Abstract
Ventrolateral prefrontal cortex (VLPFC) has long been linked to language production, but the precise mechanisms are still being elucidated. Using neuropsychological case studies, we explored possible sub-specialization within this region for different linguistic and executive functions. Frontal patients with different lesion profiles completed two sequencing tasks, which were hypothesized to engage partially overlapping components. The multi-word priming task tested the sequencing of co-activated representations and the overriding of primed word orders. The sequence reproduction task tested the sequencing of co-activated representations, but did not employ a priming manipulation. We compared patients’ performance on the two tasks to that of healthy, age-matched controls. Results are partially consistent with an anterior-posterior gradient of cognitive control within lateral prefrontal cortex (Koechlin & Summerfield, 2007). However, we also found a stimulus-specific pattern, which suggests that sub-specialization might be contingent on type of representation as well as type of control signal. Isolating such components functionally and anatomically might lead to a better understanding of language production deficits in aphasia.
Keywords: language production, sequencing, cognitive control, prefrontal cortex, aphasia
Introduction
Language production involves the conversion of a message or thought into a string of words. This process may include several component processes, including the selection of appropriate words, the sequencing of those words in a grammatical order, and the programming of articulatory motor movements. The ventrolateral prefrontal cortex (VLPFC), which includes Broca’s area, has long been associated with language production. While the original hypothesis, traced back to Broca, tied this region to speech per se, more recent accounts have hypothesized that VLPFC is involved in higher-level cognitive and linguistic functions (see e.g., Cabeza & Nyberg, 2000). It is possible that different sub-regions within VLPFC support different sub-components that are involved in producing language. In this article, we explore the issue of sub-specialization from a neuropsychological perspective.
Previous neuroimaging and neuropsychological studies have suggested some possible subdivisions of prefrontal mechanisms for language. One dominant idea is that the posterior and dorsal part of VLPFC (Brodmann areas (BA) 44/6) might be responsible for processing phonological information and the anterior and ventral part (BA 47) for processing semantic information, with the intermediate region (BA 44/45) being responsible for processing syntax (Bookheimer, 2002). This is consistent with known connectivity patterns between posterior and frontal brain regions in the macaque monkey: a dorsal pathway from posterior temporal and inferior parietal regions - thought to be involved in phonological processing in humans - primarily targets posterior VLPFC while a ventral pathway from more anterior temporal regions – thought to be involved in semantic processing in humans – primarily targets anterior VLPFC (Hickok & Poeppel, 2004; Petrides & Pandya, 2009). Thus, it seems plausible that different parts of frontal cortex support higher-level cognitive processing of different kinds of linguistic representations that reside in temporal and parietal areas.
Direct evidence for the anterior-posterior semantic-phonological distinction in the frontal cortex of humans comes from a number of studies (Fiez, 1997; Hamilton, Martin, & Burton, 2010; McDermott, Petersen, Watson, & Ojemann, 2003; Poldrack et al., 1999). For example, McDermott et al. (2003) presented participants with 16-word lists and asked them to attend either to the meaning or the rhyme relations between words. Results showed preferential activation for the semantic condition in BA 47 and BA 44/45 and preferential activation for the phonological condition in frontal regions posterior to those found for the semantic condition (BA 44/6). Based on evidence from patients with brain damage, Martin and colleagues have suggested that semantic and phonological short-term memory (STM) components are separable both functionally and anatomically (Martin & He, 2004; Martin & Romani, 1994). A recent fMRI study from the same group showed increased activation in parts of VLPFC anterior to BA 44/6 when comparing high and low semantic STM load conditions (Hamilton et al., 2010). Analysis within a posterior BA 44 region of interest detected no such effect of semantic STM load, consistent with the hypothesis that this sub-region is not associated with semantic processing.
There is also evidence for anterior versus posterior semantics versus syntax parcellation within VLPFC. Dapretto and Bookheimer (1999) examined activation during a semantic condition where a same/different judgment between two sentences relied on the processing of word substitutions and that during a syntactic condition where the same/different judgment relied on the processing of syntactic alternations. The findings implicated BA 44 in syntactic processing and BA 47 in semantic processing (Dapretto & Bookheimer, 1999). Other fMRI studies have reported increased frontal activation for some syntactic structures over others, particularly in BA 44 (Caplan, Alpert, & Waters, 1998; Newman, Ikuta, & Burns, 2010). Such evidence is broadly consistent with specialization of posterior VLPFC for some sub-component of syntax, although the interpretation of the data with respect to the domain specificity or generality of the underlying mechanisms is still controversial (Novick, Trueswell, & Thompson-Schill, 2005).
In sum, evidence from language studies has suggested a gradient between semantic processing in anterior VLPFC and phonological processing in posterior VLPFC with syntactic processing somewhere in between. However, the functions of lateral PFC (LPFC), or subdivisions among them, are clearly not limited to the domain of language. There is general consensus that this region is involved in cognitive control or the coordination of thoughts and actions in accordance with goals. Koechlin and colleagues have proposed an influential “cascade model” with respect to the organization of cognitive control processes for selecting actions (Koechlin, Ody, & Kouneiher, 2003; Koechlin & Summerfield, 2007). Under this model, cognitive control involves at least three nested levels of processing whose anatomical loci are hypothesized to lie on a posterior-to-anterior axis within LPFC: lateral premotor areas support sensory control or the selection of motor actions in response to stimuli; posterior LPFC supports contextual control or the selection of motor actions that takes into account (immediate) contextual signals; and anterior LPFC supports episodic control or the selection of motor actions that takes into account the broader temporal episode. Put another way, the integration of more and more temporally distant events is thought to require the operation of more and more anterior portions of LPFC. Further, the processes are supposed to be hierarchically organized such that increased activation of more anterior regions modulates the activation of less anterior regions but not vice versa (Koechlin et al., 2003; Koechlin & Summerfield, 2007. See Badre and D’Esposito (2007) for a different hierarchical proposal).
It might be worth exploring whether these broad organizing principles that have been proposed for dorsolateral PFC and action selection can explain the observed subspecialization within ventrolateral PFC for different linguistic processes. For example, the processing of semantic and discourse information might require the integration of current input into a broader context (“episodic control” in the cascade model) while the processing of phonological or local syntactic information might require lower-level cognitive control (“sensory control” or “contextual control” in the cascade model). This could explain the localization of semantic processing to anterior PFC and phonological and syntactic processing to more posterior regions (Bornkessel-Schlesewsky, Grewe, & Schlesewsky, 2012).
Our motivation in exploring the sub-specialization issue was to better understand the language impairments in aphasia that follow damage to the frontal cortex. Although the relation between frontal damage and production impairments in aphasia is often taken for granted, the two do not always go together (Dick et al., 2001; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 1994). It is possible that different sub-components of language production rely on different neural substrates, leading to the inconclusive findings. Thus, our approach has been to isolate particular cognitive components in patients with focal frontal lesions.
