Abstract
Adoptive T cell therapy utilises the specificity of the adaptive immune system to target cancer and virally infected cells. Yet the mechanism and means by which to enhance T cell function are incompletely described, especially in the skin. Here, we utilise a murine model of immunotherapy to optimise cell-mediated immunity in the skin. We show that in vitro derived central but not effector memory-like T cells bring about rapid regression of skin expressing cognate antigen as a transgene in keratinocytes. Local inflammation induced by the TLR7 receptor agonist, imiquimod, subtly yet reproducibly decreases time to skin graft rejection elicited by central but not effector memory T cells in an immunodeficient mouse model. Local CCL4, a chemokine liberated by TLR7 agonism, similarly enhances central memory T cell function. In this model, IL-2 facilitates the development of in vivo of effector function from central memory but not effector memory T cells. In a model of T cell tolerogenesis, we further show that adoptively transferred central but not effector memory T cells can give rise to successful cutaneous immunity that is dependent on a local inflammatory cue in the target tissue at the time of adoptive T cell transfer. Thus, adoptive T cell therapy efficacy can be enhanced if CD8+ T cells with a central memory T cell phenotype are transferred and IL-2 is present with contemporaneous local inflammation.
Keywords: Rodent, T Cells, cytotoxic, Inflammation, Memory, Skin
Introduction
Epithelial cancers and chronic viral infections at epithelial surfaces represent a significant cause of morbidity and mortality worldwide (1, 2), for which adoptive transfer of ex vivo activated, antigen-specific T cells is mooted as potential therapy. Adoptive T cell transfer, which utilises the specificity of the T cell receptor, conveys an advantage over immunisation, as individual antigen specific CD8+ T cells of high affinity can be expanded ex vivo to produce large numbers of cytotoxic effector precursors. Adoptive cell therapy holds great promise for the treatment of epithelial cancers and chronic viral infections (3). However, the reported success rates for adoptive cell therapy in humans are highly variable, ranging between 20% and 70% (4–6). The variable response observed to adoptive immunotherapy suggests a need to better understand the requirements for optimisation of in vivo outcomes after adoptive transfer of T cells.
Once activated, naïve CD8+ T cells differentiate into short-lived CD44hi CD62Llo effector and effector memory T cells (TEFF/TEM), or lymphoid organ-residing CD44hi CD62Lhi central memory T cells (TCM) (3). Previous work investigating the means by which to optimise T cell therapy has shown that, whilst TEFF/TEM demonstrate the capacity for rapid target lysis, the in vivo durability of TCM make this cell type best suited for use in adoptive cell therapy (7, 8). However, the mechanisms underlying enhanced outcomes with TCM and the means by which to further augment the function of TCM are poorly understood. Furthermore, the relative potential of TEM and TCM in adoptive cell therapy targeted to the skin has not been described.
CD8+ T cell-mediated immunity occurs in a tissue-specific manner. CD8+ T cell activation via subcutaneous immunisation, for example, induces activated T cells that home to skin (9). Thus, optimisation of immunotherapy for cutaneous viral infection and malignancy requires an understanding of the immunobiology of adoptively transferred T cells in skin-specific immunotherapy. We have utilised a system in which murine skin expressing a model antigen is grafted onto a naïve host to study the requirements for effective skin-targeted immunotherapy (10–13). In this system a model antigen, such as ovalbumin or the viral oncogene E7, is expressed as a transgene in keratinocytes under the control of basal keratinocyte-specific promoters for the genes keratin 5 or keratin 14. In the case of ovalbumin, this system models the immune consequences of antigen expression in epithelial cells without the confounding effects of tumour-related immune modulation. Rejection of the transgenic graft is easily visualised and is a measure of local immune effector function (12). This system allows investigation of induction of immune responses by antigen expressed in skin and effector functions elicited within this site by endogenous or adoptively transferred naïve T cells (10–13). Here, we investigate the capacity of different populations of ex vivo activated memory T cells to elicit immune effector functions in skin and the requirements for an optimal response.
Materials and methods
Mice
C57BL/6 and Rag1−/− mice were sourced from the Animal Resources Centre (WA, Australia). T cell receptor transgenic mice specific for H-2kb-bound SIINFEKL peptide (OT-I mice) were originally sourced from William R. Heath (University of Melbourne, VIC, Australia). K5mOVA transgenic C57BL/6 mice, in which ovalbumin (OVA), is expressed under control of the keratin 5 promoter, were provided by H. Azukizawa (Osaka Univeristy, Osaka, Japan) (14). CD11c.OVA mice, in which OVA expression is under the CD11c promoter, were bred at the Biological Resources Facility (BRF; QLD Australia). All mice were housed under specific pathogen-free conditions, used at 6–10 weeks of age and sex-matched for all experiments. All animal procedures were approved by the University of Queensland Animal Ethics Committee.
Flow cytometry and intracellular antigen staining
Monoclonal antibodies (mAb) to murine CD8, CD3, CD44, CD62L, interferon-γ, T-bet, IL-2 and associated immunoglobulin controls were purchased from BD Bioscience (CA, USA) and eBioscience (CA, USA) and used as per manufacturer protocol. For intracellular cytokine staining, cells were stimulated with 25ng/ml phorbol 12-myristate 13-acetate (Sigma; NSW, Australia) and 1μg/ml ionomycin (Sigma; NSW, Australia) and monensin (BioLegend; CA, USA). Permeabilisation, fixation and staining were performed as per manufacturer’s instructions (Mouse Intracellular Cytokine Staining Kit; BD Biosciences). Staining of the transcription factor T-bet was performed with fixation and permeabilisation reagents from the Foxp3 staining kit as per manufacturer’s instructions (BD Biosciences). Data was acquired using the FacsCalibur flow cytometer (BD Bioscience) and analysed using FlowJo version 8.7.3 (Tree Star; CA, USA).
Skin grafting
Ear skin was grafted onto the flanks as previously described (12, 15). Briefly, donor ear epidermis was placed onto graft beds of 1cm2 on the flanks of anaesthetised mice and fixed with Elastoplast fabric strips (Beiersdorf; NSW, Australia). Bandages were removed 7 days later and grafts allowed to heal completely for 6 weeks during which time all inflammatory cytokines return to resting levels (16, 17). Grafts were considered rejected when >80% of the graft area was visibly ulcerated and necrotic.
Imiquimod treatment
Skin grafts were treated with 30mg of 5% imiquimod cream (Aldara™; iNova Pharmaceuticals; NSW, Australia) under occlusive dressing for 10 consecutive days or until rejection occurred (15). Aqueous cream (Sorbolene; Redwin Skincare, VIC, Australia) was applied as a control.
Central and effector memory T cell culturing and adoptive transfer
An existing protocol was modified for in vitro differentiation of central (TCM) and effector memory-phenotype cells (TEM) from wild-type, antigen-naive OT-I mice at 6–8 weeks of age (18). For differentiation of TEM, splenocytes from naïve OT-I mice were cultured overnight with 100ng/ml of SIINFEKL peptide, washed and then grown with 20ng/ml of recombinant IL-2 (Peprotech; NJ, USA). TCM were differentiated similarly using lymphocytes from naïve OT-I mice and, following peptide exposure, were cultured with 10ng/ml of recombinant IL-7 and IL-15 (Peprotech; NJ, USA). All cells were grown in RPMI Medium 1640 (Gibco; CA, USA) supplemented with 10% foetal calf serum (Bovogen; VIC, Australia). Prior to tail vein adoptive transfer, non-viable cells were eliminated by centrifugation with Histopaque 1077 as per manufacturer’s instructions (Sigma; NSW, Australia).
