Abstract
Background
The cognitive effects of postmenopausal hormone therapy (HT) have been studied extensively, but little is known about the relationship between premenopausal hormone use and cognition. Hormonal contraceptive use vs. nonuse may be a potential factor influencing cognitive processes in midlife. The aim of this study is to explore the effect of modification of hormone milieu through use of hormonal contraception in premenopausal women and midlife cognitive function.
Methods
Subjects were 261 cognitively normal women, aged 40–65 (mean μ=52), enrolled in the Wisconsin Registry for Alzheimer's Prevention. All women completed the Women's Health History Questionnaire and a self-report health history questionnaire and were administered a battery of neuropsychologic tests. Cognitive results were analyzed using summary scores for the domains of Verbal Ability, Visuo-spatial Ability, Working Memory, Verbal Learning & Memory, and Speed & Flexibility derived using a confirmatory factor analysis.
Results
Hormonal contraceptive ever users performed significantly better than never users in the domains of Visuo-spatial Ability (μ=0.75, 95% confidence interval [CI] 0.23-1.28, p=0.005) and Speed & Flexibility (μ=0.52, 95% CI −0.16-1.04, p=0.007), with duration-dependent increases in performance, especially in ever users with ≥15 years of use.
Conclusions
These data provide preliminary evidence that hormonal contraceptive use may influence cognitive outcomes, even years after use is discontinued. Hormonal contraceptive users scored better in domains of Visuo-spatial Ability and Speed & Flexibility than never users, with a duration-dependent trend. Further research is needed to explore the use of hormonal contraceptives to prevent or delay cognitive decline and to clarify the physiologic basis of this phenomenon.
Introduction
In our aging population, the incidence of Alzheimer's disease (AD) and dementia is increasing.1,2 The social and economic burden these diseases place on the healthcare system, patients, and family members makes them a significant public health concern.3 There is an urgent need for research focused on earlier recognition and prevention of these degenerative diseases in addition to continued efforts to develop new treatment strategies.
Some research has shown that hormone therapy (HT), specifically estrogen, can have a protective effect against the onset of dementia in women, depending on the type of therapy and age at the onset of use.4–6 However, a Cochrane review found that there is little evidence that estrogen or combined estrogen and progestin therapy protects against a decline in overall cognitive functioning of healthy older postmenopausal women.7 A substudy of the Women's Health Initiative (WHI) looked at the effects of postmenopausal HT on a variety of outcomes, including cognition. In subjects receiving conjugated equine estrogens (CEE) plus progestin, an almost doubled risk of all-cause dementia was shown.8,9 Given that all the women enrolled in the WHI study were ≥65 years of age, some researchers have speculated that there is a critical period during menopause characterized by a relatively rapid estrogen decline, during which therapy must be applied in order to see cognitive benefits.10–12
Studies have begun to look at the link between premenopausal hormone use and cognition, specifically the use of hormonal contraceptives.13,14 Hormonal contraceptive use allows for the study of premenopausal women exposed to controlled levels of exogenous hormones, in contrast to those whose hormone levels vary naturally across the menstrual cycle. The various generations of contraceptive HTs employed different dosages and combinations of hormones.15 Little is know about the cognitive effects of these hormones, and further research is needed to explore the association between the history of hormonal contraceptive use and cognitive functioning in midlife and old age. Other factors that need to be evaluated include family history and apolipoprotein E4 (ApoE4) status and how these variables play a part in cognitive decline along with concurrent or past hormone use.
In this study, we sought to characterize the cognitive effect of manipulating ovulation through exogenous hormone exposure in women by modification of estrogen and progestin levels. We hypothesized that this performance was duration dependent, with longer duration being correlated with better performance.
Materials and Methods
Study population
Institutional Review Board approval was obtained to do a cross-sectional review of 261 female subjects in the Wisconsin Registry for Alzheimer's Prevention (WRAP) database through the Wisconsin Alzheimer's Institute. Inclusionary criteria for the database required documented positive or negative parental history of AD. Subjects who met the parental history requirement for AD were between the ages of 40 and 65 and had a parent diagnosed with AD, either autopsy confirmed or verified through medical record review by a multidisciplinary diagnostic consensus conference. Patients enrolled without the parental history requirement were between the ages of 40 and 65 and had a mother who survived to age ≥75 and a father who survived to age ≥70 without evidence of dementia. All participants were volunteers who provided informed consent for study enrollment as well as cognitive testing.