In a preliminary study with aphasic patients, we reported that some frontal patients had difficulty in flexibly sequencing words (Thothathiri, Schwartz, & Thompson-Schill, 2010). We used a simple two-word picture-naming task that isolated sequencing from other sentence processing components such as verb retrieval and syntactic-semantic mappings. Post-hoc anatomical analysis tied a sub-region within LPFC, namely BA 44/6, to exaggerated interference when participants were primed with a noun in one phrasal position and then had to produce that same noun in the other phrasal position. Frontal patients who had BA 44/6 damage showed this pattern while others, whose lesions spared this sub-region, did not. We interpreted these data within a larger framework wherein lateral PFC supports cognitive control, particularly the biasing of competing representations during the selection of a single representation. Invoking this framework, Robinson and colleagues have suggested that the inability to select amongst verbal responses under conditions of high competition is central to what Luria termed “dynamic aphasia” (Luria, 1970; Robinson, Blair & Cipolotti, 1998; Robinson, Shallice & Cipolotti, 2005). Our more specific suggestion was that the BA 44/6 sub-region within PFC might be associated with sequencing or “selection for position” (Thothathiri et al., 2010). When multiple words or items are co-activated, selecting the right word or item at the right time might require an intact BA 44/6.
Other studies have also tied sequencing operations to the posterior-most region of VLPFC, adjoining and including the premotor cortex (Gelfand & Bookheimer, 2003; Grewe et al., 2006). For example, Gelfand and Bookheimer (2003) reported that the posterior part of Broca’s area was activated during sequence manipulation irrespective of whether the stimuli were phonemes or hummed notes. Within the domain of language, Bornkessel-Schlesewsky and colleagues have suggested a functional gradient within VLPFC for sequencing, wherein activation in posterior sub-portions correlates with sentence-internal or local aspects of sequencing and activation in anterior sub-portions is sensitive to the relation between the current sentence and the broader discourse (Bornkessel-Schlesewsky et al., 2012). This interpretation seems consistent with the cascade model described above. Sequencing or ordering different items with respect to one another (e.g., say X after Y) is a contextual process, which might be supported by posterior LPFC. Additionally, the modulation of sequencing by semantic or discourse constraints might require the recruitment of episodic control supported by anterior LPFC.
In the current study, we sought to establish the causal links between different subregions within LPFC and the different components that might be involved in producing a sequence of words. We chose patients with different lesion profiles primarily affecting the premotor cortex, posterior VLPFC or anterior VLPFC, and examined the impact of such lesions on two different sequencing tasks. In the multi-word priming task, participants were required to sequence two nouns during spoken production. On critical trials, we manipulated whether a primed noun was produced in the same position or a different position from the previous trials. In the sequence reproduction task, we manipulated sequencing demands not by a priming manipulation but by presenting the entire sequence all at once or item by item. Patients with different lesion profiles showed different patterns of deficits on the two tasks. Our results speak to the architecture of cognitive control in LPFC and the implications of this architecture for language processing in aphasic patients and neurotypical adults.
Patient Information
Selection Criteria
We recruited five patients with post-stroke aphasia from the Moss Rehabilitation Research Registry for whom clinical behavioral data and high quality brain scans were available from prior research participation. Our main selection criterion was lesion profile. Two of the patients had participated in the previous multi-word priming study (Thothathiri et al., 2010). Both had lesions to posterior VLPFC (BA 44/6). In addition, we recruited two other patients with VLPFC lesions. Because we wanted to examine the possible selectivity of BA 44/6 for sequencing mechanisms, we chose one patient who had damage to anterior VLPFC (BA 45/47) and not posterior VLPFC, and another patient who had extensive damage to BA 6 and a small lesion in BA 44. The fifth patient had damage to non-frontal areas only.
Our secondary selection criterion was high accuracy in single picture naming because we wanted to isolate patients’ difficulty in sequencing multiple words from other naming related difficulties. We chose patients with > 80 % accuracy on the Philadelphia Naming Test or PNT (see Standardized Behavioral Measures).
Imaging Methods
All scans were obtained at least 6 months post-onset and within 6 years of testing. Patients P1 and P3 underwent magnetic resonance imaging (MRI) scans (P1: 3 Tesla, T1-weighted, TR = 1620 ms, TE = 3.87 ms, FOV = 192×256 mm, slice thickness = 1 mm; P3: 1.5 Tesla, T1-weighted, TR = 3000 ms, TE = 3.54 ms, FOV = 24 cm, slice thickness = 1 mm). Their lesions were segmented manually on a 1×1×1 mm T1-weighted structural image, after which the scan and lesion were sequentially registered to a standard template (“Colin27”; Holmes et al., 1998) using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann, & Gee, 2006; see also http://www.picsl.upenn.edu/ANTS/). The final lesion map was quantized to produce a 0/1 map, using 0.5 as the cutoff value.
MRI was contra-indicated for the other three patients (P2, P4, P5). They underwent whole brain CT scans without contrast (60 axial slices, 3 mm thick) on a 64–Slice SOMATOM Sensation Scanner. Their lesions were drawn directly onto the common Colin27 volume, after rotating (pitch only) the template to approximate the slice plane of the patient’s scan. The lesions maps were all drawn or approved by an experienced neurologist, who had no knowledge of the behavioral data.
For each patient, total lesion volume and percent damage to several a priori ROIs were calculated using VoxBo (http://www.voxbo.org) and MRICron (http://www.sph.sc.edu/comd/rorden/mricron).
Anatomical Differences and Predictions
Table 1 shows the percent damage in selected Brodmann areas and a summary classification for each patient. Here and elsewhere, the frontal patients are presented in the order of posterior to anterior damage.
Table 1.
Patient lesion information
| P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|
| Summary Classification | Non-frontal | Premotor | Posterior LPFC | Posterior & Anterior LPFC | Anterior LPFC |
| Total lesion volume (cc) | 78.52 | 50.98 | 46.28 | 51.86 | 17.86 |
| Posterior VLPFC (%) | |||||
| BA 6 | 0 | 56.4 | 21 | 11.8 | 0 |
| BA 44 | 0 | 6.1 | 91.8 | 87.3 | 0 |
| Anterior VLPFC (%) | |||||
| BA 45 | 0 | 0 | 5 | 42.9 | 38.8 |
| BA 47 | 0 | 0.1 | 0.2 | 19 | 28.3 |
| DLPFC (%) | |||||
| BA 9 | 0 | 3.7 | 21.2 | 13.4 | 0 |
| BA 46 | 0 | 0 | 6.9 | 6.2 | 6.5 |
| Temporal/Parietal (%) | |||||
| BA 21 | 10.8 | 0 | 0 | 0 | 0 |
| BA 22 | 18.8 | 0 | 0 | 0 | 0 |
| BA 39 | 90.7 | 0 | 0 | 0 | 0 |
| BA 40 | 73 | 0 | 0 | 0 | 0 |
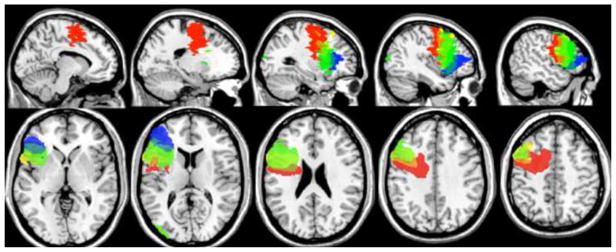
The differences between the frontal patients’ lesions are illustrated graphically in Figure 1. P2 (shown in red) has additional damage to medial BA 6 as well as posterior lateral BA 6 compared to all the other patients. This lesion, which includes the supplementary motor area, might lead to widespread impairments in this patient on any task that requires the planning and execution of a sequence of movements. P5 (shown in blue) has the anterior-most profile with a lesion that spared posterior LPFC and premotor areas entirely. Thus, this patient might be expected to be impaired only on tasks that require higher-level or “episodic” cognitive control. The two other patients, P3 (yellow) and P4 (green), have lesion profiles that are in between those of P2 and P5. They have damage to BA 44 in common. P3 has additional damage to the junction of BA 44/6 compared to P4 while P4 has additional damage to BA 45/47 compared to P3. The relatively more posterior profile in P3 might lead to wider impairments, even on tasks that require sensory or contextual control only. In contrast, P4, who has a combination of posterior and anterior LPFC damage, might show the strongest impairments in higher-level cognitive control tasks that recruit anterior LPFC and cascade down to posterior LPFC. She might also show milder impairments on tasks that only involve lower-level cognitive control.
