Abstract
Cytotoxic lymphocytes and dendritic cells infiltrating human renal cell carcinoma (RCC) are not sufficient to prevent tumor progression. Our studies identified alterations of the immune cell infiltrate as well as some of the underlying mechanisms. This knowledge should facilitate the development of anti-RCC therapies that achieve better tumor control.
Keywords: dendritic cells, immune escape, renal cell carcinoma, tumor milieu, tumor-infiltrating lymphocytes
Renal cell carcinoma (RCC) exhibit a prominent immune cell infiltrate consisting of T cells, natural killer (NK) cells, dendritic cells (DCs) and macrophages. Intratumoral CD8+ T and NK cells are differentiated effector cells with lytic granules.1 Moreover, some CD8+ T cells express a tumor-reactive T-cell receptor (TCR)2 and mediate antitumor reactivity when analyzed ex vivo following exposure to interleukin (IL)-2.1 A high frequency of NK cells among the lymphocytic infiltrate seems to predict a better prognosis.3 Still, tumors grow despite the infiltration of potentially tumor-reactive cytotoxic effector cells indicating that their antitumor activity is compromised within the tumor microenvironment.
Using immune histology and ex vivo analysis of tumor-infiltrating leukocytes (TILs), we identified alterations in RCC-infiltrating T cells, NK cells and DCs that may be relevant for the loss of local immune competence and ensuing in tumor immunoescape.1,4
DCs are central regulators of immune responses with the capacity to induce immunity or tolerance depending on their differentiation state. Thus, targeting this cell population would constitute an effective means for tumors to alter the immune response toward immunosuppression. Indeed, we identified a DC subtype that is enriched within RCC (ercDC), co-expressing markers of DCs (CD209) and macrophages (CD14).4 Tumor-secreted factors (CXCL8 plus IL-6 and the vascular endothelial growth factor, VEGF) were sufficient to induce the ercDC differentiation state. ErcDCs were often found in close proximity to T cells; yet in the absence of evidence of direct T-cell inhibition suggests that they are different from classical myeloid-derived suppressor cells. While not inhibiting T cells directly, ercDCs nevertheless showed characteristics related to tumor immunoescape: they intrinsically produced high levels of matrix metalloproteinase 9 (MMP-9) and in T-cell cross-talk experiments they induced milieu changes that are known to promote tumor cell proliferation (elevated secretion of tumor necrosis factor α, TNFα) and to limit the recruitment of TH1-polarized lymphocytes (reduced levels of CXCL10, CCL5) (Fig. 1).
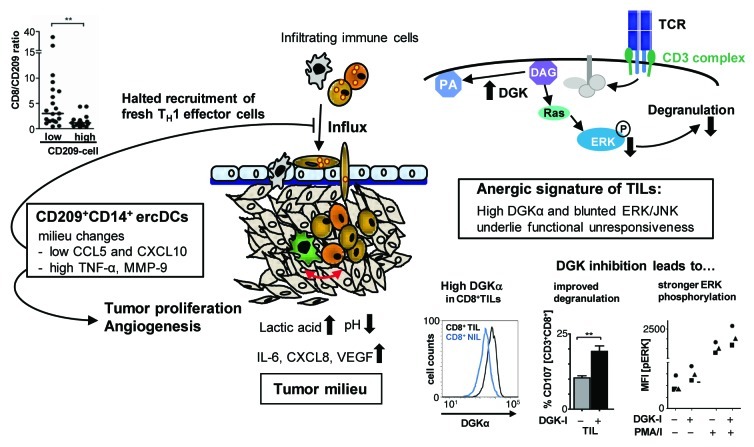
Figure 1. Intratumoral alterations of dendritic-cell differentiation and CD8+ T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Various immune cell populations, including CD8+ T cells, natural killer (NK) cells and dendritic cells (DCs) infiltrate clear cell renal cell carcinoma (RCC). These effector cells experience alterations within the tumor milieu that interfere with their capacity to exert antitumor effector function. CD8+ T cells isolated from RCC tissues (CD8+ TILs) are non-responsive to CD3-stimulation, lacking degranulation and cytokine production. An anergic signature with high diacylglycerol kinase α (DGKα) levels and poor activation of TCR distal MAPK pathways (ERK, JNK) was identified underlying these deficits. Depicted in the upper right corner is a simplified scheme of the TCR/CD3-signaling pathway leading to degranulation via the stimulation-induced production of second messenger diacylglycerol (DAG), which, besides other activities, phosphorylates and activates RAS/ERK. It was observed that CD8+ TILs express high levels of DGKα which catabolizes DAG to phosphatic acid (PA), thus