Abstract
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-associated malignancy that is highly prevalent in Southern China and South-East Asia. EBV-targeted immunotherapy remains a goal in the development of novel treatment strategies. A novel adenoviral polyepitope-based immunotherapy has been developed to rapidly generate high frequency EBV-specific T cells to treat patients with refractory or metastatic disease.
Keywords: EBV, HLA, T cells, adenovirus, epitope, immunotherapy, nasopharyngeal carcinoma
Nasopharyngeal Carcinoma and Epstein-Barr Virus: A Target for Adoptive Cellular Therapy
Nasopharyngeal carcinoma (NPC) is common in South-East Asia, and occurs with an annual incidence as high as 25–50 cases per 100,000 people in Southern China and Hong Kong. Current therapeutic approaches are highly effective at treating stage I and II disease, with an overall 5 y survival greater than 80%. However, in spite of increasing public awareness, many patients are still diagnosed with advanced stage III or IV disease. Current standard therapy for stage III and IV NPC is a combination of chemotherapy and radiotherapy, and overall 5 y survival ranges from 50 to 60%. In the setting of recurrent or metastatic disease, chemotherapy is generally used as palliative therapy, and overall survival is low.1 There is therefore an urgent need to develop targeted therapies for advanced/metastatic NPC which complement standard treatment options.
As a likely etiological agent for NPC, the Epstein-Barr virus (EBV) remains an important target for novel therapeutic approaches. Immunotherapy based on autologous EBV-specific T cells has recently emerged as an effective tool for the treatment of EBV-associated malignancies and its efficacy in lymphoid malignancies such as post-transplantation lymphomas (PTLs) has been convincingly demonstrated. Emerging data support the potential application of cytotoxic T lymphocyte (CTL)-based therapy to NPC, on the backbone of chemotherapy and as salvage treatment. Several phase I and II clinical studies have been completed using approaches based on those employed to generate CTLs for PTLs, and have shown some efficacy against NPC.2-4 However, unlike PTL, NPC occurs in immunocompetent individuals, and—as in other EBV-associated malignancies that occur in an immunocompetent setting—immunological pressure results in the expression of a limited array of EBV antigens, namely, the latent membrane protein (LMP)-1 and -2 and the EBV nuclear antigen 1 (EBNA1).5 These proteins are likely critical for maintaining cellular transformation in malignant cells and their poor immunogenicity likely plays a key role in promoting immunoevasion by EBV-positive malignant cells.6,7 The focus of our research has therefore been the development of an immunotherapeutic approach that only targets LMP1, LMP2 and EBNA1 in order to improve the specificity of CTLs for use in adoptive cell transfer therapy and to avoid the requirement to generate patient derived EBV-transformed lymphoblastoid cell lines.
An Adenoviral Vector Polyepitope-based Adoptive Immunotherapy Approach for Recurrent or Metastatic Nasopharyngeal Carcinoma
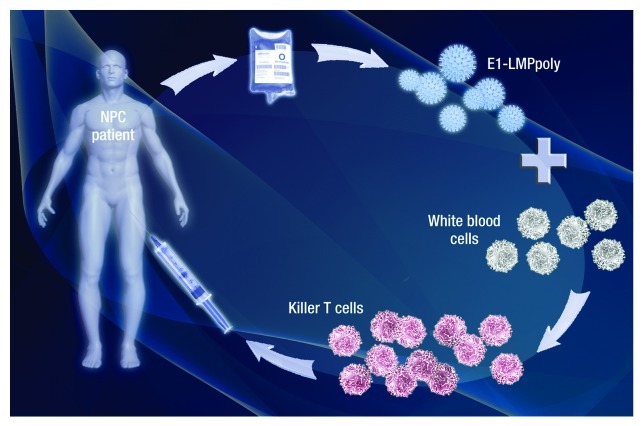
We have developed an adenoviral based vector, referred to as E1-LMPpoly, which encodes multiple CTL epitopes from LMP1 and LMP2 fused to a truncated EBNA1 without an internal glycine-alanine repeat sequence. This approach has been designed to optimise the immunogenicity of these antigens by avoiding the poor antigen processing associated with their full-length variants, and to limit the potential oncogenicity associated with full-length LMPs. Our preliminary studies clearly showed the efficacy of the polyepitope approach, demonstrating that E1-LMPpoly can be used to rapidly expand LMP1-, LMP2- and EBNA1-specific T cells from cancer patients.8 We have recently completed a formal clinical assessment of the E1-LMPpoly vector as a therapeutic tool for recurrent or metastatic NPC (Fig. 1).9 Twenty-two NPC patients who had loco-regional recurrence or distant metastatic disease, and who progressed after standard palliative radiotherapy, chemotherapy and/or surgery were recruited into this study. As is common for LMP1-, LMP2- and EBNA1-specific T-cell populations, the majority of donors showed no detectable ex vivo reactivity against these antigens. Nevertheless, antigen-specific T cell lines were successfully generated using the E1-LMPpoly vector from 