Lymphocyte purification from skin and liver
Skin grafts were excised and incubated in 1mg/ml collagenase at 37°C for 3 hours with mechanical dissociation. Cells were then strained and resuspended in buffer containing 5% foetal calf serum. Single cell suspensions of liver cells were purified with a 32% Percoll gradient (GE Healthcare; Uppsala, Sweden) as per manufacturer’s instructions and red blood cells removed with the use of ACK (Ammonium-Chloride-Potassium) lysis buffer (Life Technologies; CA, USA).
Monoclonal antibody production and treatment
Anti-CD8β (antibody 53-5.8) was produced from hybridoma cell lines using a sequential serum dilution technique as previously described (15). For grafting experiments 100μg of 53-5.8 in PBS was injected intraperitoneally on the day prior to skin grafting. On days 2 and 7 post-grafting animals were treated with a further 150μg of 53-5.8. On day 9 post-grafting, CD8+ T cell depletion was assessed by analysis purification of leukocytes from a peripheral eye bleed stained with CD3 and CD8β. Anti-IL-2 rat IgG monoclonal antibodies from clones S4B6 and Jes6-1A12 were sourced from BioXcell (NH, USA) a fermentation and purification service which produces endotoxin-negative, in vivo verified antibodies. 100μg of both S4B6 and Jes6-1A12 were injected daily for 10 days from the day of cell transfer as previously described (19).
In vitro cytotoxicity assay
Cytotoxicity assay was performed as described previously (20). EL4 H-2Kb expressing thymoma cells were labelled with 100μCi of Chromium51 with or without 1μM SIINFEKL peptide. Labelled cells were then incubated for 2.5 or 5 hours with TEM, TCM, media (spontaneous release control), or 5% Triton-X100 (maximum release control). Cells were then spun down and supernatant dried onto Lumaplate membrane overnight (Packard; CT, USA) and scintillation detected by TopCount NXT (Perkin Elmer; MA). Percent lysis was calculated from means of triplicate rows as follows: [(sample − spontaneous)/(maximum − spontaneous)] × 100%.
Chemokine injection and chemokine receptor antagonists
All recombinant chemokines were sourced from Peprotech (NJ, USA). The chemokine antagonist, CCL5(9–68), and inert control peptide, CCL2(4Ala), were synthesized as previously described (21). Mice were treated with 100μg of CCL5(9–68) or the inert control peptide CCL2(4Ala) via i.p. injection every other day following day 4 post-grafting. The in vivo activity of this antagonist has been validated as previously described (22).
Statistics
Skin graft survival curves were depicted on Kaplan-Meier curves and log-rank tests performed to assess statistical significance differences in survival. All data analysed by Prism Version 4 (Graphpad Software; CA, USA).
Results
Antigen specific CD8+ T cells are necessary and sufficient for K5mOVA skin graft rejection
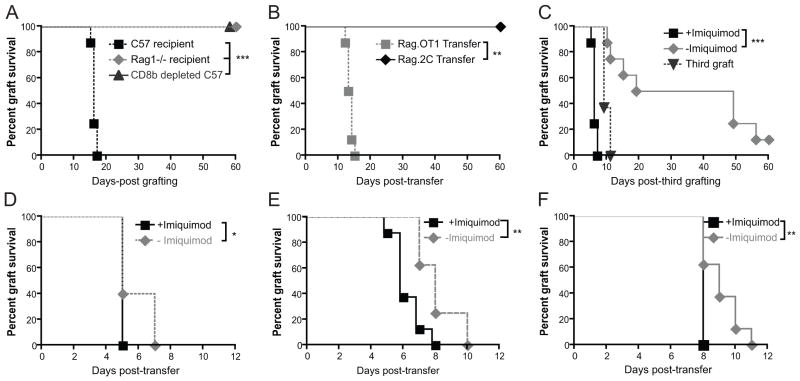
Rejection of skin grafts expressing non-self antigen from a keratin promoter has been used to study T cell immunotherapy (12, 15, 23). We studied the capacity of T cells to mediate rejection of skin grafts expressing ovalbumin from the keratin 5 promoter (henceforth, K5mOVA mice) (14). Newly-placed K5mOVA skin grafts were rejected spontaneously by naïve, immunocompetent C57BL/6 animals but not by lymphodeplete Rag1−/− animals, nor by wild-type animals depleted of cells expressing the CD8β chain, confirming that K5mOVA graft rejection is dependent on CD8+ T cell effector function (Figure 1A). Transfer of TCR transgenic T cells specific for the H-2Kb-bound dominant ovalbumin epitope, SIINFEKL (Rag.OT-I), but not of TCR transgenic T cells specific for the H-2Kb allo-epitope, SIYRYYGL (Rag.2C), induces graft rejection in Rag1−/− animals bearing healed K5mOVA grafts, confirming that antigen-specific CD8+ T cells are sufficient for induction of graft rejection (Figure 1B).
Figure 1. K5mOVA skin graft rejection is CD8+ T cell dependent and is enhanced by local TLR7 ligation.
Kaplan-Meier survival curves of membrane-bound ovalbumin-expressing skin grafts (K5mOVA). A. C57BL/6, Rag1−/− mice and C57BL/6 mice treated with anti-CD8β monoclonal antibody were grafted with K5mOVA expressing skin and grafts monitored for rejection. n=8, ***p<0.001 by log rank test. B. Rag1−/− mice bearing K5mOVA skin grafts received an intravenous transfer of 106 SIINFEKL-specific Rag.OT-I splenocytes or SIYRYYGL-specific Rag.2C splenocytes. Grafts were then monitored for rejection. n=7, **p<0.01 by log rank test. C. C57BL/6 mice were depleted of CD8β+ T cells by treatment with the monoclonal antibody 53–5.8 and grafted with two K5mOVA skin grafts. 10 weeks later, animals received a third, contralateral graft, and one of the two original grafts were treated for 10 days with imiquimod whilst the other received control cream. n=7, ***p<0.001 by log rank test. D–F. Rag1−/− mice bearing bilateral, well-healed K5mOVA skin graft were adoptively transferred with 107 (D), 106 (E), or 105 (F) naïve Rag.OT-I splenocytes. At the same time all animals received subcutaneous immunisation with the adjuvant OVA in Quil A, whilst one of the two grafts was treated with imiquimod and the other with the control cream. n=8 for all groups, *, p < 0.05, **p<0.01 by log rank test.
A local inflammatory cue enhances skin graft rejection by activated CD8+ T cells
Using a papillomavirus antigen, HPV16 E7 protein, expressed as a transgene in skin grafts, we have previously demonstrated that healed grafts are less effectively rejected by transferred antigen-specific T cells than newly-placed grafts (12). To investigate whether local inflammation alters the efficiency of antigen-experienced CD8+ T cells in skin graft rejection, mice were primed to OVA by placement of a K5mOVA graft, which was rejected. Subsequently, these mice were depleted of CD8β+ T cells, and given two further K5mOVA grafts, which were not rejected. After T cell recovery, a third K5mOVA graft was given and one of the two well-healed grafts was treated with imiquimod. The newly placed graft was rejected confirming restoration of functional OVA-specific T cells. The imiquimod treated healed graft was rejected more rapidly than the untreated graft (Figure 1C), demonstrating that local inflammation contributes to immune effector function. To confirm that imiquimod alone does not induce rejection of K5mOVA skin, Rag1−/− mice bearing well-healed K5mOVA grafts were treated with imiquimod or control cream, neither resulted in grafts being rejected (Supplementary Figure 1).