limiting the amount of DAG available to promote ERK phosphorylation. The lower right panels show: representative histograms of DGKα fluorescence of CD8+ TILs (black line) and CD8+ T cells from non-tumor kidney (CD8+ NILs, blue line); degranulation and ERK phosphorylation of TILs, either untreated or treated with a DGK inhibitor (DGK-I, R59022). CD3-stimulation of TILs in the presence of DGK-I allowed more CD8+ T cells to degranulate as detected by FACS analysis of surface-mobilized CD107. Depicted is the mean percentage of CD107+ (degranulating) cells among the gated CD3+CD8+ population of TILs. Concomitantly with an improved degranulation, the treatment of CD8+ TILs with DGK-I increased the basal (unstimulated, PMA/I-) and the PMA/I-stimulation-induced (PMA/I+) level of phosphorylated ERK. Shown is the mean fluorescence intensity of phosphorylated ERK (MFI [pERK]) detected by phosphoflow analysis. Each symbol represents the TILs of one patient. The RCC milieu is rich in various cytokines and chemokines. Moreover, as a consequence of the von Hippel-Lindau protein inactivation, RCC cells are highly glycolytic resulting in lactic acidosis (high lactic acid plus low pH). We found that lactic acidosis strongly suppressed interferon γ (IFNγ) production by CD8+ T cells and reduced their degranulation capacity. Moreover, CXCL8 in combination with interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) altered DC differentiation. The resulting ercDCs (CD209+CD14+) produce high matrix metalloproteinase 9 (MMP-9) and engage in a T-cell cross-talk resulting in increased tumor necrosis factor α (TNFα) and reduced TH1-cell recruiting chemokines (CXCL10, CCL5). Thus, ercDCs promote tumor proliferation and angiogenesis (via high levels of TNFα and MMP-9), and stall the continuous influx of fresh immune effector cells (by limiting the amount of CXCL10 and CCL5), which would be required to sustain an antitumor response within the hostile tumor milieu. Shown is an immunohistochemical assessment of the proportion of CD8+ T cells in RCC tumors that contained either high or low numbers of CD209+ cells. Tumors with high numbers of CD209+ cells had a significantly lower CD8 to CD209 ratio consistent with lower recruitment of CD8+ T cells. Collectively, the tumor milieu alters infiltrating immune cells by inducing anergy-related genes in CD8+ T cells and influencing DC differentiation, thereby limiting antitumor reactivity.
A TH1/Tc1-polarized infiltrate is associated with good prognosis in many tumor types.5 The immune infiltrate of RCC is indeed TH1/Tc1-polarized, as indicated by CXCR3 expression and the presence of lytic granules.1,4 Thus, why are RCCs not rejected? We addressed this question by analyzing the functional status of tumor-infiltrating CD8+ T cells (CD8+ TILs) and NK cells ex vivo, directly after isolation from the tumor tissue. They were non-responsive to stimulation, lacking granule mobilization, cytolytic activity and cytokine production.1,6 Deficits in the activation of TCR distal signaling molecules were found causative for the functional unresponsiveness of CD8+ TILs. Among other alterations, we observed high diacylglycerol kinase α (DGKα) expression, low basal phosphorylation of extracellular signal-regulated kinase (ERK) as well as reduced stimulation-induced activation of ERK, c-Jun N-terminal kinase (JNK) and AKT (Fig. 1). These features were caused by the tumor microenvironment as they were not observed in CD8+ T cells from normal kidney tissues (CD8+ NILs), which were functionally active. A signature similar to that observed for CD8+ TILs has been described for anergic CD4+ T cells, but it was not found in profiles of exhausted CD8+ T cells during chronic viral infection,1 in spite of the fact that the latter functionally resemble CD8+ TILs.