Each participant completed an entry assessment that included neuropsychologic testing, ApoE genotyping (Athena Diagnostics, Worcester MA, and Atwood Lab, Madison, WI), and a health history questionnaire assessing demographics; medical and psychiatric status; self-reported history of cardiovascular, neurologic, psychiatric, and other major medical disorders; current medications; selected lifestyle variables (exercise, tobacco, and alcohol use); and depressive symptoms (20-item Center for Epidemiologic Studies-Depression scale [CES-D]).16 The content of the health history questionnaire was based on the questionnaire used in the WHI Memory Study.17 Potential participants were excluded if they had incomplete data on cognitive tests or hormonal contraceptive use or if they self-reported (1) stroke or probable dementia, (2) multiple sclerosis, (3) epilepsy/seizures, (4) meningitis, or (5) Parkinson disease. These criteria were intended to exclude conditions affecting cognition. To verify that patients with cognitive impairment were excluded, cognitive testing data were reviewed by the study investigators. All patients spoke fluent English.
Hormonal contraceptive use
Hormonal contraceptive use history and duration of use were obtained from the health history questionnaire, and users were categorized as ever users and never users.
Cognitive testing
Each WRAP participant completed neuropsychologic assessment at entry into the study. Patients completed a battery of 17 cognitive tests designed to test a broad range of specific cognitive functions and affect. Patients were evaluated on an individual basis by a trained psychometrician. Tests included the Wechsler Abbreviated Scale of Intelligence, (WASI)18 Boston Naming Test,19 Controlled Oral Word Fluency,20 Benton's Judgment of Line Orientation,20 Wide Range Achievement Test-3rd ed. (WRAT-3)21 Auditory Verbal Learning Test,22 Wisconsin Card Sort-64,23 Wechsler Adult Intelligence Scale (WAIS-3) Working Memory,24 Trail Making Tests A and B,25 and the Stroop Color Word Test.26
Analysis
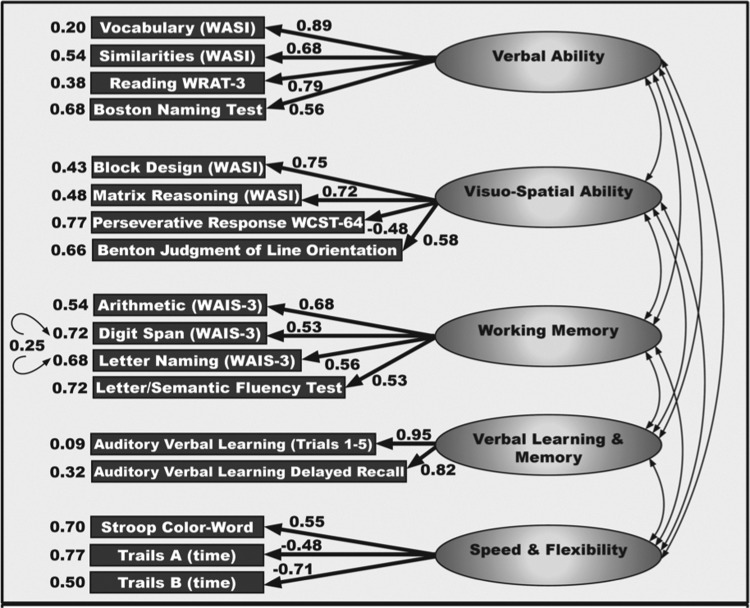
Outcomes of the cognitive testing were summarized into a five-factor structure. This structure compiles all administered tests by loading significantly high on a single factor (loading varied, in absolute value, from 0.09 to 0.95) (Fig. 1). The strength of the factor loadings suggested that each of the subscales effectively measured its respective construct. The magnitude of the correlations between the factors (0.26–0.55) indicated a high level of interconnectedness among measures. The confirmatory factor analysis accounts for 65.5% of the total variance among tests. This allowed the WRAP neuropsychologic test battery to be sorted into five multitest domains labeled Verbal Ability, Visuo-spatial Ability, Working Memory, Verbal Learning & Memory, and Speed & Flexibility. The confirmatory factor analysis allows a global measure of five factor domains to be used to represent a common trait of potential clinical relevance in studying cognitive aging.27
FIG. 1.
Confirmatory factor analysis of cognitive variables obtained with the Wisconsin Registry for Alzheimer's Prevention (WRAP) neuropsychologic battery, using the full sample. Fit indices are as follows: root mean square error of approximation (RMSEA)=0.07, normed fit index (NFI)=0.94, comparative fit index (CFI)=0.96, and goodness of fit index (GFI)=0.91. Model tested with subgroups: Males vs. females; young (age 30–54) vs. old (55–67); parental history positive vs. parental history negative; parental history and apolipoprotein E4 (ApoE4)+vs. parental history negative and ApoE4−. In all cases, fit indices were in acceptable ranges. WAIS, Wechsler Adult Intelligence Scale, 3rd ed.; WASI, Wechsler Abbreviated Scale of Intelligence; WCST, Wisconsin Card Sort Test-64; WRAT-3, Wide Range Achievement Test, 3rd ed.