To investigate the mechanism by which local inflammation could enhance CD8+ effector function, we first studied whether activated, adoptively transferred, antigen-specific CD8+ T cells could promote graft rejection in the absence of other lymphocytes. Rag1−/− animals received two K5mOVA skin grafts and were allowed to heal for 6 weeks. Following healing, grafted animals received an adoptive transfer of OT-I splenocytes and subcutaneous immunisation with OVA/Quil A. When one of the two healed K5mOVA grafts was treated with topical imiquimod, rejection of the treated graft was significantly faster than rejection of the untreated graft across a range of OT-I cell precursor frequencies (Figure 1D–F). The effect of imiquimod on graft rejection was most pronounced at the lowest number (105) of OT-I transferred cells indicating the capacity of imiquimod to enhance suboptimal responses. In these immunised animals, two distinct populations of activated CD8+ T cells could be demonstrated: CD44hi CD62Llo T cells of effector memory T (TEM) phenotype, and CD44hi CD62Lhi T cells of central memory T cell (TCM) phenotype (Supplementary Figure 2).
Local TLR7 ligation augments cutaneous immunotherapy elicited by TCM but not TEM
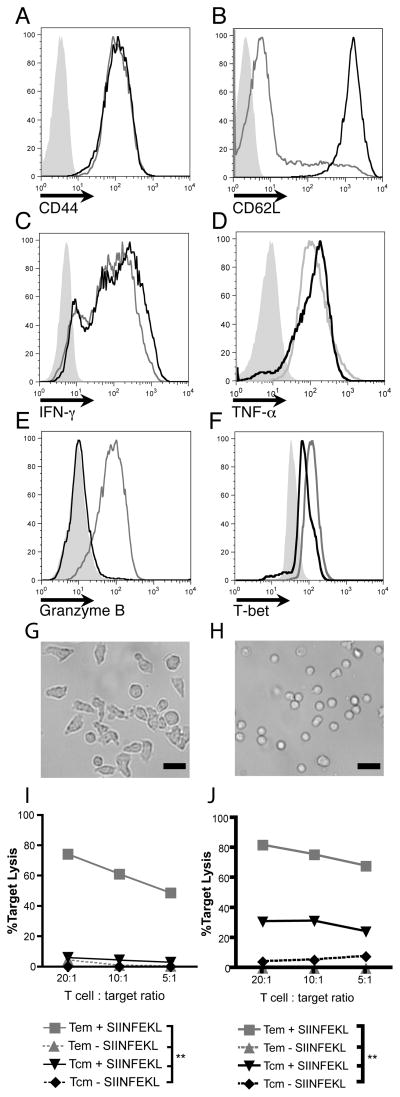
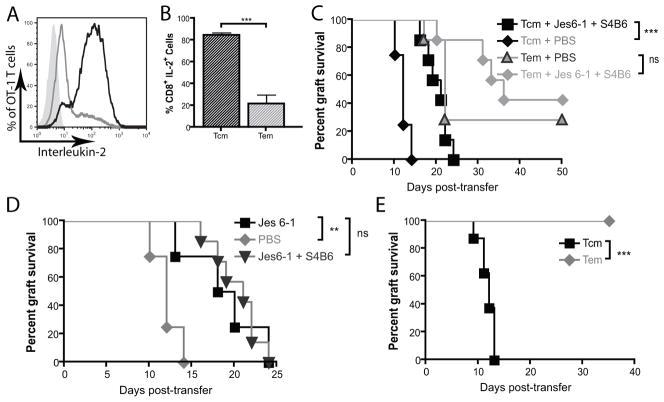
We next aimed to determine which subset of adoptively transferred, activated CD8+ T cells, (TEM or TCM-phenotype cells) were responsible for the accelerated rejection induced by topical imiquimod. Modification of an existing protocol (18) using non-immunised wild-type OT-I lymphocytes was implemented for the ex vivo production of TCM and TEM. OT-I cells differentiated in the presence of IL-7 and IL-15 were CD44hi CD62Lhi and expressed interferon-γ and tumour necrosis factor-α but failed to express granzyme B after stimulation with PMA/ionomycin, consistent with a TCM-like phenotype (Figure 2A–E). In contrast, cells differentiated in the presence of IL-2 showed a morphology consistent with fully differentiated effector/effector memory T cell (compare Figure 2G and H) and were CD44hi and CD62Llo TEM-like cells and expressed high levels of interferon-γ, granzyme B and tumour necrosis factor-α (Figure 2A–E). The putative TEM population expressed high levels of T-bet, whilst the TCM and naïve cell populations expressed intermediate and low levels of T-bet, respectively (Figure 2F). In vitro differentiated TEM exhibited enhanced in vitro killing capacity as compared to TCM (Figure 2I–J). In vitro differentiated memory T cell populations, therefore, demonstrated the functional and phenotypic attributes held characteristic of TEM and TCM-like cells.
Figure 2. Ex vivo differentiated TCM and TEM exhibit extracellular markers, intracellular molecules and killing capacity characteristic of TCM and TEM.
A&B. CD44 and CD62L staining of OT-I in vitro derived TCM (black lines) and TEM (grey lines) cultured as per materials and methods. Isotype control shown in filled grey histograms. C–E. Intracellular Interferon-γ, tumour necrosis factor-α, granzyme B expression in in vitro differentiated TCM and TEM following stimulation with PMA/ionomycin. TCM shown as black lines, TEM as grey lines and unstimulated cells shown as filled grey histograms. F. Intracellular expression of the T-box transcription factor T-bet. TCM shown as black lines, TEM as grey lines and naïve cells shown as filled grey histograms. All vertical axes in histograms are frequency of cells as percentage of total cells analysed. Flow cytometry plots represent one three individual experiments conducted with pooled lymphocytes from OT-I mice. G&H. Micrograph of OT-I TEM (G) and TCM (H). Photos represent one of two individual experiments using pooled lymphocytes. Scale bar = 20μm. I & J. In vitro cytotoxic killing assays of TCM (black lines) and TEM (grey lines) demonstrating chromium release following 2.5 hours (K) and 5 hours (L) of exposure to SIINFEKL-pulsed (sold lines) and non-pulsed targets (broken lines). **p<0.01 by one-way ANOVA test with Bonferroni post-hoc analysis. Target lysis calculated by [(sample − spontaneous)/(maximum − spontaneous)] × 100%.
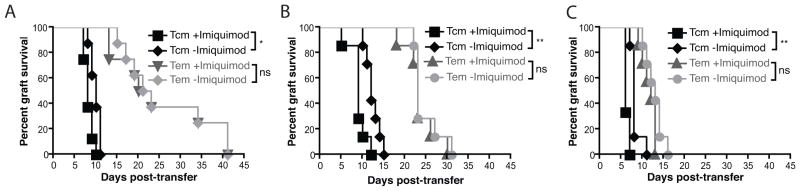
To compare the capacity of TEM and TCM T cell subsets to induce skin graft rejection, and the extent to which this was enhanced by local inflammation, Rag1−/− mice bearing two well-healed K5mOVA grafts received in vitro differentiated TCM or TEM, and one of the two grafts was treated with topical imiquimod. Rejection of the K5mOVA skin grafts was significantly quicker in recipients of TCM than in recipients of equivalent numbers of TEM (Figure 3A–C). Further, grafts treated with topical imiquimod on animals recipient of TCM showed accelerated rejection compared with untreated grafts, whereas this was not observed for animals recipient of TEM. The effect of rapid TCM-mediated graft rejection augmented by imiquimod was consistently observed across a range of transferred OT-I TCM and TEM frequencies.