16 out of 22 NPC patients following an in vitro culture period of 14 d. Importantly, these T-cell lines had a high specificity for LMP1, LMP2 and/or EBNA1, with 11 out of 16 T-cell lines being reactive to both LMP- and EBNA1-encoded CD8+ T-cell epitopes. The adenoviral-based polyepitope approach therefore allows for the rapid generation of T cells from a very low precursor frequency, which can potentially be administered within a month after the initial blood drawing. As it has previously been reported for the majority of cellular approaches against EBV-associated malignancies, the infusion of EBV-specific T cells was safe and only grade 1 and/or 2 toxicities including flu-like symptoms, malaise, dry cough and low blood pressure were observed. Of the 14 patients treated with T cell-based therapy in the study, 10 showed stable disease following treatment, and the time to the diagnosis of progressive disease ranged from 38 to 420 d. Of particular note was the potential impact that T-cell therapy had upon the median overall survival of patients, which was 523 d for patients who received CTLs and 220 d for patients who did not. Overall survival in a corresponding institutional cohort was 10.3 mo. While further evidence on the efficacy of this treatment strategy is required, these observations indicate that E1-LMPpoly-based cellular therapy provides potential clinical benefit to patients with refractory or metastatic disease.
Figure 1. Schematic representation of the generation of EBV-specific T cells using E1-LMPpoly for adoptive therapy in NPC patients. PBMC are purified from 200-400ml of patient peripheral blood, stimulated with the adenoviral vector encoding E1-LMPpoly and incubated for 14 days in the presence of interleukin-2. Following microbiological testing, the E1-LMPpoly T cells are ready to be reinfused into the patient within 4 weeks after the blood is drawn.
Combining Chemotherapy with EBV-specific CTL Therapy
Among the 14 patients that received T-cell therapy in our study, one patient had an unexpected good response to subsequent chemotherapy. This donor had 3 lines of palliative chemotherapy before T-cell infusion and the disease was chemoresistant. When disease progressed after T-cell infusion, the patient was started on palliative chemotherapy that induced rapid regression of the tumor after 1 cycle. While the response was transient and he subsequently succumbed to disease, this preliminary observation supports the notion that the antitumor efficacy of CTLs can potentially be improved by decreasing the tumor burden, possibly creating immunological space and reducing the inhibitory tumor microenvironment, with chemotherapy. These observations provide another important platform to explore the potential therapeutic efficacy of EBV-specific T cells in combination with chemotherapy for advanced/metastatic NPC.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21286
References
- 1.Ma BB, Chan AT. Systemic treatment strategies and therapeutic monitoring for advanced nasopharyngeal carcinoma. Expert Rev Anticancer Ther. 2006;6:383–94. doi: 10.1586/14737140.6.3.383. [DOI] [PubMed] [Google Scholar]
- 2.Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–9. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 3.Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–90. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 5.Brooks L, Yao QY, Rickinson AB, Young LS. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–97. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith C, Beagley L, Khanna R. Acquisition of polyfunctionality by Epstein-Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression. J Virol. 2009;83:6192–8. doi: 10.1128/JVI.00239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Wakisaka N, Crough T, Peet J, Yoshizaki T, Beagley L, et al. Discerning regulation of cis- and trans-presentation of CD8+ T-cell epitopes by EBV-encoded oncogene LMP-1 through self-aggregation. Blood. 2009;113:6148–52. doi: 10.1182/blood-2009-02-203687. [DOI] [PubMed] [Google Scholar]
- 8.Smith C, Cooper L, Burgess M, Rist M, Webb N, Lambley E, et al. Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J Immunol. 2006;177:4897–906. doi: 10.4049/jimmunol.177.7.4897. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Tsang J, Beagley L, Chua D, Lee V, Li V, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012;72:1116–25. doi: 10.1158/0008-5472.CAN-11-3399. [DOI] [PubMed] [Google Scholar]