Comparability of hormonal contraceptive ever users vs. never users was assessed based on age at screening, race, years of education, socioeconomic status (SES), depression, parental history of AD, self-rated health, hysterectomy, HT use, ApoE4 status, and parity using analysis of variance (ANOVA) models for continuous variables and chi-square analyses for categorical variables.
The primary comparison of interest was group (users and never users of hormonal contraceptives) differences on five cognitive factors. Secondary comparisons of interest were differences on the five cognitive factors between users and never users based on duration of hormonal contraceptive use using a grouping variable. Differences between groups were assessed using the two-sample multivariate analysis of covariance (MANCOVA) models. This statistic examines group differences on all dependent variables (differences on five cognitive factors) simultaneously to reduce the risk of false positive results that is associated with performing a series of univariate analyses of covariance (ANCOVA). The grouping variable was never vs. ever use of hormonal contraceptives. Age at study enrollment, years of education, and SES, all known modifiers of cognitive performance, were entered into the model as covariates. Ever users were dichotomized into three groups based on duration of exposure (<5 years, 5 to <15 years, 15+years). A post-hoc pairwise comparison MANCOVA was performed to determine if there was a difference in effect based on duration of use.
Because not all women in our study were postmenopausal, not all of them qualified for HT use. Therefore, a subanalysis of postmenopausal women with known HT status was conducted to look for confounding effects of this endogenous hormone application. A Bonferroni correction was used to maintain the familywise error rate. Finally, potential interaction effects resulting from the participant variables of parental history of AD and ApoE4 status were assessed by including the appropriate interaction term in the model in two secondary analyses. Parental history and ApoE4 status were included in the model as an additional grouping variable in separate MANCOVA analyses. A Bonferroni correction was used to maintain the familywise error rate.
Analyses were conducted using SPSS for Mac (version 17.0, release 17.0.2, SPSS Inc., Chicago, IL). A nominal two-sided p value of 0.05 was regarded as statistically significant.
Results
Characteristics of hormonal contraceptive users and never users are shown in Table 1. The average age of the 262 women was 52.4 years (standard deviation [SD] 6.2 years). ANOVA comparisons showed that subjects at screening were of comparable age (p=0.11), race/ethnicity (p=0.89), years of education (p=0.19), SES (p=0.47), history of depression (p=0.58), and self-reported health status (p=0.88). There were no differences in variables affecting hormone levels, such as hysterectomy (p=0.83) and history of HT use (p=0.79). Distribution of the ApoE4 allele and parental history of AD were comparable in users and never users (p=0.21 and 0.90, respectively). Significantly fewer hormonal contraceptive ever users than never users had a history of pregnancy (p=0.002). Prevalence of underlying health characteristics/behaviors (heart disease, hypertension, hypercholesterolemia, diabetes mellitus, depression, self-rated health, smoking, and alcohol use) also did not differ across groups (data not shown) (all p>0.10).
Table 1.
Baseline Characteristics of Female Wisconsin Registry for Alzheimer's Prevention Study Participants
| Characteristic | Hormonal contraceptive ever users (n=227) | Hormonal contraceptive never users (n=34) | p valuea(2-sided) |
|---|---|---|---|
| Age at screening, mean (SD) | 52.6 (6.0) | 50.8 (7.4) | 0.11b |
| Race/ethnicity, n (%) | 0.89 | ||
| American Indian | 1 (0.4) | 0 (0.0) | |
| Black | 2 (0.9) | 0 (0.0) | |
| White | 223 (98.2) | 34 (100.0) | |
| Hispanic | 1 (0.4) | 0 (0.0) | |
| Years of education, mean (SD) | 15.9 (2.6) | 16.5 (2.8) | 0.19b |
| SES, n (%) | 0.47 | ||
| ≤ $19,000 | 5 (2.2) | 0 (0.0) | |
| $20,000–$39,000 | 19 (8.4) | 6 (17.6) | |
| $40,000–$59,000 | 53 (23.3) | 8 (23.5) | |
| $60,000–$79,000 | 42 (18.5) | 8 (20.6) | |
| > $80,000 | 100 (44.1) | 13 (38.2) | |
| Not reported | 8 (3.5) | 0 (0.0) | |
| Depression, n (%) | 55 (24.2) | 6 (17.6) | 0.58 |
| Self-rated health, mean (SD)c | 3.9 (0.8) | 3.8 (0.8) | 0.88b |
| Hysterectomy, n (%) | 48 (21.1) | 5 (14.7) | 0.38 |
| Postmenopausal, n (%) | 177 (78.0) | 22 (64.7) | 0.69 |
| HT use, n (%)d | 0.69 | ||
| Ever | 112 (63.3) | 11 (50.0) | |
| Never | 59 (33.3) | 11 (50.0) | |
| Don't know | 6 (3.3) | 0 (0.0) | |
| ApoE4, n (%) | 0.21 | ||
| Yes | 139 (61.2) | 17 (50.0) | |
| No | 88 (38.8) | 17 (50.0) | |
| Parent with AD, n(%) | 206 (90.7) | 31 (91.2) | 0.90 |
| Parity, n (%) | 204 (89.9) | 33 (97.1) | 0.002 |
Based on chi-square tests (categorical variables) or t tests (continuous variables).
