Abstract
Myeloid derived suppressor cells (MDSCa) are a heterogeneous population of cells that promote an immunosuppressive environment in tumor-bearing hosts. Recently, B7-H1 signaling has been reported to be critical for the maintainancer of regulatory T cells (Tregs), another immunosuppressive cell population. Here, we discuss the immunosuppressive function of B7-H1 on MDSCs, and the functional crosstalk between Tregs and MDSCs.
Keywords: B7-H1, IL-10, MDSC, Tregs, melanoma
We have recently described a novel strategy whereby regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) functionally crosstalk, through the B7-H1 pathway, during the development of murine melanomas.1 Together with Tregs, MDSCs contribute to establishing an immunosuppressive tumor microenvironment in multiple solid neoplasms including skin cancer.1-3
B7-H1 is known to suppress T cell proliferation directly and its expression levels are reported to correlate with a bad prognosis in malignant melanoma patients.4 Further evidence suggests that the tumor microenvironment greatly influences B7-H1 expression. For example, tumor-derived factors can stimulate B7-H1 expression by myeloid dendritic cells (DCs).5 As a consequence, the tumor stroma profoundly decreases the capacity of DC to activate antitumor T-cell responses. Moreover, tumors can directly induce the expression of B7-H1 in T cells, in turn leading to reduced interleukin (IL)-12 production by myeloid DCs and hence in impaired priming of tumor-specific T cells. Our present report demonstrates that, in addition to DCs and T cells, MDSCs also exhibit increased surface expression of B7-H1 molecules in melanoma-bearing mice.1 Thus, at late stages of tumor growth, the ret transplantable melanoma maintains a suppressive tissue microenvironment by driving an increased expression of B7-H1 in CD11b+ MDSCs.
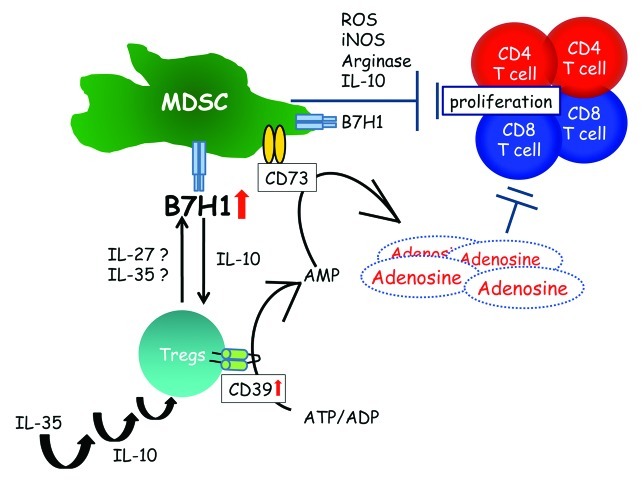
In parallel with MDSCs, another major subpopulation of immunosuppressive cells, namely Tregs, is capable of downregulating the function of immune cells in tumor-bearing hosts, by cell-to-cell contact (due to the expression of inhibitory molecules at their surface) as well as upon the secretion of soluble factors. The accumulation of Tregs in the tumor microenvironment has been shown to correlate with an unfavorable prognosis in several neoplasms.6 One molecule that frequently exerts inhibitory functions in this setting is B7-H1. In contrast to the stimulatory B7–1/B7–2-CD28 pathway, the interaction of B7-H1 with its receptor provides signals that quench the activation of T-cell responses. In myeloid cells such as DCs, B7-H1-induced pathways are able to inhibit DC functions by stimulating IL-10 production and by decreasing the expression of activatory receptors such as B7–1 and B7–2.