Figure 1.
Red = P2; Yellow = P3; Green = P4; Blue = P5; Orange = overlap between P2 and P3; Yellow+Green = overlap between P3 and P4; Blue+Green = overlap between P4 and P5. Top: from left to right, medial to lateral sagittal slices at MNI X coordinates = −10, −20, −30, −40 and −50. Bottom: from left to right, ventral to dorsal axial slices at MNI Z coordinates = 0, 12, 24, 36 and 48.
Standardized Behavioral Measures
Table 2 shows a subset of the behavioral measures collected from the patients as part of a standardized battery at the Moss Rehabilitation Research Institute. All patients were classified as anomic based on the Western Aphasia Battery (WAB. Kertesz, 1982). Their WAB fluency scores were 8 or 9 out of a maximum of 10. Accuracy on the Philadelphia Naming Test (PNT. http://www.ncrrn.org; Roach, Schwartz, Martin, Grewal & Brecher, 1996) ranged between 82–97% suggesting that the patients were quite accurate at naming single pictures.
Table 2.
Patient standardized behavioral measures
| Controls | Patient cohort | P1 | P2 | P3 | P4 | P5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||||||
| WAB Fluency | 107 | 6.65 | 2.61 | 9 | 9 | 8 | 9 | 8 | |||
| PNT | 21 | 97.2 | 2.67 | 107 | 64 | 29 | 97 | 93 | 95 | 91 | 82 |
| Category probe span | 21 | 5.39 | 1.28 | 107 | 2.18 | 1.28 | 2.83 | 4.29 | 3.2 | 3.5 | 2.45 |
| Rhyme probe span | 21 | 6.45 | 1.57 | 107 | 2. 85 | 1.69 | 4.35 | 2.38 | 6.27 | 5 | 6.55 |
Table 2 also shows two different short-term memory (STM) span scores (Freedman & Martin, 2001). The category probe span measures the list length for which participants can accurately judge whether any item from the list was in the same semantic category as the probe item. Thus, it is considered to be a measure of semantic STM (Freedman & Martin, 2001). Testing starts at list length 1 and proceeds to the next length if the participant scores better than 75% correct. The maximum score is 7. The rhyme probe span measures the list length for which participants can accurately judge whether the probe rhymes with one of the items in the list. Thus, it is considered to be a measure of phonological STM (Freedman & Martin, 2001). Testing starts at list length 1 and advances to the next length if the participant scores above 75% correct. The maximum score is 10.
Individual Patient Summary
Patient P1
P1 is a right-handed female, who was 58 years old and 118 months post onset at the time of testing. Her structural MRI revealed damage to temporal and parietal regions; she had no damage to LPFC (Table 1). P1 had high WAB fluency and PNT scores, and her category and rhyme probe spans were within 2 standard deviations (SDs) of the control mean (Table 2).
Patient P2
P2 is a right-handed male, who was 49 years old and 113 months post onset at the time of testing. His CT revealed cortical damage mainly limited to premotor areas, with some additional damage in the bordering region of posterior LPFC (Table 1). P2 had high WAB fluency and PNT scores, and a category probe span within 1 SD of the control mean. His rhyme probe span was more than 2 SDs from the control mean, suggesting possible impairment to phonological STM (Table 2).
Patient P3
P3 is a right-handed male, who was 72 years old and 24 months post onset at the time of testing. His structural MRI revealed damage that was largely confined to posterior LPFC (Table 1). P3 had high WAB fluency and PNT scores, and his category and rhyme probe spans were within 2 SDs of the control mean (Table 2).
Patient P4
P4 is a right-handed female, who was 41 years old and 88 months post onset at the time of testing. Her CT revealed damage to posterior and anterior LPFC (Table 1). P4 had high WAB fluency and PNT scores, and her category and rhyme probe spans were within 2 SDs of the control mean (Table 2).
Patient P5
P5 is a right-handed female, who was 72 years old and 20 months post onset at the time of testing. Her CT revealed damage to anterior but not posterior LPFC (Table 1). P5 had high WAB fluency and PNT scores, and a rhyme probe span that was within 1 SD of the control mean. Her category probe span was more than 2SDs from the control mean, suggesting possible impairment to semantic STM (Table 2).
Multi-word Priming Experiment
Participants
In addition to the five patients, seven healthy controls (4 female. Mean age = 55.9 years) participated. All were native English speakers from the Philadelphia area, right-handed, scored 26 or higher on the Mini-mental State Examination (MMSE), and reported no history of neurodegenerative disease, learning disorders, or ADHD.
Stimuli
We used 40 color pictures adapted from Snodgrass & Vanderwart’s line drawings (Rossion & Pourtois, 2004). The 40 items belonged to eight semantic categories (see Appendix). All had name agreement >90% (Snodgrass & Vanderwart, 1980).
Appendix.
| Edible things | Clothing | Kitchen things | Animals | Toys/Games | Body parts | Hardware/Tools | Misc. |
|---|---|---|---|---|---|---|---|
| apple | belt | bottle | cat | football | ear | ladder | cloud |
| carrot | hat | spoon | fish | kite | heart | chain | star |
| lemon | ring | cup | owl | swing | nose | ruler | candle |
| onion | sock | knife | snake | bell | eye | pen | bus |
| grapes | shoe | chair | zebra | drum | hand | saw | pipe |
Procedure
We employed a modified version of the multi-word naming task reported in Thothathiri, et al. (2010). On each trial, participants were presented with two pictures appearing side by side. They were instructed to name the pictures from left to right using the phrase “the x and the y”. The two pictures were enclosed together in a box to promote concurrent planning of both names. Unlike the previous version (Thothathiri et al., 2010), the pictures stayed on the screen until the experimenter pressed a key to move to the next trial. Thus, working memory demands were minimal.