Equal variance assumed based on Levene's test for equality of variances.
Based on 5-point scale of self-reported health.
Postmenopausal women only, n=199.
AD, alzheimer's disease; ApoE4, Apolipoprotein E e4 allele; HT, hormone therapy; SD, standard deviation; SES, socioeconomic states.
There was a significant group difference between users and never users in history of pregnancy, and this factor was included in the adjusted model. Age at study enrollment, years of education, and SES were also included in adjusted MANCOVA analysis, as these factors have all been associated with cognitive performance in prior studies. As reported in Table 2, there were significant differences in cognitive performance across the model associated with hormonal contraceptive use (p=0.029, MANCOVA). Hormonal contraceptive ever users had higher scores than nonusers in Speed & Flexibility (μD=0.52, 95% confidence interval [CI]−0.16-1.04, p=0.007) and in Visuo-spatial Ability (μD=0.75, 95% CI 0.23-1.28, p=0.005). Scores were similar between ever users and never users in Verbal Ability (μD=0.44, 95% CI −0.16-1.04, p=0.007), Working Memory (μD=0.40, 95% CI 0.15-0.95, p=0.15), and Verbal Learning & Memory (μD=0.01, 95% CI −0.51-0.53, p=0.97). (μD=mean of the differences.)
Table 2.
Ever Use of Hormonal Contraceptives and Cognitive Outcomes in Female Wisconsin Registry for Alzheimer's Prevention Study Participants
| Cognitive domain | Hormonal contraceptive users (n=227) Mean (SE)a | Hormonal contraceptive never users (n=34) Mean (SE)a | Difference between users and never users Mean (95% CI)a,b | p Valuea,b(MANCOVA=0.029) |
|---|---|---|---|---|
| Verbal Ability | 0.46 (0.11) | 0.01 (0.28) | 0.44 (−0.16-1.04) | 0.15 |
| Visuo-spatial Ability | 0.48 (0.10) | −0.38 (0.25) | 0.75 (0.23-1.28) | 0.005 |
| Working Memory | 0.28 (0.10) | −0.12 (0.26) | 0.40 (−0.15-0.95) | 0.15 |
| Verbal Learning & Memory | 0.43 (0.10) | 0.42 (0.25) | 0.01 (−0.51-0.53) | 0.97 |
| Speed & Flexibility | 0.51 (0.07) | −0.01 (0.18) | 0.52 (0.14-0.90) | 0.007 |
Adjusted for age at intake, SES, years of education, and parity.
A Bonferroni adjustment was adopted for multiple comparisons.
CI, confidence interval; MANCOVA, multivariate analysis of covariance; SE, Standard Error.
A secondary analysis (n=259) was performed to look for main effect differences in cognitive outcomes based on length of time of hormonal contraceptive use according to four categories (never user,<5 years of use, 5 to <15 years of use, 15+ years of use) (Table 3). Cognitive performance increased as a function of use-duration of hormonal contraceptives but was not significant across all five domains (p=0.096, MANCOVA). A pattern of cognitive performance could be discerned from this analysis, however, especially in the domains of Speed & Flexibility and Visuo-spatial Ability. In post-hoc pairwise comparisons, ever users with the most hormonal contraceptive exposure (15+ years) performed the best against never users in the domain of Speed & Flexibility (μD=0.68, 95% CI 0.39-0.96, p=0.02) vs. those with<5 years of use (μD=0.48, 95% CI 0.25-0.73, p=0.16 and 5 to <15 years of use (μD=0.54, 95% CI 0.33-0.74, p=0.07). There was also a trend toward better performance in subjects with 15+ years of use in the domain of Visuo-spatial Ability (μD=0.51, 95% CI 0.11-0.92, p=0.054).
Table 3.