Figure 3. In vitro differentiated central but not effector memory CD8+ T cell rejection of skin grafts is enhanced by a local inflammatory stimulus.
Rag1−/−mice bearing two well-healed K5mOVA grafts received either in vitro differentiated TCM (black lines) or TEM (grey lines). Grafts were then treated with either imiquimod or control cream and graft rejection plotted on a Kaplan-Meier survival curve. A. 104 adoptively transferred TCM and TEM CD8 T cells. Data analysed by log rank test, n=7 for each group, *p<0.05, ns = not significant. B. 105 adoptively transferred TCM and TEM CD8 T cells. Data analysed by log rank test, n=7 for each group, **p<0.01, ns = not significant. C. 106 adoptively transferred TCM and TEM CD8 T cells. Data analysed by log rank test, n=8 for each group, **p<0.01, ns = not significant.
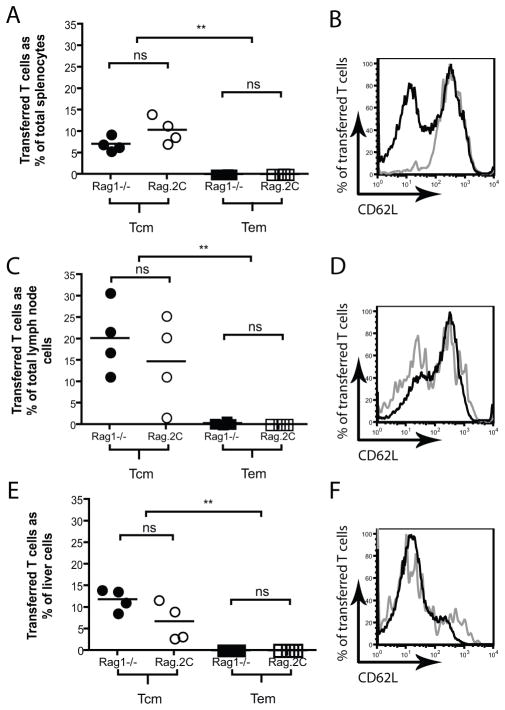
TCM persist to a greater extent than TEM in both lymphodeplete and lymphoreplete environments
We next investigated whether TCM-mediated graft rejection was a consequence of prolonged OT-I T cells survival in vivo. In vitro differentiated TCM and TEM were transferred into Rag1−/− mice bearing well-healed K5mOVA skin grafts. Given that lymphodeplete environments can prolong survival of transferred T cells through the availability of homeostatic cytokines (25), TCM and TEM were also transferred into lymphoreplete Rag.2C mice bearing well-healed K5mOVA skin grafts – Rag.2C mice possess a complement of functional T cells solely specific for the SIYRYYGL alloepitope. 45 days following transfer spleen, lymph nodes and liver were harvested, processed into single cell suspensions, stained for CD8, Vα2 and CD62L and analysed by flow cytometry. Figure 4A , C and E show that TCM are present in larger numbers than TEM 45 days following transfer into either lymphodeplete Rag1−/− or lymphoreplete Rag.2C mice. Consistent with memory T cell phenotype, transferred TCM and TEM differentiate into CD62Lhi (present in lymph nodes) and CD62Llo (present in liver) T cells indicating that these two cell types can trans-differentiate in vivo following transfer (Figure 4 B, D and F).
Figure 4. TCM persist to greater extent than TEM in both Rag1−/− and Rag.2C mice.
Rag1−/− (filled symbols) and Rag.2C (open symbols) mice bearing well-healed K5mOVA grafts were adoptively transferred with 106 TCM (circles, black lines on histogram) or TEM (squares, grey lines on histogram). 45 days following transfer spleen (panels A&B), pooled inguinal, brachial, axillary and mesenteric lymph nodes (panels C&D) and liver (panels E&F) were harvested and cells stained for CD8, Vα2, and CD62L. A, C and E. CD8+ Vα2+ cells as percent of total cells in each organ. n=4per group, **p<0.01 when comparing Rag1−/− to Rag1−/− and Rag.2C to Rag.2C, ns = not significant; analysed by one-way ANOVA analysis with post-hoc multiple comparison test. B, D and F. Representative CD62L expression of CD8+ Vα2+ CD44+ in various organs analysed by flow cytometry 45 days following transfer into Rag1−/− mice.
Inhibition of IL-2 mitigates enhanced rejection by TCM
Autocrine production of IL-2 has been shown to drive the secondary expansion and survival of central memory T cells (26). Figure 5A and B show that in vitro differentiated TCM but not TEM produce large amounts of IL-2 upon stimulation with PMA/Ionomycin. We thus hypothesised that IL-2 played a role in enhancing TCM mediated graft rejection. Treatment with IL-2 inhibiting monoclonal antibodies JES6-1 and S4B6 was associated with significantly delayed well-healed K5mOVA graft rejection in recipients of TCM but not TEM (Figure 5C). Furthermore, inhibition of IL-2 binding to the high affinity IL-2 receptor α, CD25, by JES6-1 alone also delayed TCM-mediated skin graft rejection to that same extent as combination of JES6-1 and S4B6 which blocks IL-2 completely (Figure 5D). This confirms a role for autocrine IL-2 signalling through the trimeric high affinity IL-2 receptor complex in the acquisition of effector function by TCM but not TEM.
Figure 5. IL-2 inhibition delays TCM mediated-rejection of well-healed K5mOVA grafts.
A. Intracellular expression of interleukin-2 by flow cytometry following stimulation of in vitro differentiated central memory T cells (T CM, black line) and effector memory T cells (TEM, grey line) with PMA/Ionomycin. B. Quantitation of percent of IL-2-expressing in vitro differentiated TCM and TEM. Data from three individual experiments. Data analysed by Student’s t-test. Error bars represent S.E.M. ***p<0.001. C & D. Kaplan-Meier survival curve of well-healed K5mOVA skin grafts on Rag1−/− mice which received an adoptive transfer of either 105 TCM or TEM treated for 10 days with intraperitoneal injections of PBS or 100μg of the IL-2-blocking monoclonal antibodies Jes6-1 and S4B6 (C); or Jes6-1 alone or PBS (D). Animals were then monitored for graft rejection. n=8 for TCM and n=7 for TEM. **p<0.001, ns = not significant. Data analysed by log rank test. E. Rag.2C mice bearing well-healed K5mOVA skin grafts were adoptively transferred with 105 TCM or TEM. Following transfer, grafts were monitored daily for graft rejection. n=8 per group, ***p<0.001. Data analysed by log rank test.
Lymphoreplete environments have been reported to inhibit adoptive cell therapy by decreasing the availability of γ-chain cytokines to adoptively transferred, effector-phenotype cells (25). Given that TCM are able to produce the γ-chain cytokine, IL-2, we proposed that TCM but not TEM would reject skin grafts in lymphoreplete environments. Figure 5E shows that TCM but not TEM rejected well-healed K5mOVA skin grafts in lymphoreplete Rag.2C animals.