Each participant completed four blocks of 80 trials each, spread across two days (two blocks per day). Each block consisted of 20 filler trials and 20 experimental triads (60 trials) in a fixed randomized order. Within a triad, one noun was repeated in three consecutive trials. In the first two trials of the triad (primes), the repeated noun appeared in the same position (e.g., the apple and the cup followed by the apple and the shoe). In the third trial of the triad (target), the position of the repeated noun was either consistent (e.g., the apple and the star) or inconsistent with the primes (e.g., the star and the apple). Each block contained 10 “consistent” and 10 “inconsistent” targets. Half the trials in each type contained the repeated noun in the first position, half in the second position.
Each noun appeared the same number of times within a given experimental block. No two nouns within a trial belonged to the same semantic category, or had the same phonological onset or rhyme. This was done to minimize semantic and phonological interference.
In each testing session, participants were first familiarized with the pictures and their expected names. We presented pictures in isolation and provided feedback if the participant could not respond or used a different name. Subsequently, participants practiced the multi-word naming task. Once we were confident that they understood the task, we administered the actual experiment.
Dependent Measure
We used the same dependent measure as in Thothathiri, et al. (2010). Onset latencies were measured from the onset of the beep at the beginning of the trial to the onset of the first spoken word. We excluded trials where one or both nouns were missing or wrong, or there were extraneous phonemes or major disfluencies. We also excluded trials where latencies were more than 2 SDs from each participant’s average filler latency. For each participant, we computed a priming interference percent score, which was the difference in mean onset latency on inconsistent minus consistent trials, expressed as a percentage of the participant’s mean latency on filler trials.
Results and Discussion
Table 3 shows aggregate behavioral measures for the controls and individual behavior measures for the patients. Patients showed longer latencies and/or made more errors compared to controls. Four out of the five patients made more errors during inconsistent compared to consistent targets (Table 3: shown as a percent of all trials of a given type). This difference appeared strongest in P4 and P5.
Table 3.
Behavioral measures from the multi-word priming task
| Measure | Controls Mean (SD) |
P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Errors | |||||||
| Total % | 2.86 (1.74) | 3.44 | 18.75 | 4.72 | 15.63 | 19.06 | |
| Consistent % | 1.43 (1.34) | 5 | 22.5 | 2.5 | 15 | 10 | |
| Inconsistent % | 1.79 (1.89) | 7.5 | 22.5 | 5.13 | 22.5 | 17.5 | |
| Difference % | 0.36 (1.73) | 2.5 | 0 | 2.63 | 7.5 | 7.5 | |
|
| |||||||
| Filler RT | 852.68 (160.94) | 1009.67 | 1420.74 | 1871.31 | 1951.50 | 883.37 | |
|
| |||||||
| Consistent RT | 785.55 (111.40) | 1013.06 | 1233.79 | 1421.62 | 1404.62 | 766.66 | |
|
| |||||||
| Inconsistent RT | 827.88 (152.98) | 1048.39 | 1582.97 | 1855.36 | 2121.93 | 921.33 | |
|
| |||||||
| Priming Interference % | 4.36 (5.43) | 3.50 | 24.58 | 23.18 | 36.76 | 17.51 | |
Our main dependent measure was the priming interference percent score calculated from correct trials, which was normalized by filler latencies because participants who have longer latencies often tend to show larger effects. On average, controls were 4.36% slower on inconsistent compared to consistent target trials. This effect was marginally significant [t(6)=2.13, p=.08]. Non-frontal patient P1’s interference score (3.5%) was comparable to that of the controls, while the scores of the four frontal patients (17.5–36.8%) were much higher (Table 3).
We used a modified t-test to evaluate whether each patient showed significantly larger interference relative to controls (Crawford & Howell, 1998). Table 4 shows the results of this test as well as the effect sizes for each patient (Crawford, Garthwaite, & Porter, 2010). As can be seen, P1, whose lesion was restricted to non-LPFC regions, did not show significantly larger interference compared to controls. In the frontal patients, in the order of more posterior to more anterior lesion profiles: P2, who had damage mainly to premotor areas, and P3, who had damage mainly to posterior LPFC, both showed significantly larger interference scores relative to controls. Their percent scores were more than 3 SDs from the control mean. P4 who had damage to posterior as well as anterior LPFC showed an even larger effect, which was more than 5 SDs from the control mean. The patient with the most anterior LPFC profile (P5) showed a marginally significant effect. Her interference score was between 2 and 3 SDs from the mean for controls.
Table 4.
Inferential statistics and effect sizes for the multi-word priming task
| Controls | Patient | Score | Sig. test | Est. effect size (z score) | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | t | p | Point | 95% CI | ||
| 7 | 4.36 | 5.43 | P1 | 3.5 | −0.15 | .89 | −0.16 | −0.9 to 0.6 |
| P2 | 24.58 | 3.48 | <.05 | 3.72 | 1.5 to 5.9 | |||
| P3 | 23.18 | 3.24 | <.05 | 3.47 | 1.4 to 5.5 | |||
| P4 | 36.76 | 5.58 | <.05 | 5.97 | 2.6 to 9.3 | |||
| P5 | 17.51 | 2.27 | .06 | 2.42 | 0.9 to 3.9 | |||
The results of the multi-word priming experiment are consistent with the known role for LPFC in cognitive control. The task presumably tapped cognitive control at multiple levels, including sensory control related to response selection (say “apple” or “star”), contextual control related to sequencing (say “apple and star”), and episodic control related to the priming manipulation (override recently primed order if necessary and name pictures from left to right). We found that while the non-frontal patient was unimpaired relative to control participants, patients with frontal cortex damage showed larger interference scores. This was most evident in the three patients with some posterior LPFC damage but a weak effect was also seen in the patient whose lesion was restricted to anterior LPFC. As described above, these impairments could have arisen at multiple levels of the proposed cognitive control hierarchy. To tease apart the different contributions of the different LPFC sub-regions, we tested the four frontal patients on an alternative sequence manipulation task. This task did not use the priming manipulation and presumably required no episodic control. Based on the cascade model, we predicted that the three patients with premotor and/or posterior LPFC damage (P2, P3, P4) would be impaired in this task and that the anterior LPFC patient (P5) would not.
Sequence Reproduction Experiment
In this experiment, we manipulated sequencing-related cognitive control demands by presenting the items to be sequenced all at once rather than one by one. Presenting items simultaneously rather than sequentially might result in the planning of multiple responses at once. This planning ahead is expected to have a “cost”, in the form of slowed sequence initiation times relative to the sequential condition. However, prior planning should also result in a future “benefit”, in the form of speeded up responses to subsequent items of the sequence. In a previous study, Lepage and Richer (1996) reported such a pattern of slower reaction time for the first item and faster reaction times for subsequent items in healthy control participants. In comparison, patients with heterogeneous left and right frontal lesions showed markedly slower reaction times for the first item but no speeding up of subsequent responses (Lepage & Richer, 1996).
