoxic stress, both due to an adverse microenvironment (often featuring hypoxia and acidosis) and to cell-intrinsic alterations including conformationally aberrant oncoproteins, an unusually hight need for protein synthesis, elevated levels of reactive oxygen species (ROS), spontaneous DNA damage, DNA replicative stress and genomic instability. Thus, cancer cells require massive chaperone support to prevent oncoprotein degradation and sustain cell survival. Importantly, during oncogenesis, the normal function of HSP90 is ubiquitously subverted for the maintenance of malignant transformation. Cancer-specific alterations of the HSP90 system include a massive upregulation of HSP90 (in part through heat-shock factor 1-mediated gene transactivation) that temporally correlates with malignant transformation. Thus, HSP90 plays a key role in the conformational stabilization of mutant and overexpressed client oncoproteins and exerts powerful anti-apoptotic effects in cancer.2 For example, HSP90 protects mutant p53 proteins from the E3 ligase activity of endogenous MDM2 and CHIP, operating as a large protective ‘cage’ against p53 degradation.3
Several strategies exist to interfere with HSP90 function. Geldanamycin or its derivative 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) operates by binding to the ATP-binding pocket of HSP90, thus inactivating its enzymatic functions. Importantly, tumor HSP90 has a 100-fold higher affinity for 17-AAG than HSP90 from normal tissues. This generates a therapeutic window for the use of HSP90 inhibitors in cancer therapy.2 Another compound, suberoylanilide-hydroxamic-acid (SAHA), interferes with the deacetylation of cytoplasmic HSP90 by blocking its obligatory positive regulator, the cytoplasmic histone deacetylase 6 (HDAC6). Thus, SAHA operates by blocking HSP90 in an acetylated, enzymatically-inactive status. Generally, HDAC inhibitors interfere with transcriptional regulations due to their ability to promote histones acetylation. Among several HDACs, only HDAC6 is cytoplasmic and promotes the deacetylation of HSP90.
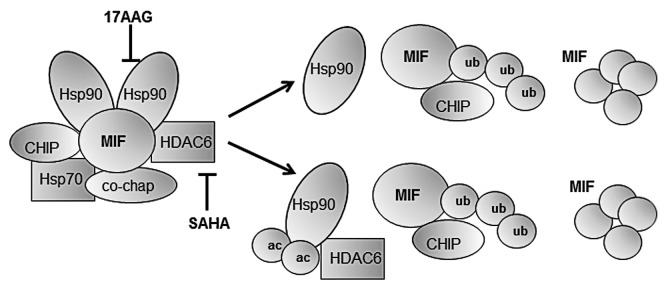
HSP90 inhibitors have been shown to effectively kill tumor cells in several model systems. Second-generation HSP90 inhibitors and specific HDAC6 inhibitors have recently been developed, and have been associated with durable clinical responses in clinical trials.4,5 However, it is difficult to predict how individual tumors will respond. Answering this question or identifying predictive biomarkers will be facilitated by the identification of client proteins that are stabilized by HSP90 and are critically required for tumor cell survival and proliferation (Fig. 1). Oncoproteins such as HER2, AKT, RAS, p53 and EML4-ALK are among such clients.1,2
Figure 1. HSP90 inhibition by blockade of HSP90 ATP-binding site (with 17-AAG), and HSP90 de-acetylation (with SAHA). HSP90 stabilizes client proteins, shielding them from normal degradation (left). HSP90 inhibition by 17-AAG or SAHA dissociates this complex, leading to release and activation of E3-ubiquitin ligases that initiate the degradation of HSP90 client proteins (right).
Recently, our group identified macrophage migration inhibitory factor (MIF) as another important protein stabilized by HSP90 in cancer.6 We observed elevated MIF levels in the ErbB2 mouse model of breast cancer. Upon HSP90 inhibition by the systemic administration of 17-AAG, MIF degradation was initiated by the E3 ubiquitin ligase CHIP and tumor progression was strongly impaired. In line with these results, 17-AAG-induced apoptosis and the growth defects of cancer cells treated with 17-AAG in vivo were significantly rescued by excess ectopic MIF. Likewise, in Mif−/− mice, the development of ErbB2 breast tumors was delayed, and these mice survived longer than their Mif+/+ counterparts. Altogether, these observations indicate that HSP90 stabilizes MIF in cancer cells, in turn constituting an essential contributor to ErbB2-driven tumor progression. Surprisingly, at least in this model, the HSP90 inhibitor 17-AAG acted largely through the destabilizion of MIF (rather than ErbB2), and this function was crucial for its anti-tumor activity.
Why does elevated MIF promote tumor progression? MIF, a pleiotropic tumor stimulator, influences several signaling pathways.7,8 For example, MIF acts as a pro-inflammatory/pro-angiogenic cytokine in an autocrine and paracrine fashion, inducing the stabilization of HIF1α and the secretion of multiple interleukins. MIF also binds to the CD74 cell surface receptor and activates the mitogen-associated protein kinase (MAPK) pathway in tumor and stromal cells. Furthermore, high MIF levels accumulate intracellularly in the nucleus and the cytoplasm. Finally, MIF interferes with the oncosuppressive functions of p53 and pRB.9,10 Thus, MIF degradation as a result of HSP90 inhibition should abolish most, if not all, MIF-dependent tumor-promoting activities.
The value of MIF as predictor of the clinical efficacy of HSP90 inhibitors may depend on tumor type and on the specific HSP90 inhibitor. Additional HSP90 clients may constitute predictive biomarkers similar to MIF. Thus, identifying critical client proteins of HSP90 in a given cancer may allow for the prediction of the clinical efficacy of treatments based on HSP90 inhibitors. This would result in the selective and individualized use of a pleiotropic drug, potentially translating a “dirty drug” in clean clinical responses.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21173
References
- 1.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 2.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, et al. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res. 2011;9:577–88. doi: 10.1158/1541-7786.MCR-10-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–9. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 5.Wong K, Koczywas M, Goldman JW. E.H. P, Horn L, Lufkin JM, et al. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) as monotherapy in patients with advanced non-small cell lung cancer (NSCLC). 2011 ASCO Annual Meeting 2011; Abstract No: 7500. [Google Scholar]
- 6.Schulz R, Marchenko ND, Holembowski L, Fingerle-Rowson G, Pesic M, Zender L, et al. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012;209:275–89. doi: 10.1084/jem.20111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemajerova A, Moll UM, Petrenko O, Fingerle-Rowson G. Macrophage migration inhibitory factor coordinates DNA damage response with the proteasomal control of the cell cycle. Cell Cycle. 2007;6:1030–4. doi: 10.4161/cc.6.9.4163. [DOI] [PubMed] [Google Scholar]
- 8.Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26:281–5. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Petrenko O, Fingerle-Rowson G, Peng T, Mitchell RA, Metz CN. Macrophage migration inhibitory factor deficiency is associated with altered cell growth and reduced susceptibility to Ras-mediated transformation. J Biol Chem. 2003;278:11078–85. doi: 10.1074/jbc.M211985200. [DOI] [PubMed] [Google Scholar]
- 10.Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol Cell. 2005;17:225–36. doi: 10.1016/j.molcel.2004.11.052. [DOI] [PubMed] [Google Scholar]