Hormonal Contraceptives and Cognitive Outcomes According to Years of Use in Female Wisconsin Registry for Alzheimer's Prevention Study Participants
| Cognitive domain | Duration of use (years) | Score Mean (95% CI)a,b | p valuea,b(Overall p=0.096) |
|---|---|---|---|
| Verbal Ability | Never user (n=34) | −0.09 (−0.65-0.46) | 0.16 |
| <5 (n=69) | 0.64 (0.27-1.02) | ||
| 5–<15 (n=99) | 0.33 (0.02-0.65) | ||
| 15+ (n=51) | 0.50 (0.06-0.93) | ||
| Visuo-spatial Ability | Never user | −0.35 (−0.86 to 0.16) | 0.05 |
| <5 | 0.41 (0.06-0.75) | ||
| 5–<15 | 0.30 (0.00-0.59) | ||
| 15+ | 0.51 (0.11-0.92) | ||
| Working Memory | Never user | −0.09 (−0.61-0.45) | 0.38 |
| <5 | 0.13 (−0.23-0.49) | ||
| 5 to <15 | 0.27 (−0.03-0.57) | ||
| 15+ | 0.48 (0.06-0.90) | ||
| Verbal Learning & Memory | Never user | 0.49 (0.00-0.99) | 0.48 |
| <5 | 0.63 (0.29-0.96) | ||
| 5–<15 | 0.34 (0.05-0.62) | ||
| 15+ | 0.27 (−0.13-0.67) | ||
| Speed & Flexibility | Never user | −0.01 (−0.37-0.35) | 0.03 |
| <5 | 0.48 (0.24-0.73) | ||
| 5–<15 | 0.54 (0.33-0.74) | ||
| 15+ | 0.68 (0.39-0.96) |
Adjusted for age at intake, SES, years of education, and parity.
A Bonferroni adjustment was adopted for multiple comparisons.
To look for a potential confounding effect of HT use, a subanalysis of cognitive scores in perimenopausal and postmenopausal subjects was conducted (n=199) (Table 4). There was no significant difference in performance across all five domains between hormonal contraceptive users and never users (p=0.101, MANCOVA). However, hormonal contraceptive users continued to score significantly higher in the domains of Visuo-spatial Ability (μD=0.79, 95% CI 0.10-0.91, p=0.03) and Speed & Flexibility (μD=0.59, 95% CI 0.10-1.07, p=0.02). There were no significant differences in the domains of Verbal Ability (μD=0.35, 95% CI −0.40-1.09, p=0.36), Working Memory (μD=0.27, 95% CI 0.43-0.80, p=0.45), and Verbal Learning & Memory (μD=0.03, 95% CI−0.66-0.72, p=0.90).
Table 4.
Use of Postmenopausal Hormone Therapy and Cognitive Outcomes in Female Wisconsin Registry for Alzheimer's Prevention Study Participants
| Cognitive domain | Hormonal contraceptive usersa(n=177) Mean (SE) | Hormonal contraceptive never usersa(n=22) Mean (SE) | Mean difference (Users−never users)a | p valuea(Overall p=0.101) |
|---|---|---|---|---|
| Verbal Ability | 0.53 (0.12) | 0.18 (0.36) | 0.35 | 0.36 |
| Visuo-spatial Ability | 0.25 (0.11) | −0.53 (0.33) | 0.79 | 0.03 |
| Working Memory | 0.15 (0.12) | −0.12 (0.34) | 0.27 | 0.45 |
| Verbal Learning & Memory | 0.30 (0.12) | 0.27 (0.33) | 0.03 | 0.90 |
| Speed & Flexibility | 0.34 (0.08) | −0.24 (0.23) | 0.59 | 0.02 |
Adjusted for age at intake, SES, years of education, parity, and HT use; Bonferroni for multiple comparisons.
In order to determine if an interaction was present between family history of AD and hormonal contraceptive use, an interaction term was included in the model. Parental history did account for some of the effect of history of hormonal contraceptive use in the model. The impact of hormonal contraceptive use on cognitive performance differed based on parental history of AD (p=0.022, MANCOVA). Although there was no significant difference in Working Memory performance between never and ever users, the mean difference in Working Memory between users and never users was greater in women with no parental history of AD (μD=2.67) than in women with a parental history of AD (μD=0.19, p=0.01). Analysis was limited by the small sample size (n=3) in parental history-negative never users. Differences in other domains between users and never users did not differ based on parental history of AD (Verbal Ability, p=0.25; Visuo-spatial Ability, p=0.44; Verbal Learning & Memory, p=0.78). There was, however, a trend suggesting that hormonal contraceptive users with a parental history of AD performed better than those without a parental history of AD in the domain of Speed & Flexibility (p=0.08).
