Abstract
Activated lymphocytes secrete dendritic cell (DC)-activating cytokines including tumor necrosis factor α and interferon γ, and induce Type-1-polarized DCs (DC1s). Lymphocyte-polarized DC1s secrete high levels of biologically active interleukin-12 (IL-12p70) and CXCL10 and show enhanced CTL-inducing activity. Our data demonstrate the feasibility of using autologous lymphocytes to enhance the immunogenic properties of DCs in a low-cost clinically-compatible process.
Keywords: IL-12p70, dendritic cells, effector CD8+ T cells, polarization
Dendritic cells (DCs) are potent antigen-presenting cells (APCs), specialized in initiating and regulating T cell responses.1 The induction of different forms of antigen-specific immune responses in tumor-specific T cells by DCs requires peptide:MHC complexes (signal 1), co-stimulatory signals (signal 2) and the secretion of specific cytokines (signal 3).2 The ability of ex-vivo-generated DCs to provide such signals has resulted in the development of DC-based cancer immunotherapy.1,2 Since the conditions of maturation of DCs affect their ability to induce different forms of immunity,3,4 various DC maturation protocols have been designed to optimize the pattern of anti-tumor T cell responses. These protocols utilize different combinations of clinical grade recombinant cytokines such as interferon (IFN) IFNα and γ or tumor necrosis factor α (TNFα) and/or toll-like receptor (TLR) agonists including monophosphoryl lipid A (MPLA), polyinosinic:polycytidylic acid (poly-I:C) and the imidazoquinoline resiquimod (R-848), in order to induce mature type-1 polarized DCs (DC1s) with a high capacity to produce biologically active interleukin-12 (IL-12p70), a critical factor for the immunologic and clinical efficacy of cancer vaccines and for the induction of type-1 immunity.5-8
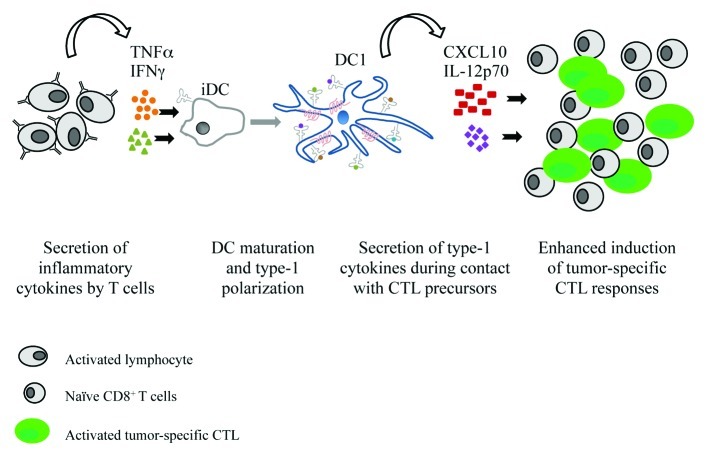
In an attempt to limit the need for costly clinical-grade cytokines, we have tested the feasibility of using autologous lymphocytes to induce DC1s.9 Anti-CD3 and anti-CD28 activated bulk lymphocytes isolated from healthy individuals or cancer patients (mostly CD4+ and CD8+ T cells) efficiently expanded and rapidly produced high levels of the DC1-inducing cytokines IFNγ and TNFα upon restimulation (Fig. 1). CD3-(re)activated lymphocytes and their supernatants (which are preferable for use in clinical settings) induced the maturation of autologous immature (i)DCs. Autologous lymphocyte-matured and supernatant-matured DCs showed an enhanced ability to produce IL-12p70 as compared with iDCs or DCs matured using a “conventional” cytokine cocktail composed of IL-1β, IL-6, TNFα and prostaglandin E2 (PGE2). Furthermore, both the lymphocyte-, and supernatant-matured DCs exhibited an enhanced production of interferon-inducible protein 10 (IP-10/CXCL10), which is also important for the optimal induction of type-1 immune responses.10
Figure 1. Activated lymphocytes induce the maturation and type-1 polarization of autologous dendritic cells. Expanded lymphocytes restimulated with anti-CD3 antibodies or anti-CD3 plus anti-CD28 microbeads rapidly secrete high levels of interferon γ (IFNγ) and tumor necrosis factor α (TNFα), which induce the maturation and type-1 polarization of autologous dendritic cells (DCs). The type-1 DCs (DC1s) induced by restimulated lymphocytes or their culture supernatant, express the lymph node-homing chemokine receptor CCR7 and migrate in response to CCL21. Upon CD40L stimulation, lymphocyte- and supernatant-matured DCs secrete high levels of biologically functional interleukin-12 (IL-12p70) and IP-10. Tumor-peptide-loaded supernatant-matured DCs efficiently induce the expansion of tumor-specific cytotoxic T lymphocytes (CTLs).
Lymphocyte supernatant-induced DC1s showed elevated expression of the lymph node-homing chemokine receptor CCR7 and an enhanced responsiveness to the lymph node-directing chemokine CCL21 compared with iDCs, although lower than that of DCs matured in the presence of PGE2. Supernatant-matured DCs contained both mature (CD83+ CCR7+) and immature (CD83- CCR7-) cells, suggesting that, when used as cancer vaccines, only a part of such DCs would migrate to lymphoid organs, and indicating a venue for optimization of the proposed DC maturation protocol.
Loaded with tumor-associated peptides, supernatant-matured DC1s and mature DCs generated in the presence of PGE2 induced a comparable expansion of MART-1-specific CD8+ T cells. However, naïve CD8+ T cells primed by supernatant-matured DC1s contained enhanced numbers of functional tumor-specific cytotoxic T lymphocytes (CTLs) as compared with CD8+ T cells induced by non-polarized PGE2-matured DCs.
Our current data demonstrate that patient-derived autologous lymphocytes can be used to induce the maturation and type-1 polarization of DCs. Since T cells constitute a high proportion of peripheral blood mononuclear cells that can be easily expanded and activated in clinical settings, the proposed method allows for the generation of high numbers of DCs for repetitive cycles of vaccination, reducing the need for clinical-grade cytokines and the overall cost of the generation of type-1 polarized DCs.
Glossary
Abbreviations:
- APCs
antigen presenting cells
- DCs
dendritic cells
- PGE2
prostaglandin E2
- TCR
T cell receptor
- TLR
toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21295
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Kalinski P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1, 2, 3 and 4. Curr Opin Investig Drugs. 2009;10:526–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Kaliński P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 4.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield LH, Gooding W, Whiteside TL. Development of a potency assay for human dendritic cells: IL-12p70 production. J Immunother. 2008;31:89–100. doi: 10.1097/CJI.0b013e318158fce0. [DOI] [PubMed] [Google Scholar]
- 7.DeBenedette MA, Calderhead DM, Tcherepanova IY, Nicolette CA, Healey DG. Potency of mature CD40L RNA electroporated dendritic cells correlates with IL-12 secretion by tracking multifunctional CD8(+)/CD28(+) cytotoxic T-cell responses in vitro. J Immunother. 2011;34:45–57. doi: 10.1097/CJI.0b013e3181fb651a. [DOI] [PubMed] [Google Scholar]
- 8.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with alpha-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berk E, Muthuswamy R, Kalinski P. Lymphocyte-polarized dendritic cells are highly effective in inducing tumor-specific CTLs. Vaccine. 2012;••• doi: 10.1016/j.vaccine.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, et al. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells--significant roles of CXCL10. Cancer Res. 2009;69:1587–95. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]