Intradermal CCL4 but not CCL2 nor CXCL2 is sufficient to recapitulate the effect of imiquimod on TCM-mediated graft rejection
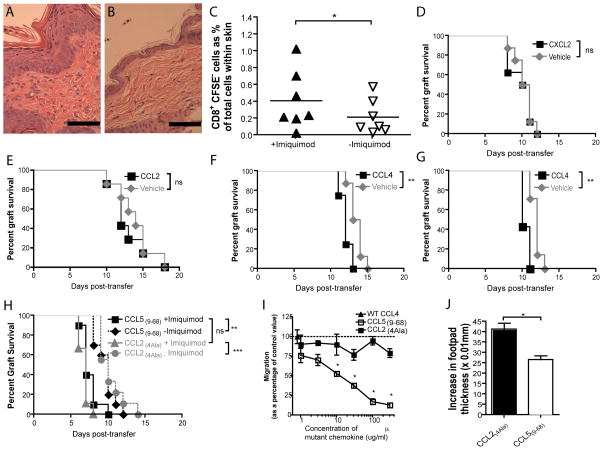
We next examined the mechanism by which imiquimod enhances TCM-mediated graft rejection. Imiquimod treatment of the flank of C57BL/6 mice induces a dense inflammatory infiltrate into the epidermis and dermis (Figure 6A–B). To assess if imiquimod increased trafficking of TCM-derived cells into inflamed skin, Rag1−/− mice bearing two well-healed K5mOVA skin grafts were adoptively transferred with 105 CFSE-labelled TCM and one of the two grafts was treated daily with imiquimod. Analysis of single cell suspensions of skin grafts by flow cytometry on day 5 post-transfer showed imiquimod increased T cell traffic into imiquimod-inflamed skin. All cells analysed were CFSE negative indicating that these cells were divided progeny of transferred TCM, as Rag1−/− mice have no endogenous T cells (Figure 6C).
Figure 6. Imiquimod enhances inflammatory infiltrate and traffic of adoptively transferred CD8+ T cells into inflamed grafts. The effects of imiquimod on TCM-mediated graft rejection are recapitulated by the chemotactic molecule, CCL4.
A & B. Haematoxylin and eosin micrograph of flank skin of C57BL/6 mice treated for 5 days with imiquimod (A) or control cream (B). Scale bars represent 50μm. C. Rag1−/− mice bearing two, bilateral K5mOvA received an adoptive transfer of 105 CFSE-labelled, in vitro-derived TCM 6 weeks following grafting. At time of transfer one of the two grafts was treated with imiquimod daily for 5 days, whilst the other received control cream. Grafts were removed, processed into single cell suspension. Cells were then analysed by flow cytometry for CD3, CD8 and CFSE staining. n=7 per group, *p<0.05 by paired Student’s t test. D–G. Rag1−/− bearing two well-healed K5mOVA skin grafts were adoptively transferred with 105 TCM and received intradermal injections of 200ng of recombinant murine CCL2 (D) or CXCL2 (E) or CCL4 (F) or 1μg of CCL4 (G) or vehicle control on days 4, 5, 7 and 9 post-transfer. Grafts were then monitored for rejection. n=8 for each experiment, **p<0.01, ns. not significant by log rank test. H. Two groups of Rag1−/− mice bearing bilateral, well-healed K5mOVA skin grafts were adoptively transferred with TCM and treated with the CCR5 inhibiting peptide CCL5(9–68) or the non-functional mutated chemokine CCL2(4Ala) whilst one of the two grafts was treated with imiquimod. Grafts were then monitored for rejection and plotted on a Kaplan-Meier survival curve. n=8 for all groups, **p<0.01, ***p<0.001, ns = not significant by log rank test. I. Transwell migration assay of purified CD8+ T cells stimulated with CD3 and CD28 monoclonal antibodies, migrating in response to CCL4. Inhibition of T cell migration by CCL59–68 was evaluated by increasing concentration of CCL59–68 and assessing percent of migrated cells relative to wild-type CCL4. Non-functional CCL24Ala peptide was used as control. n=2, *p<0.05 by one-way ANOVA. J. Delayed-type hypersensitivity reaction was established by tail-base immunisation of C57BL/6 mice with Ovalbumin in complete Freund’s adjuvant. 7 days later, recall was conducted in footpad and increase in footpad thickness was assessed by callipers. To assess for in vivo activity of CCL59–68, one day prior to recall animals were treated with CCL59–68 or control peptide CCL24Ala. Data are presented as mean + S.E.M. Data analysed by Student’s t-test. n = 12 per group, *p<0.05.
We next hypothesised that local inflammation could enhance graft rejection in part via inflammatory chemokines We have previously shown in a microarray screen that local imiquimod application up regulates the expression of the chemokines CCL2, CXCL2 and CCL4 all of which are known to induce T cell trafficking (15, 27, 28). To determine whether chemokines alone could enhance graft rejection, one of two grafts on Rag1−/− animals bearing well-healed K5mOVA grafts that received in vitro differentiated TCM, were injected subcutaneously with recombinant CCL2, CXCL2 or CCL4, and the other with vehicle control. Only CCL4 accelerated rejection of grafts mediated by TCM and this was in a dose-dependent fashion (Figure 6D–G).
If CCL4 was the only determinant of imiquimod-enhanced TCM-mediated graft rejection, then we predicted that blockade of the dominant CCL4 receptor, CCR5, would reverse imiquimod-enhanced graft rejection. However, inhibition of CCR5, using a small peptide antagonist (CCL5(9–68)) failed to alter the effects of imiquimod on TCM-mediated graft rejection (Figure 6H), even though this antagonist is able to inhibit CCL4-mediated migration of CD8+ T cells in vitro and T cell-dependent delayed type-hypersensitivity reactions in vivo (Figure 6I–J).
Local TLR7 stimulation and adoptive transfer of T cells are sufficient to overcome tolerance of skin expressed antigen
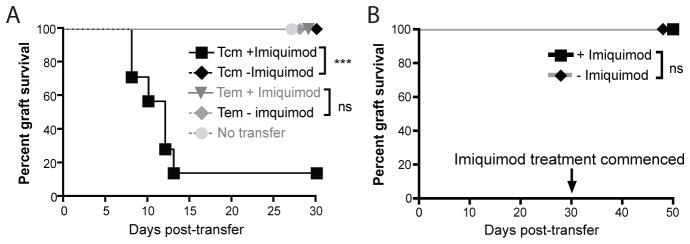
Therapy mediated by adoptively transferred antigen specific T cells may be inhibited by tolerance (29–34). To investigate whether local inflammation might overcome tolerance induced in adoptively transferred T cells, we utilised CD11c.OVA mice, in which expression of OVA in DC from the CD11c promoter in DC induces tolerance in adoptively transferred OVA specific memory T cells (35–37). K5mOVA skin grafts were not rejected by CD11c.OVA mice consistent with the impaired response of this transgenic mouse strain to ovalbumin (Figure 7A) (35). CD11c.OVA mice bearing bilateral well-healed K5mOVA skin grafts received an adoptive transfer of either TCM or TEM and one graft was treated daily with imiquimod. Animals that received OVA-specific TCM rejected only the grafts treated with imiquimod whereas animals that received OVA-specific TEM did not reject grafts, whether or not treated with imiquimod.
Figure 7. Local TLR7 ligation and TCM transfer is able to overcome tolerogenic environments and instate cutaneous immunotherapy.
A. CD11c.OVA mice bearing two well-healed K5mOVA skin grafts were adoptively transferred with 105 TEM or TCM 6 weeks following grafting. At time of transfer one of the two grafts was treated with imiquimod whilst the other was treated with control cream. Data analysed by log rank test, n=7 for TCM, n=8 for TEM. ***p<0.001, ns = not significant. B. CD11c.OVA mice bearing well-healed K5mOVA skin grafts were adoptively transferred with 105 TCM. 30 days later grafts were treated with either imiquimod or control cream for 10 days. Data analysed by log rank test, n=8, ns = not significant.