We hypothesized that cognitive control might be necessary to resolve interference amongst multiple responses during the simultaneous presentation condition. The effectiveness of this interference resolution process may be measured by the “net cost” of planning items together, which is the sum of the slowing down of the first response and the speeding up of subsequent responses. The less efficient or successful the interference resolution, the larger the net cost. Based on the cascade model, we predicted that patients with lesions in posterior LPFC and premotor cortex would show abnormal net costs relative to controls.
Participants
In addition to the four frontal patients, seven healthy controls (4 female. Mean age = 58.14 years) participated. All were native English speakers from the Philadelphia area, right-handed, scored 27 or higher on the Mini-mental State Examination (MMSE), and reported no history of neurodegenerative disease, learning disorders, or ADHD. Five out of the seven controls had also participated in the multi-word priming experiment.
Stimuli & Procedure
Participants used a keyboard to reproduce four-item sequences displayed on a computer screen. The sequences consisted of letters (A, B, C) or color squares (red, yellow, blue). For each stimulus type, the three unique items were mapped to the j, k and l keys respectively. Participants were asked to use their left hand, with the ring finger on j, middle finger on k and index finger on l. All sequences appeared centered on the screen against a black background. Letters were capitalized and in white, bold, 40 pt. Arial font; colors were 1.75″ (width) by 1.4″ (height) rectangles. Inter-trial interval was 3 seconds, during which a fixation cross appeared centered on the screen.
Items within the sequences appeared either sequentially or simultaneously. In the sequential condition, items in the sequence appeared one at a time. When participants entered a response to item 1, item 2 appeared to the right of item 1 (which remained on the screen), and so on. The trial ended after four responses were made. In the simultaneous condition, all four items appeared together at the beginning of the trial and remained on the screen until the participant made four key presses and the trial ended. For both conditions, we instructed participants to replicate the sequence from left to right as accurately and as quickly as possible.
For each stimulus type, we constructed 18 four-item sequences from the three unique items (e.g., ABCA, BABC) with the constraint that immediate repetition of an item (e.g., ABBC) was not allowed. Each item appeared at least once and possibly twice in a sequence.
Participants completed 50 trials in each of the 4 experimental conditions – letters presented sequentially, letters presented simultaneously, colors presented sequentially, and colors presented simultaneously. Each of the 18 possible sequences appeared between two and four times within a set of 50 trials. Testing with letters and colors was done on different days, with at least 2 days in between. For a given stimulus type, there were 50 trials each in the sequential and simultaneous conditions. These were administered in two blocks of 25 trials for a total of 4 blocks. We presented the four blocks in an ABBA order e.g., Sequential-Block 1, Simultaneous-Block 1, Simultaneous-Block 2, Sequential-Block 2. Which block and condition was presented first was counterbalanced across participants. For a given participant, this order remained constant across stimulus types.
Each testing session began with 25 mapping-practice trials where single items were presented to allow participants to practice mapping a visual stimulus to the appropriate key. Subsequently, participants completed at least 5 sequencing-practice trials before each sequential or simultaneous condition block. We allowed participants to practice as many times as necessary until they felt comfortable with the task.
Dependent Measure
Participants’ responses and reaction times were recorded using E-prime. Trials containing one or more errors were eliminated from the analysis. We also excluded trials with RTs greater than 2 SDs from the participant’s mean for a given item and condition.
For each participant, we computed an interference score, which was the net cost for planning multiple responses at once. We restricted our analysis to the first two items in order to eliminate the possibility of performance differences between patients due to differences in STM. All patients studied had phonological and semantic STM spans greater than 2 (Table 2). The formula for the interference score was as follows:
The first parenthetic term in the numerator represents the possible cost incurred due to the sequencing of multiple items and the planning of multiple responses. The second parenthetic term in the numerator represents the possible benefit obtained from prior sequencing and planning. The sum of these two terms represents the net cost for planning multiple responses together. In order to account for baseline latency differences, we normalized this net cost by each participant’s mean latency to respond to the first two items in the sequential presentation condition.
Results and Discussion
Controls were highly accurate on the task. Mean error rates in the four conditions were as follows: letters-sequential = 1.14% (SD=1.57), letters-simultaneous = 2.86% (SD=3.02), colors-sequential = 2.00% (SD=2.31) and colors-simultaneous = 1.43% (SD = 1.51). The error rates for the patients in the four conditions were as follows: letters-sequential (P2=2%, P3=10%, P4=2%, P5=0%); letters-simultaneous (P2=2%, P3=4%, P4=4%, P5=0%); colors-sequential (P2=6%, P3=8%, P4=10%, P5=0%); and colors-simultaneous (P2=18%, P3=6%, P4=8%, P5=2%). Thus, with the possible exception of P2 in the colors-simultaneous condition, patients were also reasonably accurate on the task.
We excluded trials containing errors from our analysis of response latencies. In addition, we excluded outlier trials where response latencies were more than 2 SDs away from each participant’s mean per condition and item. For controls, this eliminated an average of 16–18% of the trials in the different experimental conditions. Similar percentages of trials were excluded for the same reason in the patients (P2: 12–20%; P3: 14–20%; P4: 14–18%; P5: 12–22%).
The raw response latencies for each patient and the corresponding means for the controls are shown in Table 5 and depicted visually in Figures 2 and 3. Figure 2 shows the response latencies for sequencing letters when they were presented sequentially versus simultaneously. Qualitatively, most patients showed a similar pattern to controls: when comparing the simultaneous to the sequential condition, latencies for item 1 were longer (presumably due to the planning of multiple responses) and latencies for subsequent items were shorter (presumably a benefit from planning ahead). P2 clearly looks like an exception with a large cost on item 1 but little to no benefit for subsequent items. It is possible that other patients have more subtle impairments that might become evident in our quantitative analyses. Figure 3 shows the response latencies for sequencing colors when they were presented sequentially versus simultaneously. The pattern looks similar to that described above for letters.
Table 5.
Raw response latencies in the sequence reproduction task
| Condition | tem | Controls Mean | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sequential - Letters | 795.69 | 958.86 | 1023.17 | 866.9 | 930.86 | ||
| 624.89 | 986.44 | 829.92 | 827.56 | 813.17 | |||
| 620.4 | 954.7 | 798.22 | 841.46 | 799.95 | |||
| 651.93 | 1071.23 | 890.5 | 881.46 | 903.79 | |||
|
| |||||||
| Sequential - Colors | 890.54 | 1079.9 | 1248.53 | 977.64 | 951.6 | ||
| 684.12 | 964.76 | 800.08 | 977.06 | 826.15 | |||
| 662.73 | 961.95 | 802.45 | 832.69 | 737.35 | |||
| 704.52 | 1025.07 | 877.16 | 940.83 | 893.55 | |||
|
| |||||||
| Simultaneous - Letters | 999.43 | 1288.69 | 1537.73 | 1149.22 | 1038.92 | ||
| 393.46 | 975.26 | 567.07 | 686.54 | 608.9 | |||
| 430.2 | 994.97 | 576.29 | 695.8 | 583.92 | |||
| 424.87 | 1013.9 | 616.41 | 477.39 | 646.15 | |||
|
| |||||||
| Simultaneous - Colors | 1180.19 | 1488.58 | 1542.73 | 1261.44 | 1211.12 | ||
| 476.99 | 896.97 | 557.41 | 775.05 | 755.65 | |||
| 478.28 | 1028.32 | 611.41 | 829.05 | 751.74 | |||
| 467.91 | 1046.61 | 622.14 | 661.03 | 762.77 | |||
Figure 2.