DGKs are physiological inhibitors of TCR signaling.1 Indeed, we were able to link high DGKα levels to suppressed ERK phosphorylation and to inhibition of CD8+ TIL function, as TILs showed stronger ERK activation and better degranulation when stimulated in the presence of a DGKα inhibitor (Fig. 1). Moreover, we observed that the in vivo-repressed CD8+ TIL functions were reversible by ex vivo culture in the presence of IL-2. IL-2 is known to regulate DGKα and to restore responsiveness of anergic CD4+ T-cells.1 Indeed, functional recovery of CD8+ TILs occurred concomitantly with a decrease in DGKα levels and an increase in basal and stimulation-induced phosphorylation of ERK and AKT. In addition, culture in IL-2 reduced the levels of p27KIP1 and increased those of cyclin E1 suggesting that it promotes cell cycle progression, which is critical for T cells to overcome anergy.
IL-2 therapy has a long history in the treatment of RCC. However, response rates are low and complete responses are limited to a small subgroup of RCC patients7 indicating that some mechanisms of immunoescape within the RCC environment cannot be overcome by IL-2.
Specific features of clear cell RCC, including loss-of-function mutations of the von Hippel-Lindau gene and overexpression of carboxic anhydrases, contribute to a tumor microenvironment that is rich in lactate and acidic.8 We observed that tumor lactic acidosis inhibits cytokine production and impairs lytic granule exocytosis of effector lymphocytes by inhibiting the phosphorylation of JNK, c-Jun and MAPK p38, which are required for T-cell effector function.9 Of note, the inhibition of effector lymphocytes could be prevented by neutralizing acidosis even in the continuous presence of lactate.9
Collectively, our studies provide insights into the paradoxical situation whereby RCCs are generally not rejected despite being strongly infiltrated by various immune effector cells. Among other alterations, we observed tumor-induced changes in DC differentiation and the induction of anergy-associated genes in T cells, preventing the infiltrate to mediate antitumor functions. Considering the tumor-specific immune context (i.e., type, density and functional orientation of TLs) and adjusting therapy according to the composition of the infiltrate could result in improved response rates. Patients carrying tumors with a high ercDC frequency may benefit best from the administration of tyrosine kinase inhibitors, as these agents have the potential to re-polarize myeloid cells to support antitumor responses.10 However, patients with high numbers of tumor-infiltrating NK and CD8+ T effector cells should be considered for IL-2 therapy. The response rates to IL-2-based approaches may be improved by DGKα inhibition and anti-acidosis treatment, as these interventions may re-establish and prolong the functional responsiveness of the cytotoxic cell infiltrate.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21356
References
- 1.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. Oberneder, Noessner E. High DGK-α and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol. 2012;188:5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 2.Leisegang M, Turqueti-Neves A, Engels B, Blankenstein T, Schendel DJ, Uckert W, et al. T-cell receptor gene-modified T cells with shared renal cell carcinoma specificity for adoptive T-cell therapy. Clin Cancer Res. 2010;16:2333–43. doi: 10.1158/1078-0432.CCR-09-2897. [DOI] [PubMed] [Google Scholar]
- 3.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med (Berl) 2012;90:55–66. doi: 10.1007/s00109-011-0806-7. [DOI] [PubMed] [Google Scholar]
- 4.Figel AM, Brech D, Prinz PU, Lettenmeyer UK, Eckl J, Turqueti-Neves A, et al. Human renal cell carcinoma induces a dendritic cell subset that uses T-cell crosstalk for tumor-permissive milieu alterations. Am J Pathol. 2011;179:436–51. doi: 10.1016/j.ajpath.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Gröne EF, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. 2006;12:718–25. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pract Oncol. 2007;4:497. doi: 10.1038/ncponc0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morais C, Johnson DW, Gobe GC. The VHL-HIF signaling in renal cell carcinoma: promises and pitfalls. In: Amato RJ, ed. Emerging Research and Treatments in Renal Cell Carcinoma. InTech, 2012:57-82. [Google Scholar]
- 9.Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131:633–40. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JP, Emens LA. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE₂ in murine macrophages. Int Immunopharmacol. 2010;10:1220–8. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]