OVA specific T cells transferred to CD11c.OVA mice more than 4 weeks previously are fully tolerised (35). To demonstrate whether such tolerance could be overcome by local pro-inflammatory signals, we grafted CD11c.OVA mice with K5mOVA skin. When grafts were healed, animals received OVA specific TCM by adoptive transfer. After a further 30 days grafts were treated with imiquimod, and no rejection was observed (Figure 7B). Thus, once tolerance is established, local inflammation can no longer establish a successful adaptive immune response mediated by TCM.
Discussion
In this study we demonstrate that cells differentiated in vitro to acquire TCM characteristics exhibit more rapid in vivo cutaneous immune effector function, manifest as rejection of transgenic skin, than those differentiated to TEM. Rapid TCM-but not TEM-mediated graft rejection is mitigated by the inhibition of IL-2. We further show that TCM-mediated graft rejection is enhanced by local inflammation induced by a TLR7 ligand or by the local provision of the chemokine, CCL4. In contrast, graft rejection mediated by TEM is not significantly enhanced by TLR7 ligation. Local inflammation in skin allows TCM effector capacity in a tolerogenic environment that is otherwise induced by presentation of cognate antigen by APC.
The long lived nature and rapid responses of memory T cells, has led to the proposal that these cells are the most appropriate for T cell-based immunotherapies (38). The ability of local inflammation to enhance the development of fully competent T effector cells from memory T cell populations, as demonstrated here using parallel transgenic graft targets, has only recently been recognised, yet no studies have investigated the effect of local inflammation on TCM and TEM-mediated immune responses (39–42). Local TLR7 ligation augments rejection of human growth hormone (hGH) transgenic skin grafts from a graft primed animal (15). Well-healed grafts expressing the E7 protein of human papillomavirus are, unlike newly placed and inflamed grafts, protected against rejection by E7 specific CTL passively transferred and primed in vivo (12). Here we show that time to effective TCM-mediated immune response in skin is decreased by local inflammation, whereas TEM-mediated immune responses are not affected. Traditionally, TEM, which express cytotoxic molecules, such as granzyme B, and demonstrate enhanced in vitro cytotoxicity, have been considered the ideal cell type for adoptive immunotherapy (43, 44). Yet, TCM have been reported to mediate disease in mouse model of skin graft versus host disease (GVHD) (45), and central memory T cells mediate regression of transplanted melanoma in mice more effectively than effector memory T cells (24), which is considered a product of their prolonged in vivo persistence (46). Likewise, recently described human and murine T memory stem cells (TSCM) possess prolonged in vivo persistence that has been attributed to comprehensive regression of established tumours in mice and may hold future therapeutic promise in humans (47, 48).
We have previously demonstrated generation of enhanced effector function for skin graft rejection following systemic administration of the TLR4 agonist LPS in wild-type mice (49). In this study we show that an inflammatory cue provided at the effector site decreases the time to CD8+ TCM-mediated attack in immunodeficient mice. Although the effect of imiquimod in enhancing TCM but not TEM-mediated graft rejection is subtle, the effect is consistently reproducible across multiple different cell precursor frequencies and multiple conditions. Rejection of skin grafts in the tolerogenic CD11c.OVA environment is, furthermore, dependent on a local inflammatory cue. This may indicate that the primary role of inflammation in augmenting effector T cell responses is in influencing other adaptive immune cells. Local enhancement of effector function by local pro-inflammatory signalling through TLRs might also reflect increased recruitment of effectors (42), increased proliferation and maturation (50) of effector precursors, and/or induction of helper functions that override local inhibitors in skin (51). Local inhibitors include the anti-inflammatory cytokines IL-10 and TGF-β; NKT cells are also inhibitory in skin through an IFN-γ dependent mechanism (10, 11, 52, 53).
Imiquimod can induce T cell migration to inflamed sites presumably through upregulation of chemokines and cellular adhesion molecules (15, 54–57). CCL4, CCL2 and CXCL2 expression is increased in imiquimod-inflamed skin (15), we show here that only intradermal injections of CCL4 but not CCL2 nor CXCL2 is able to recapitulate the effects of imiquimod. However, inhibition of CCL4 through a small peptide antagonist that binds to the dominant CCL4 receptor, CCR5, does not reverse imiquimod-augmented graft rejection. The lack of effect of the CCR5 antagonist indicates a potential redundancy in the chemo-attractive molecules dictating effector T cell migration to the skin (58). Further analyses of the differential expression of chemokines and chemokine receptors seen on TCM effector progeny and TEM may further elucidate such redundancy.
Enhancement of graft rejection by local inflammation was specific to TCM, and inhibited by blockade of IL-2 signalling. A role for IL-2 in augmenting TCM but not TEM-mediated effector function has been proposed but not experimentally shown (59). Here we show that CD8+ T cells differentiated in the presence of IL-15 and IL-7 but not IL-2 produce IL-2 upon stimulation. Blocking IL-2 mediated signalling with monoclonal antibodies prolongs TCM-mediated effector function, manifest as delayed graft rejection where the time to rejection is similar that observed in recipients of TEM. Given that autocrine production of IL-2 has been shown to drive secondary expansion of memory T cells (26), the likely effect of IL-2 in this model is to induce TCM proliferation. Signalling through the trimeric high affinity IL-2 receptor complex has been shown to cause proliferation as opposed to maintain T cell number (19). This is of consequence given that we have shown there that inhibition of signalling through the high affinity trimeric IL-2 receptor complex prolongs TCM-mediated graft survival.
TCM-phenotype cells induce more complete regression of transplantable melanoma than TEM (24), and it has been postulated that enhanced in vivo reactivity against tumours by TCM is a product of lymph node homing and prolonged in vivo survival (24, 46). In this study we confirm that TCM persist to a greater extent in vivo than TEM. We further previous observations in showing that persistence of TCM is seen across multiple lymphoid organs. This is significant given that the liver has been shown to be a niche for TEM/TEFF T cells (60), yet in our study the number of TCM in liver at day 45 following transfer is greater than that of TEM. We further show that 45 days following transfer into grafted hosts, TCM possess the capacity to differentiate into CD62Lhi central memory phenotype cells. Maintenance of large populations of in vitro derived T cells following transfer is thought to be a product of homeostatic gamma-chain cytokines present in lymphopaenic hosts (25). We transferred TCM into both lymphodeplete (Rag1−/−) and lymphoreplete (Rag.2C) hosts and failed to see a significant difference in the persistence of TCM, whilst the number of persisting TEM was too small to conclude any significant difference Rag1−/− and Rag.2c mice. Moreover, we show that TEM failed to reject grafts in Rag.2C lymphoreplete mice. Thus the capacity of TCM to persist and reject skin grafts in both lymphodeplete and lymphoreplete environments may be a consequence of intrinsic TCM programming and cytokine production.
Jensen and colleagues (2011) recently proposed that host-produced IL-15 contributes to persistence of adoptively transferred human TCM in a lymphodeplete mouse model (8). In our study, TCM differentiated in the presence of IL-15 produced IL-2, whereas TEM differentiated in the presence of IL-2 did not produce autocrine IL-2. Our data indicated that autocrine IL-2 production is a consequence of IL-15 stimulation and may play a pivotal role in the effector function of TCM progeny. In current adoptive cell therapy strategies used in humans, IL-2 is administered along with effector-phenotype cells to prolong the persistence adoptively transferred cells (2). IL-2, however, has significant adverse effects including cardiomyopathy and pleural effusion (61). The use of TCM which self-produce IL-2 and display enhanced in vivo persistence may obviate the need for exogenous use of IL-2 in the clinic.