Because it is a known risk factor for AD, ApoE4 status was also included in the model to determine possible interaction with hormonal contraceptive use; no interaction was found between ApoE4 and hormonal contraceptive use (p=0.68, MANCOVA). Hormonal contraceptive use did not appear to differentially affect cognition in women depending on their ApoE4 status.
Discussion
Through analysis of a cognitively healthy, middle-aged group of women, we aimed to elucidate the effects of hormonal contraceptive use on cognitive outcomes, comparing women who had no history of hormonal contraceptive use (never users) to two groups of past users of hormonal contraceptives. Specifically, we compared cognitive outcomes between groups of women with three duration levels of exposure. We also explored the data for possible interactions between hormonal contraceptive use and risk factors for AD, such as parental history and presence of a genetic risk factor for AD, the ApoE4 allele, both potential modifiers of cognitive outcomes.
When adjusting for age, SES, parity, and years of education, hormonal contraceptive use was found to be associated with significantly better performance in the cognitive domains of Visuo-spatial Ability and Speed & Flexibility. This association was supported by the finding that the effect was dose dependent. Surprisingly, even those users with very brief exposures (<5 years) outperformed never users. The same effect was still present in a secondary analysis of perimenopausal and postmenopausal women with a history of HT use.
An interaction was found between parental history of AD and hormonal contraceptive use. Performance in the domain of Working Memory between ever users and never users was better in women with no parental history of AD than in women with a parental history of AD. There was no differential effect of hormonal contraceptive use depending on ApoE4 status. However, the analysis of interactions between AD risk factors and cognition was limited by small sample size. These results are only preliminary findings and are presented to prompt further research.
Our finding that hormone contraceptive use has a duration-dependent effect on cognitive performance has important clinical relevance. In developed countries, many women have an extensive history of exogenous hormone exposure.28 Hormonal contraceptive use is often started at a young age for everything from acne control to alleviation of premenstrual symptoms. Considering HT along with hormone contraceptives, a woman could potentially have upward of 40 years of exogenous hormone exposure. The implications of this modification of the hormonal milieu are not well understood. Moreover, subtle group differences in cognition related to hormone exposure earlier in life may play out in a meaningful way later in life. In other words, our study population is relatively young; small differences in cognitive performance at this age may portend marked changes decades later if the trajectory continues. Likewise, subtle changes in cognitive reserve could influence age of onset.29,30 Finally, possible protective effects of early life exogenous hormone use could protect cognitive reserve in an older woman.
Our results suggesting that hormonal contraceptive use affects Speed & Flexibility are consistent with other findings that estrogen modulates and even improves this area of function.31–33 Speed & Flexibility has been labeled a female-specific task, meaning women tend to outperform men in this area. Women perform better on female-oriented tasks when estrogen levels are high, for example, during the midluteal stage of the menstrual cycle.34 Women taking hormonal contraceptives have controlled levels of estrogen that are higher than the body's normal levels, meaning that improved performance in this domain is biologically plausible.
Although exact mechanisms are unknown, the presence of estrogen receptors in the basal forebrain cholinergic nuclei has lead to suggestions that interactions with the cholinergic system are involved.35,36 In addition, estrogen has been shown to increase the density of hippocampal cell connections in animal models.37,38 The location of estrogen receptors near nerve growth factor in the brain may imply that estrogen facilitates neurotrophic responses.39 Detailed discussion of other potential mechanisms of the effects of estrogen on cognition is beyond the scope of this article. Comprehensive reviews can be referenced for further information on this topic.40–42
Our finding that hormonal contraceptive ever users outperform never users in the domain of Visuo-spatial Ability is surprising. Better performance on visuo-spatial tasks has been associated with androgenic hormones and has been labeled a male-oriented task.43 The androgen receptor is expressed in various brain regions, including the cerebral cortex, amygdale, and hypothalamus.44 Studies of genetically male individuals born with complete androgen insensitivity syndrome, which is characterized by nonfunctional androgen receptors, have found that these individuals have impaired visuo-spatial cognition.45,46 This suggests that androgen receptor activation may influence visuo-spatial cognitive differences between males and females. Although estrogen levels are similar in all types of hormonal contraceptives, levels of progesterone vary depending on the preparation used. New research has found that preparations of hormonal contraceptives with the most androgenic activity are associated with the strongest performance on visuo-spatial tasks.47 In addition, a study looking at HT with estrogen alone and HT including progestin found that both are positively associated with visuo-spatial performance.48 Detailed information on the types of hormonal contraceptive preparations our subjects used was not available, but it is possible that better performance in the domain of Visuo-spatial ability could be related to the progestin in these preparations. This exposure to exogenous progestins makes better performance in the domain of Visuo-spatial ability plausible. Further research is warranted to explore these findings.