Among the possible mechanisms by which MDSCs suppress immune reactions, the secretion of IL-10 and the release of reactive nitrogen and oxygen species may play an important role, as they are known to inhibit T-cell proliferation.2 In our experimental setup, we observed the downregulation of IL-10 production by MDSCs after the depletion of Tregs. This may readily impede the suppressive function of MDSCs. Indeed, in parallel with decreased production of IL-10 by MDSCs in Treg-depleted hosts, we frequently observed a substantial upregulation of MHC class II molecules on other immune cells like DCs. This may be the result of reduced IL-10 levels and could faciliate the activation CD4+ and CD8+ cells. IL-10 is also known to increase the expression of co-inhibitory molecules such as B7-H1 within the tumor microenvironment. Likewise, we observed that the depletion of Tregs reduced the expression of B7-H1, B7-H3 and B7-H4 on MDSCs, suggesting that the reduction of IL-10 secretion by CD11b+ cells may account for this effect.
More recently, several groups shed light on the effects of IL-35-producing Tregs on immunosuppressive cells via the B7-H1 pathway.7,8 IL-35 is a heterodimer of EBV-induced gene 3 (EBI3) and of the p35 subunit of IL-12, and has been identified as an inhibitory cytokine produced by natural Tregs. Exogenous IL-35 treatment suppresses Th1 and Th17 cells and promotes the expression of CD39 and the production of IL-10 by CD4+ T cells.8 Interestingly, the combination signal transduced via B7-H1 and CD169 is indispensable for the induction of IL-35+ Tregs.7 These findings might help explain the crosstalk between Tregs and MDSCs via B7-H1 in the ret melanoma model in vivo.
Tregs in tumors not only suppress effector T cells directly, but modify the phenotype of the tumor-infiltrating macrophages to express inhibitory B7-H molecules and to produce IL-10. As we discussed above, the depletion of Tregs significantly downregulates the expression of immunosuppressive molecules such as B7-H1 on MDSCs and reduced tumor growth, indicating a concerted immunosuppressive activity of Tregs and MDSCs.1 In humans, MDSCs are a less defined and phenotypically more heterogenous group of cells than in mice, having only immunosuppressive activities in common. In this aspect, MDSCs in humans correspond to CD163+ alternative activated tumor-associated macrophages (TAM) instead.3,9 Interestingly, CD4+CD25+Foxp3+ Tregs produce IL-10, IL-4 and IL-13, and are able to steer monocyte differentiation toward alternative activated CD163+ M2 macrophages.10 In addition, several reports demonstrate therapeutic benefits following the selective reduction of MDSCs by chemotherapeutic agents like gemcitabine or 5-fluorouracil.2 Thus, together with Tregs, immunosuppressive cells such as MDSCs and tumor-associated macrophages (TAMs) contribute to the establishment of an immunosuppressive tumor microenvironment in skin cancer.1-3,9
Taken together, our recent results suggest the existence of a crosstalk between Tregs and MDSCs. Since Treg depletion is increasingly used in clinical settings, it is critically important to investigate the interactions between Tregs and MDSCs in various tumor entities. Therefore, combinatorial regimens targeting both Tregs and MDSCs may be beneficial for boosting the immune system and elicit anticancer responses in patients.
Figure 1. Myeloid-derived suppressor cells (MDSCs) are a heterogenous population of immature myeloid cells both in humans and mice. Together with regulatory T cells (Tregs), MDSCs suppress antitumor immunity through multiple different mechanisms.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21176
References
- 1.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J Invest Dermatol. 2012;132:1239–46. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 2.Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura T, Kambayashi Y, Hidaka T, Hashimoto A, Haga T, Aiba S. Comparison of Foxp3+ regulatory T-cells and CD163+ macrophages in invasive and non-invasive extramammary Paget’s disease. Acta Derm Venereol. 2012 doi: 10.2340/00015555-1453. In press. [DOI] [PubMed] [Google Scholar]
- 4.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–8. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125(Suppl 2):S272–83. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, et al. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol. 2010;40:321–9. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 8.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–53. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishibashi M, Fujimura T, Hashimoto A, Haga T, Onami K, Tsukada A, et al. Successful treatment of MMP9-expressing angiosarcoma with low-dose docetaxel and biphosphonate. Case Rep Dermatol. 2012;4:5–9. doi: 10.1159/000335999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJC, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]