Failure of adoptive immunotherapeutic regimes may be accounted for by the tolerogenic effects of malignant disease (29, 30, 32–36, 62, 63). To address the effects of tolerance induction we utilised the CD11c.OVA murine model. This mouse strain lacks a functional OVA-responsive repertoire and induces tolerance in adoptively transferred naïve and memory CD8+ T cells (35–37). In this model, we show that adoptive transfer of antigen specific T cells alone did not reject K5mOVA skin grafts. Rather, only skin grafts that were inflamed by imiquimod in animals that had received TCM were rejected. Imiquimod treatment could not overcome established tolerance as treating skin grafts with imiquimod after TCM had undergone tolerance induction failed to induce graft rejection. Other approaches to breaking or preventing tolerance have used CD40, IL-2 and CD4+ T cell-based regimens. Our method using contemporaneous pro-inflammatory signals and T cell transfer may complement these (39, 64–66) and provides a means to enhance the efficacy of skin-directed adoptive immunotherapy in a potentially tolerogenic environment. Holcmann et al. (2009) reported that skin inflammation alone does not break tolerance and cause skin disease when naïve CD8+ T cells are adoptively transferred into animals that express tamoxifen-inducible K5mOVA (68). This disparity may be accounted for by the observation that memory and naïve CD8+ T cells display differing sensitivities to tolerance induction (35, 36, 62).
The mechanisms by which imiquimod breaks tolerance in the skin are currently under investigation. Imiquimod-inflamed skin tumours in humans possess decreased number of TReg cells (57). Furthermore, we have shown recently that TReg expression of CD25 limits memory CD8+ T cell expansion by limiting availability of IL-2 (68). We show here that CD25 plays a crucial role in the rapid response of TCM in Rag1−/− model system shown here. Therefore, in the CD11c.OVA mice imiquimod may decrease TReg number thus liberating available IL-2 for local memory T cell expansion. Mast cells may also play an important role in breaking tolerance in skin. Imiquimod increases mast cell number in the skin, whilst injection of the mast cell stimulating compound 48/80 induces rejection of skin grafts in CD11c.OVA mice recipient of TCM (S.F. unpublished observations).
To our knowledge, this work presents the first comparative analysis of TCM and TEM-mediated regression directed against skin and provides a mechanism underlying the rapid rejection of skin grafts expressing a foreign antigen by TCM. Furthermore, this is the first work we are aware of that provides a simple and clinically available intervention that differentially optimises in vitro derived TCM but not TEM function in the skin in a tolerogenic environment. The clinical implications of this work for future approaches to immunotherapy are significant. Understanding the in vivo function of adoptively transferred memory T cells in the skin is particularly important given the significant morbidity and mortality posed by cutaneous chronic viral infection in the skin and the potential of adoptive cell therapy to address this clinical problem (1, 3). This work posits an effective strategy for the use of ex vivo differentiated TCM in combination with a local inflammatory cue for immunotherapy in the skin in tolerogenic hosts.
Supplementary Material
Imiquimod does not cause graft rejection in the absence of lymphocytes. Rag1−/− mice were grafted with two K5mOVA skin grafts. 40 days later grafts were treated with either imiquimod or control cream. Grafts were monitored daily for rejection and plotted on a Kaplan-Meier survival curve. Data analysed by log rang test, n = 10, ns = not significant.
Rag1−/− mice received an intravenous transfer 106 Rag.OT-I splenocytes and were immunised with either Quil A alone (-Immunisation) or ovalbumin with Quil A (+Immunisation). Inguinal lymph node (LN), spleen, blood and liver were harvested 7 days post-transfer and immunisation and stained for expression of the memory T cell marker (CD44) and the lymph node homing marker (CD62L). Plots were gated on Vα2+ CD8+ cells. Numbers in each quadrant represents percent of Vα2+ CD8+ cells. Plots shown are representative of one of two experiments.
Acknowledgments
Mrs Allison Choyce assisted in the running of all mouse experiments. Mrs Joanne Leerborg assisted in creation of the anti-CD8β monoclonal antibody. Assistance in animal handling was from the Biological Research Facility staff. The authors thanks Professor Leonard Harrison, Walter and Eliza Hall Institute for provision of CD11c.OVA mice.
Grant support: Funding for this work was from NHMRC Program Grant 569938 and NCI Grant 5U01CA141583. IHF is recipient of a Queensland Government Fellowship. RJS was recipient of NHMRC CDA 519768.
Abbreviations used in this article
- TCM
Central memory T cell
- TEM
Effector memory T cell
- TReg
Regulatory T cell
References
- 1.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4:46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 6.Khammari A, Labarriere N, Vignard V, Nguyen JM, Pandolfino MC, Knol AC, Quereux G, Saiagh S, Brocard A, Jotereau F, Dreno B. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J Invest Dermatol. 2009;129:2835–2842. doi: 10.1038/jid.2009.144. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. 2010;184:1242–1250. doi: 10.4049/jimmunol.0902191. [DOI] [PubMed] [Google Scholar]
- 11.Mattarollo SR, Yong M, Tan L, Frazer IH, Leggatt GR. Secretion of IFN-gamma but not IL-17 by CD1d-restricted NKT cells enhances rejection of skin grafts expressing epithelial cell-derived antigen. J Immunol. 2010;184:5663–5669. doi: 10.4049/jimmunol.0903730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto K, Leggatt GR, Zhong J, Liu X, de Kluyver RL, Peters T, Fernando GJ, Liem A, Lambert PF, Frazer IH. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J Natl Cancer Inst. 2004;96:1611–1619. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 13.Broom JK, Lew AM, Azukizawa H, Kenna TJ, Leggatt GR, Frazer IH. Antigen-specific CD4 cells assist CD8 T-effector cells in eliminating keratinocytes. J Invest Dermatol. 2010;130:1581–1589. doi: 10.1038/jid.2010.17. [DOI] [PubMed] [Google Scholar]
- 14.Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, Sumikawa Y, Okabe M, Yoshikawa K, Itami S. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 15.Zhong J, Hadis U, De Kluyver R, Leggatt GR, Fernando GJ, Frazer IH. TLR7 stimulation augments T effector-mediated rejection of skin expressing neo-self antigen in keratinocytes. Eur J Immunol. 2008;38:73–81. doi: 10.1002/eji.200737599. [DOI] [PubMed] [Google Scholar]
- 16.Bingaman AW, Ha J, Waitze SY, Durham MM, Cho HR, Tucker-Burden C, Hendrix R, Cowan SR, Pearson TC, Larsen CP. Vigorous allograft rejection in the absence of danger. J Immunol. 2000;164:3065–3071. doi: 10.4049/jimmunol.164.6.3065. [DOI] [PubMed] [Google Scholar]
- 17.Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P. Testing time-, ignorance-, and danger-based models of tolerance. J Immunol. 2001;166:3663–3671. doi: 10.4049/jimmunol.166.6.3663. [DOI] [PubMed] [Google Scholar]
- 18.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 20.Leggatt GR, Narayan S, Fernando GJ, Frazer IH. Changes to peptide structure, not concentration, contribute to expansion of the lowest avidity cytotoxic T lymphocytes. J Leukoc Biol. 2004;76:787–795. doi: 10.1189/jlb.0104026. [DOI] [PubMed] [Google Scholar]
- 21.Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, Webster J, Harrison JM, Swann J, Clark-Lewis I, Korner H, McColl SR. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol. 2009;182:3121–3130. doi: 10.4049/jimmunol.0713169. [DOI] [PubMed] [Google Scholar]