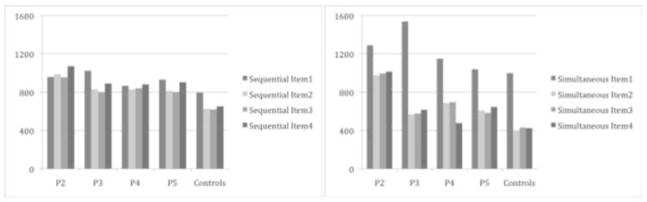
Response latencies in the sequential and simultaneous conditions for the sequencing of letters
Figure 3.
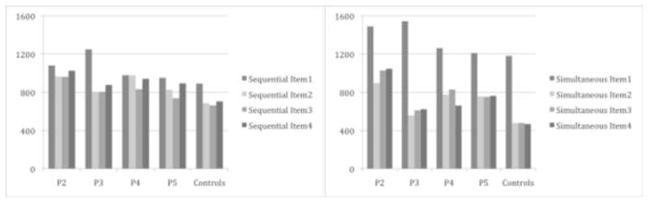
Response latencies in the sequential and simultaneous conditions for the sequencing of colors
Our main dependent measure was the normalized net cost or interference score, which was computed as the difference between simultaneous and sequential conditions for items 1 and 2 as a percent of the average latency for those items in the sequential condition. Table 6 shows the mean and standard deviation of the interference score for controls, separately for each stimulus type, along with the corresponding individual scores for patients. Controls showed significantly higher interference during the sequencing of colors compared to letters [t(6)=2.84, p<.04]. In the patient group, only the anterior LPFC patient (P5) showed a similar pattern, where the interference score was much larger for colors compared to letters. P3 and P4, who had posterior LPFC and/or anterior LPFC damage, showed the opposite pattern: they had larger interference scores for letter than for color stimuli. P2, who had a predominantly premotor lesion, showed heightened interference for both stimulus types.
Table 6.
Interference percent scores for the sequence reproduction task
| Stimulus Type | Controls Mean (SD) | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
|
| |||||
| Letters | −3.74 (11.61) | 32.76 | 27.17 | 16.68 | −11.03 |
| Colors | 10.71 (7.38) | 33.34 | 5.03 | 8.37 | 21.27 |
We evaluated these qualitative patterns using a modified t-test (Crawford et al., 2010; Crawford & Howell, 1998). Inferential statistics and effect sizes are shown in Table 7. P5, who had the anterior-most lesion profile, did not differ from the controls on either stimulus type. P3 had significantly higher interference scores than controls for the sequencing of letters but not colors. P4 had a higher interference score in the letter task, but the difference from controls did not reach significance for either stimulus type. Finally, P2, who had the posterior-most lesion profile, had significantly higher interference scores than controls for both stimulus types. In each case, his score was more than 3 SDs from the control mean.
Table 7.
Inferential statistics and effect sizes for the sequence reproduction task
| Stimulus Type | Controls | Sig. test | Est. effect size (z score) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Mean | SD | Patient | Score | t | p | Point | 95% CI | |
|
| |||||||||
| Letters | 7 | −3.74 | 11.61 | P2 | 32.76 | 2.94 | <.05 | 3.14 | 1.3 to 5.0 |
| P3 | 27.17 | 2.49 | <.05 | 2.66 | 1.0 to 4.3 | ||||
| P4 | 16.68 | 1.65 | .15 | 1.76 | 0.5 to 3.0 | ||||
| P5 | −11.03 | −0.59 | .58 | −0.63 | −1.4 to 0.2 | ||||
|
| |||||||||
| Colors | 7 | 10.71 | 7.38 | P2 | 33.34 | 2.87 | <.05 | 3.07 | 1.2 to 4.9 |
| P3 | 5.03 | −0.72 | .50 | −0.77 | −1.6 to 0.1 | ||||
| P4 | 8.37 | −0.30 | .78 | −0.32 | −1.1 to 0.5 | ||||
| P5 | 21.27 | 1.34 | .23 | 1.43 | 0.3 to 2.5 | ||||
We tested whether P3 exhibited a classic dissociation by comparing the difference between his letter and color interference scores against the difference for the control sample (Revised Standardized Difference Test (RSDT): Crawford et al., 2010). The difference between the patient’s standardized scores for the two stimulus types was marginally significant [t(6)=2.08, p=.08] suggesting a qualitative difference between his performance in sequencing letters versus colors.
The results from the sequence reproduction task are partially consistent with the predictions of the cascade model. As predicted, P5, whose lesion was restricted to anterior LPFC and spared premotor and posterior LPFC areas, did not show impairment relative to controls, for either stimulus type. This is consistent with the proposal that anterior LPFC is not necessary for lower-level cognitive control that does not require the integration of episodic information, as in the case of the sequence reproduction task. Also as predicted, P2, who had premotor and some posterior LPFC damage, showed heightened interference compared to controls, for both stimulus types. This is consistent with the idea that posterior areas within the frontal cortex support non-episodic cognitive control. Together, the pattern of results for P5 and P2 offer support for an anterior-posterior gradient of cognitive control within LPFC. However, the results for the other two patients - particularly P3 - are problematic for a simple version of this proposed gradient, where sub-specialization within the prefrontal cortex is contingent solely on the type of cognitive control but does not depend on the type of stimulus. P3 showed significantly higher interference scores than controls for letters but not for colors (note however that the difference between P3’s scores for the two stimulus types was only marginally significant). P4 showed a qualitatively similar pattern of worse performance with letters. This apparent stimulus-specific pattern cannot be explained as an artifact of task difficulty i.e., that the letter task was harder than the color task, because controls showed the opposite pattern of higher interference for colors than letters.
The pattern of results in P3 (and P4) raises the possibility of a stimulus-specific gradient within VLPFC, in addition to the proposed gradient based on the type of control signal (Koechlin & Summerfield, 2007). In order to evaluate whether this stimulus-specific gradient was linguistic versus non-linguistic or something else, we tested all participants on the sequencing of color names. Participants saw sequences of “red”, “yellow” and “blue” presented sequentially or simultaneously. As before, we computed an interference percent score for each participant. The mean score for the 7 control participants was −1% (SD=12.97). Similar to the case of letters and colors, P2 had a high score (37.5%), which was nearly 3 SDs away from the control mean. None of the other three patients showed such extreme scores (P3=17.31%, P4=6.26%, P5=−0.53%). In particular, P3, who had significantly higher interference than controls for letters, did not differ from controls on the color name version (p>.2). This suggests that the stimulus specificity might be tangential to or more fine-grained (e.g., non-semantic vs. semantic) than the linguistic-nonlinguistic distinction. However, a comparison of the difference between P3’s letter and color name interference scores against the difference for the control sample (RSDT: Crawford et al., 2010) revealed no significant difference between the two stimulus types [p>.4].