The present study's strength is its relevance and timeliness. However, it is important to acknowledge limitations so they can be addressed and their impact can be minimized. As with any cross-sectional study, if an association is found during analysis, it is impossible to determine a causal relationship. Given the limitations in suggesting a temporal relationship between exposure and outcome, this study may be useful in providing directions for future research in the form of prospective cohort studies or case-control studies that will better establish etiologic relationships and clarify questions about the efficacy of formulations, dosages, and durations of use. Although we controlled statistically for the effects of several important demographic characteristics (age at study onset, SES, parity, history or HT use, and years of education) in our analyses, other unknown or unmeasured characteristics may have contributed to our results. Cohort effects and user biases may also play a role in this study. However, our finding that duration of use significantly impacts cognitive outcomes helps to strengthen our conclusion that hormonal contraceptive use and not cohort effects is responsible for these outcomes.
Because participants for this study were volunteers, the generalizability of our database to the overall Wisconsin population may be a concern. Additionally, the WRAP database itself is inherently biased in that it is not population based. Research has shown that people who volunteer for a health study tend to be better educated, to have better SES, and to be in better overall health than those who do not participate.49 This may be a particularly influential confounding factor for an AD study. History of hormonal contraceptive use was gathered by self-report, leading to the potential for recall bias. In a previous study, however, self-report of contraceptive use was shown to be a valid and accurate data collection method.50
Lack of data on the type of hormonal contraceptive preparation used prevented us from determining if the type of hormonal preparation impacted cognition in ever users. In future research, tools to aid recall, such as pictures of different preparation types or multiple choice answers, may aid in collection of these data.
Conclusions
The relationship between hormonal contraceptive use and cognition at midlife is not well understood. Consequently, there are notable gaps in existing research. Our study examined the cognitive effects associated with hormonal contraceptive use in women with and without a history of parental AD. Our analysis indicated that hormonal contraceptive use may have a protective cognitive effect even years after use is discontinued. This is especially true in subjects with a longer duration of use. This result, however, should be interpreted with caution, given the cross-sectional study design and limited sample size. We believe our findings form the basis for further research in larger and more focused clinical studies about differences between types of hormonal preparation used that look for cause and effect relationships of this phenomenon.
Acknowledgments
This research was supported by National Institutes of Health (NIH) Research Project grant R01 AG027161 and the Wisconsin Alzheimer's Disease Research Center (NIH-NIA P50 AG033514). We thank Drs. Whitney Wharton, Mark Sagar, Asenath LaRue, and Bruce Hermann and Ms. Janet Rowley for their hard work in coordinating and carrying out data collection. We are grateful to Dr. Norca Maritza Dowling, who conducted the factor analysis and developed the factor scores, and Dr. Ron Gangnon for his advice and guidance on statistical analysis. Thank you for the support of the staff of the William S. Middleton Memorial Veterans Hospital and to all Wisconsin Registry for Alzheimer's Prevention study participants.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Older Americans 2004: Key indicators of well-being. Federal Interagency Forum on Aging Related Statistics. 2004.
- 2.Bertram L. Tanzi RE. Thirty years of Alzheimer's disease genetics: The implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 3.Ernst RL. Hay JW. The US economic and social costs of Alzheimer's disease revisited. Am J Public Health. 1994;84:1261–1264. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 5.Burg MA. Fraser K. Gui S, et al. Treatment of menopausal symptoms in family medicine settings following the Women's Health Initiative findings. J Am Board Fam Med. 2006;19:122–131. doi: 10.3122/jabfm.19.2.122. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM. Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 7.Lethaby A. Hogervorst E. Richards M, et al. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espeland MA. Rapp SR. Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 9.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Calleja-Agius J. Brincat MP. Hormone replacement therapy post Women's Health Initiative study: Where do we stand? Curr Opin Obstet Gynecol. 2008;20:513–518. doi: 10.1097/GCO.0b013e32830dfa5c. [DOI] [PubMed] [Google Scholar]
- 11.Resnick SM. Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann NY Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 12.Marder K. Sano M. Estrogen to treat Alzheimer's disease: Too little, too late? So what's a woman to do? Neurology. 2000;54:2035–2037. doi: 10.1212/wnl.54.11.2035. [DOI] [PubMed] [Google Scholar]