- 22.Vassalli G, Simeoni E, Li JP, Fleury S. Lentiviral gene transfer of the chemokine antagonist RANTES 9–68 prolongs heart graft survival. Transplantation. 2006;81:240–246. doi: 10.1097/01.tp.0000194859.98504.9e. [DOI] [PubMed] [Google Scholar]
- 23.Zhong J, Matsumoto K, De Kluyver R, Fernando GJ, Leggatt GR, Frazer IH. Human growth hormone presented by K14hGH-transgenic skin grafts induces a strong immune response but no graft rejection. Immunol Cell Biol. 2004;82:577–586. doi: 10.1111/j.1440-1711.2004.01292.x. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu ZY, Rosenberg SA, Restifo NP. Increased Intensity Lymphodepletion Enhances Tumor Treatment Efficacy of Adoptively Transferred Tumor-specific T Cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci USA. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting Edge: Tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kammertoens T, Blankenstein T. Making and circumventing tolerance to cancer. Eur J Immunol. 2009;39:2345–2353. doi: 10.1002/eji.200939612. [DOI] [PubMed] [Google Scholar]
- 32.Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119:2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beyer M, Karbach J, Mallmann MR, Zander T, Eggle D, Classen S, Debey-Pascher S, Famulok M, Jager E, Schultze JL. Cancer vaccine enhanced, non-tumor-reactive CD8(+) T cells exhibit a distinct molecular program associated with “division arrest anergy”. Cancer Res. 2009;69:4346–4354. doi: 10.1158/0008-5472.CAN-08-3796. [DOI] [PubMed] [Google Scholar]
- 34.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111:2091–2100. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- 36.Steptoe RJ, Ritchie JM, Wilson NS, Villadangos JA, Lew AM, Harrison LC. Cognate CD4+ help elicited by resting dendritic cells does not impair the induction of peripheral tolerance in CD8+ T cells. J Immunol. 2007;178:2094–2103. doi: 10.4049/jimmunol.178.4.2094. [DOI] [PubMed] [Google Scholar]
- 37.Kenna TJ, Waldie T, McNally A, Thomson M, Yagita H, Thomas R, Steptoe RJ. Targeting Antigen to Diverse APCs Inactivates Memory CD8+ T Cells without Eliciting Tissue-Destructive Effector Function. J Immunol. 2010;184:598–606. doi: 10.4049/jimmunol.0900032. [DOI] [PubMed] [Google Scholar]
- 38.Perret R, Ronchese F. Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens. 2008;72:187–194. doi: 10.1111/j.1399-0039.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 39.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, Robinson BW, Currie AJ. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182:5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 40.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 41.Garbi N, Arnold B, Gordon S, Hammerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–5869. doi: 10.4049/jimmunol.172.10.5861. [DOI] [PubMed] [Google Scholar]
- 42.Chakraverty R, Cote D, Buchli J, Cotter P, Hsu R, Zhao G, Sachs T, Pitsillides CM, Bronson R, Means T, Lin C, Sykes M. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 44.Mule JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225:1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central Memory CD8(+) T Cells Induce Graft-versus-Host Disease and Mediate Graft-versus-Leukemia. J Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frazer IH, De Kluyver R, Leggatt GR, Guo HY, Dunn L, White O, Harris C, Liem A, Lambert P. Tolerance or immunity to a tumor antigen expressed in somatic cells can be determined by systemic proinflammatory signals at the time of first antigen exposure. J Immunol. 2001;167:6180–6187. doi: 10.4049/jimmunol.167.11.6180. [DOI] [PubMed] [Google Scholar]
- 50.Huang SJ, Hijnen D, Murphy GF, Kupper TS, Calarese AW, Mollet IG, Schanbacher CF, Miller DM, Schmults CD, Clark RA. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129:2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 52.Liu XS, Leerberg J, MacDonald K, Leggatt GR, Frazer IH. IFN-gamma promotes generation of IL-10 secreting CD4+ T cells that suppress generation of CD8 responses in an antigen-experienced host. J Immunol. 2009;183:51–58. doi: 10.4049/jimmunol.0802047. [DOI] [PubMed] [Google Scholar]
- 53.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Butchi NB, Pourciau S, Du M, Morgan TW, Peterson KE. Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J Immunol. 2008;180:7604–7612. doi: 10.4049/jimmunol.180.11.7604. [DOI] [PubMed] [Google Scholar]
- 55.Wolf IH, Kodama K, Cerroni L, Kerl H. Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol. 2007;29:237–241. doi: 10.1097/01.dad.0000211531.33670.94. [DOI] [PubMed] [Google Scholar]
- 56.Torres A, Storey L, Anders M, Miller RL, Bulbulian BJ, Jin J, Raghavan S, Lee J, Slade HB, Birmachu W. Immune-mediated changes in actinic keratosis following topical treatment with imiquimod 5% cream. J Transl Med. 2007;5:7. doi: 10.1186/1479-5876-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, Lambert J, Kupper TS. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flanagan K, Kaufman HL. Chemokines in tumor immunotherapy. Front Biosci. 2006;11:1024–1030. doi: 10.2741/1860. [DOI] [PubMed] [Google Scholar]
- 59.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 61.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, Bourhis JH, Reman O, Turlure P, Contentin N, de Revel T, Rousselot P, Preudhomme C, Bordessoule D, Fenaux P, Terre C, Michallet M, Dombret H, Chevret S, Castaigne S. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28:808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg SA. Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci USA. 2008;105:12643–12644. doi: 10.1073/pnas.0806877105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafer-Weaver K, Anderson M, Malyguine A, Hurwitz AA. T cell tolerance to tumors and cancer immunotherapy. Adv Exp Med Biol. 2007;601:357–368. doi: 10.1007/978-0-387-72005-0_38. [DOI] [PubMed] [Google Scholar]
- 64.Waithman J, Gebhardt T, Davey GM, Heath WR, Carbone FR. Cutting edge: Enhanced IL-2 signaling can convert self-specific T cell response from tolerance to autoimmunity. J Immunol. 2008;180:5789–5793. doi: 10.4049/jimmunol.180.9.5789. [DOI] [PubMed] [Google Scholar]
- 65.Brandmaier AG, Leitner WW, Ha SP, Sidney J, Restifo NP, Touloukian CE. High-avidity autoreactive CD4+ T cells induce host CTL, overcome T(regs) and mediate tumor destruction. J Immunother. 2009;32:677–688. doi: 10.1097/CJI.0b013e3181ab1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holcmann M, Stoitzner P, Drobits B, Luehrs P, Stingl G, Romani N, Maurer D, Sibilia M. Skin inflammation is not sufficient to break tolerance induced against a novel antigen. J Immunol. 2009;183:1133–1143. doi: 10.4049/jimmunol.0713351. [DOI] [PubMed] [Google Scholar]
- 68.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci USA. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Imiquimod does not cause graft rejection in the absence of lymphocytes. Rag1−/− mice were grafted with two K5mOVA skin grafts. 40 days later grafts were treated with either imiquimod or control cream. Grafts were monitored daily for rejection and plotted on a Kaplan-Meier survival curve. Data analysed by log rang test, n = 10, ns = not significant.
Rag1−/− mice received an intravenous transfer 106 Rag.OT-I splenocytes and were immunised with either Quil A alone (-Immunisation) or ovalbumin with Quil A (+Immunisation). Inguinal lymph node (LN), spleen, blood and liver were harvested 7 days post-transfer and immunisation and stained for expression of the memory T cell marker (CD44) and the lymph node homing marker (CD62L). Plots were gated on Vα2+ CD8+ cells. Numbers in each quadrant represents percent of Vα2+ CD8+ cells. Plots shown are representative of one of two experiments.