Conclusions, Caveats, and Future Directions
We explored the causal link between different prefrontal regions and cognitive control by studying the performance of patients with focal frontal lesions on two different sequencing tasks. Patient P2, who had extensive damage to BA 6, showed significantly higher interference scores than controls in multi-word priming as well sequence reproduction with letters, colors and color names. Patient P3, with mostly posterior LPFC damage, had significantly higher interference scores than controls in multi-word priming, and sequence reproduction with letters but not colors or color names. Patient P4, who had a lesion that encompassed both anterior and posterior LPFC, was significantly worse than controls in multi-word priming. She also showed higher interference during the sequencing of letters than colors, a pattern that was opposite to controls, but the results were not statistically significant. Finally, patient P5, whose lesion was restricted to anterior LPFC, showed a marginally significant effect in the multi-word priming task, and no effects in sequence reproduction for any stimulus type. Below, we discuss the implications of these results for the cascade model of cognitive control and language production in aphasia.
Our results show two clear patterns that are consistent with the a priori predictions of the cascade model. First, damage to anterior but not posterior LPFC affected performance only when the task required some episodic control. Patient P5 showed marginally higher interference scores than controls in the multi-word priming task, which required the overriding of recently primed orders, but no significant effects in the sequence reproduction task, which did not require integration across events. Second, widespread damage to BA 6 affected performance whenever the task required the planning and execution of multiple responses. Patient P2 showed higher interference scores than controls in multi-word priming and in the sequencing of letters, colors and color names. Together these results support a hierarchical model of cognitive control within the frontal cortex, wherein anterior regions subserve episodic control and posterior regions subserve sensory or contextual control.
In contrast to the above two results, a third pattern found in our study was unexpected. We had predicted that patients P3 and P4, both of whom had lesions in posterior LPFC, would be impaired in lower-level as well as higher-level cognitive tasks. Consistent with our predictions, patients P3 and P4 showed significantly higher interference effects than controls in the multi-word priming task. However, in the sequence reproduction task, we found an unexpected effect of stimulus type. Both patients showed numerically worse interference in letter compared to color sequencing, a pattern that was opposite to that in controls. Patient P3 had significantly higher interference scores than controls for letters but not for colors. In addition, the difference between his standardized letter and color interference scores was marginally significant. Thus, results from the sequence reproduction task suggest that some patients might be more impaired in the sequencing of some stimuli than others. This in turn suggests that a cascade model of cognitive control within VLPFC might have to be modified to take into account not just the type of control signal but also the type of representation.
What are the relevant differences between letter and color representations? One possibility is that the letter task was harder than the color task or required more executive control. For example, it could be the case that letter sequencing was subject to interference from more alternatives (other letters) or that mapping visual letters A, B and C to keyboard letters j, k and l led to more conflict. If this were true, some patients’ worse performance on letters could be explained as a consequence of task difficulty. Our data do not support this hypothesis because controls showed the opposite pattern, with significantly higher interference scores for colors than for letters. Based on this evidence, color sequencing was the harder task and the one expected to suffer the most if patients’ performance was driven by difficulty alone. Thus, the fact that some patients struggled with what is the easier task for controls, namely letters, calls for an alternate explanation.
A second possibility is that the distinction is one of linguistic versus non-linguistic stimuli. We evaluated this hypothesis by testing patients on the sequencing of color names. Patient P3, who showed exaggerated interference for the sequencing of letters, did not show significantly higher interference scores than controls for color names. However, a direct comparison between P3’s scores for the two types was not significant. Thus, the present results are suggestive but not conclusive. It is possible that the distinction between the stimulus types is more fine-grained than linguistic versus non-linguistic, but this needs to be confirmed in future studies.
One particular fine-grained account is that the relevant distinction is non-semantic versus semantic. Letters have less semantic content than colors or color names. As reviewed in the introduction, considerable evidence links posterior VLPFC to phonological and anterior VLPFC to semantic processing. Structural connectivity studies also tie posterior and anterior VLPFC to distinct dorsal and ventral pathways to temporo-parietal and temporal areas. Since letters have phonological but not (much) semantic content, this would explain why P3, whose lesion was primarily in posterior VLPFC, was impaired in the sequencing of letters but not colors or color names. Under this account, P3’s performance in the multi-word task, which involved sequencing words with semantic content, might be explained by the fact that the task required spoken language production and thus phonological sequencing. This line of reasoning is admittedly speculative. For one thing, the evidence for stimulus-specificity in the sequencing performance of P3 and P4 did not meet all tests for statistical reliability. A summary of predictions and findings (Table 8) shows that neither a subdivision model based solely on control signal nor one based just on type of representation can account for all of the observed results. Future research should clarify how subdivision based on levels of cognitive control may be reconciled with the possibility that anterior and posterior LPFC are connected to different temporal/parietal areas and modulate the processing of different kinds of linguistic representations.
Table 8.
Predictions of different subdivision models
| Task: Components | Subdivision based on | Patient | Predicted deficit? | Observed deficit? |
|---|---|---|---|---|
|
| ||||
| Multi-word: Episodic, contextual & sensory control Semantic, phonological and motor sequencing | Control signal | P2 | Yes | Yes |
| P3 | Yes | Yes | ||
| P4 | Yes | Yes | ||
| P5 | Yes | Yes | ||
|
| ||||
| Representation | P2 | Yes | Yes | |
| P3 | Yes | Yes | ||
| P4 | Yes | Yes | ||
| P5 | Yes | Yes | ||
|
| ||||
| Letters: Contextual and sensory control Phonological and motor sequencing | Control signal | P2 | Yes | Yes |
| P3 | Yes | Yes | ||
| P4 | Yes | No | ||
| P5 | No | No | ||
|
| ||||
| Representation | P2 | Yes | Yes | |
| P3 | Yes | Yes | ||
| P4 | Yes | No | ||
| P5 | No | No | ||
|
| ||||
| Colors: Contextual and sensory control Semantic and motor sequencing | Control signal | P2 | Yes | Yes |
| P3 | Yes | No | ||
| P4 | Yes | No | ||
| P5 | No | No | ||
|
| ||||
| Representation | P2 | Yes | Yes | |
| P3 | No | No | ||
| P4 | Yes | No | ||
| P5 | Yes | No | ||
|
| ||||
| Color names: Contextual and sensory control Semantic and motor sequencing | Control signal | P2 | Yes | Yes |
| P3 | Yes | No | ||
| P4 | Yes | No | ||
| P5 | No | No | ||
|
| ||||
| Representation | P2 | Yes | Yes | |
| P3 | No | No | ||
| P4 | Yes | No | ||
| P5 | Yes | No | ||
The results from the current study bear on understanding how deficits to different prefrontally mediated components might impact language production in aphasia. Damage to premotor cortex is expected to impact the resolution of response-level competition. As such, its effect on language production would be pervasive. The current study suggests that response competition may be increased by the need to counter a primed order of responses (as indicated by the multi-word priming results), but also by the simpler exigency of ordering co-activated responses (as indicated by the sequence reproduction results). Thus, patients with premotor lesions might be expected to have difficulty – in the form of increased latencies and/or errors – in producing any multi-word utterance.