- 13.Halpern DF. Benbow CP. Geary DC, et al. The science of sex differences in science and mathematics. Psychol Sci Public Interest. 2007;8:1–49. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura D. Hampson E. Cognitive pattern in men and women as influenced by fluctuations in sex hormones. Curr Directions Psychol Sci. 1994;3:144. [Google Scholar]
- 15.Newton JR. Classification and comparison of oral contraceptives containing new generation progestogens. Hum Reprod Update. 1995;1:231–263. doi: 10.1093/humupd/1.3.231. [DOI] [PubMed] [Google Scholar]
- 16.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Shumaker SA. Legault C. Kuller L, et al. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company; New York, NY: 1999. [Google Scholar]
- 19.Kaplan EF. Goodglass H. Weintraub S. The Boston Naming Test. 2nd. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 20.Benton AL. Neuropsychological assessment. Annu Rev Psychol. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson G. WRAT3 administrative manual. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- 22.Rey A. L'examen clinique en psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 23.Kongs SEA. Wisconsin Card Sorting Test—64 card version. Cutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 24.Wechsler D. Wechsler Memory Scale. 3rd. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 25.Reitan RM. Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd. Tuscon, AZ: Neuropsychological Press; 1993. [Google Scholar]
- 26.Stroop JR. Studies of inference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 27.Dowling M. Hermann B. La Rue A, et al. An examination of the latent structure of the neuropsychological test battery of the Wisconsin Registry for Alzheimer's Prevention. Chicago, IL: ICAD; 2008. [Google Scholar]
- 28.Jones RK. Drewke J. Countering conventional wisdom: New evidence on religion and contraceptive use. New York: Guttmacher Institute; 2011. [Google Scholar]
- 29.Nithianantharajah J. Hannan AJ. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89:369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 31.Kreijkamp-Kaspers S. Kok L. Grobbee DE, et al. Dietary phytoestrogen intake and cognitive function in older women. J Gerontol A Biol Sci Med Sci. 2007;62:556–562. doi: 10.1093/gerona/62.5.556. [DOI] [PubMed] [Google Scholar]
- 32.Ross JL. Roeltgen D. Feuillan P, et al. Effects of estrogen on nonverbal processing speed and motor function in girls with Turner's syndrome. J Clin Endocrinol Metab. 1998;83:3198–3204. doi: 10.1210/jcem.83.9.5087. [DOI] [PubMed] [Google Scholar]
- 33.Hoff AL. Kremen WS. Wieneke MH, et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry. 2001;158:1134–1139. doi: 10.1176/appi.ajp.158.7.1134. [DOI] [PubMed] [Google Scholar]
- 34.Hampson E. Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- 35.Ping SE. Trieu J. Wlodek ME, et al. Effects of estrogen on basal forebrain cholinergic neurons and spatial learning. J Neurosci Res. 2008;86:1588–1598. doi: 10.1002/jnr.21609. [DOI] [PubMed] [Google Scholar]
- 36.Dumas J. Hancur-Bucci C. Naylor M, et al. Estrogen treatment effects on anticholinergic induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- 37.Woolley CS. Gould E. Frankfurt M. McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen BS. Estrogen action throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 39.Toran-Allerand CD. Singh M. Setalo G. Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 40.Maki PM. Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15:667–681. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogervorst E. Yaffe K. Richards M, et al. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst Rev. 2009;1(1):CD003799. doi: 10.1002/14651858.CD003799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baum LW. Sex, hormones, and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- 43.Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 44.Clark AS. MacLusky NJ. Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 45.Voyer D. Voyer S. Bryden MP. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 46.Imperato-McGinley J. Pichardo M. Gautier T. Voyer D. Bryden MP. Cognitive abilities in androgen-insensitive subjects: Comparison with control males and females from the same kindred. Clin Endocrinol (Oxf) 1991;34:341–347. doi: 10.1111/j.1365-2265.1991.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 47.Wharton W. Hirshman E. Merritt P, et al. Oral contraceptives and androgenicity: Influences on visuospatial task performance in younger individuals. Exp Clin Psychopharmacol. 2008;16:156–164. doi: 10.1037/1064-1297.16.2.156. [DOI] [PubMed] [Google Scholar]
- 48.Tivis LJ. Green MD. Nixon SJ, et al. Alcohol, estrogen replacement therapy, and visuospatial processes in postmenopausal women. Alcohol Clin Exp Res. 2003;27:1055–1063. doi: 10.1097/01.ALC.0000075545.28199.DF. [DOI] [PubMed] [Google Scholar]
- 49.Froom P. Melamed S. Kristal-Boneh E, et al. Healthy volunteer effect in industrial workers. J Clin Epidemiol. 1999;52:731–735. doi: 10.1016/s0895-4356(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DJ. Manson JE. Colditz GA, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of U.S. women. Contraception. 1997;56:373–378. doi: 10.1016/s0010-7824(97)00172-8. [DOI] [PubMed] [Google Scholar]