In contrast, lesions in anterior LPFC would be expected to produce the most constrained production deficits. The current results suggest that patients with such lesions may not be impaired whenever multiple responses have to be sequenced but only when the expected order of responses is counter to a primed order. Thus, such patients may be predicted to have selective difficulty in the production of non-canonical sentence structures like passives or object-relatives.
We had previously suggested that patients with posterior VLPFC lesions might be impaired specifically in sequencing or selection for position (Thothathiri et al., 2010). Patients P3 and P4 were found to have deficits in the multi-word priming task despite good performance in producing single words. This hypothesis was consistent with other evidence linking posterior VLPFC (BA 44/6) to sequencing operations (Gelfand & Bookheimer, 2003; Grewe et al., 2006). In the current study, we disentangled the need for sequencing and the need to override primed orders by testing patients on a new sequence reproduction task. Results were surprising in that patient P3 showed a deficit in sequencing letters but not necessarily colors or color names. One possibility, mentioned above, is that the proposed sequencing mechanism supported by posterior LPFC might be specific to phonological representations. If this were correct, patients like P3 might be expected to have deficits in producing spoken multi-word utterances but not in ordering semantic representations using an alternate modality e.g., ordering pictures or written words. Note however that any such spoken production deficits are likely to be far subtler than those found in patients with speech apraxia or non-fluent, agrammatic aphasia. Lesions to areas other than posterior LPFC have been associated with speech articulation deficits (Dronkers, 1996). Not all patients with VLPFC damage demonstrate non-fluent aphasia (Dick et al., 2001). Patient P3 himself was anomic and had a high WAB fluency score. Thus, circumscribed lesions to posterior LPFC alone might give rise to subtle deficits (e.g., in response latencies) and not obvious disruptions during speech. Similarly, the multiword deficits observed in patients like P3 are subtler than those observed in patients with dynamic aphasia (Robinson et al., 1998). While the two types of patients might share deficits in selection amongst competing alternatives, only the latter group exhibits dramatically reduced spontaneous speech. Future studies should clarify the reasons for these differences.
The patients’ performance in the two sequencing tasks does not map in any obvious way to their semantic and phonological STM scores. For example, patient P3 showed exaggerated interference during the sequencing of letters, but his phonological STM was well within normal range (Table 2). Conversely, patient P5 has possibly impaired semantic STM but did not show heightened interference during the sequencing of color names (or other stimuli). Thus, the link between receptive span scores and the production of semantic or phonological sequences remains to be clarified.
We close with some caveats. The data reported in this paper come from four patients who comprise a select case series in that their lesions were small, focal, and distributed along an anterior-to-posterior axis within VLPFC. This select case series affords a rare opportunity to map the control functions of LPFC; but at the same time, the small number of cases limits the strength of conclusions. For one thing, the patients varied on dimensions other than lesion location. These include age, overall lesion volume, and lesion volume within any given brain region. The patients also may have differed in anatomical localization of functionally relevant cytoarchitectonic regions (Amunts, Schleicher, Burgel, Mohlberg, Uylings & Zilles, 1999) or in the manner or degree of recovery that might have resulted from anatomically identical lesions.
A comparison between patients P3 and P4 illustrates these issues. Both patients have damage to BA 44. P3 has additional damage to the junction of BA 44/6, while P4 has additional damage to BA 45/47. We have suggested that P4’s numerically worse performance than P3 in the multiword task could be explained by the additional damage to her anterior VLPFC (a region hypothesized to be important for the episodic control and semantic sequencing aspects of this task) while her numerically better performance than P3 in letter sequencing could be explained by the lower extent of damage to her posterior VLPFC (a region hypothesized to be important for the contextual control and phonological sequencing aspects of this task) (see “Anatomical Differences and Predictions” above.) However, it is equally plausible that the differences between these two patients were due to demographic factors (P4 was younger) or to variability in how the lesion to BA 44, which they shared, impacted behavior.
Caution is also warranted in interpreting the claims for subspecialization, which is likely to be relative rather than absolute. Recent neuroimaging results indicate that the hypothesized semantic-phonological distinction between anterior and posterior VLPFC is unlikely to be all or nothing. Increased activation is observed within anterior VLPFC (BA 45/47) for demanding semantic and phonological tasks, but there is relatively more activation for the former (Gold, Balota, Kirchhoff & Buckner, 2005). Similarly, posterior portions within VLPFC (BA 44 and BA 6) show increased activation for both semantic and phonological tasks relative to a baseline, but also differences between the two tasks (Gold et al., 2005). The sub-regions within VLPFC are part of a distributed network involving connections to one another as well as to posterior brain regions. Nevertheless, it is important to investigate possible relative subspecialization within VLPFC, which might help us to better understand the source of different patients’ linguistic and cognitive deficits.
In conclusion, our results show an overall pattern that is consistent with prior hypotheses: more posterior VLPFC damage leads to more pervasive deficits and possible specialization for phonological representations while more anterior VLPFC damage leads to more constrained deficits and possible specialization for semantic representations. As with all small and select case series, it is important to confirm these observations in a new set of patients.
Highlights.
Patients with different frontal lesions show different sequencing deficits
Partly consistent with anterior-posterior gradient of cognitive control
Stimulus-specific deficits suggest additional sub-specialization
Acknowledgments
We thank Branch Coslett, Olufunsho Faseyitan and Grant Walker for lesion information, Adelyn Brecher and Benedette D’Amore fore help in recruiting participants, and Jennifer Nilan for assistance in coding audio-taped data. This work was supported by grants 5-T32-HD-007425 and 5R01DC009209 to the University of Pennsylvania, and a Pennslyvania Formula Grant to Albert Einstein Healthcare Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: Cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10(3):397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual review of neuroscience. 2002;25(1):151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Grewe T, Schlesewsky M. Prominence vs. aboutness in sequencing: A functional distinction within the left inferior frontal gyrus. Brain and Language. 2012;120:96–107. doi: 10.1016/j.bandl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. Journal of Cognitive Neuroscience. 1998;10(4):541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH, Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology. 2010;27(3):245–260. doi: 10.1080/02643294.2010.513967. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12(4):482–486. [Google Scholar]
- Dapretto M, Bookheimer SY. Form and Content:: Dissociating Syntax and Semantics in Sentence Comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman JA, Dronkers N, Gernsbacher MA. Language deficits, localization, and grammar: Evidence for a distributive model of language breakdown in aphasic patients and neurologically intact individuals. Psychological Review. 2001;108(4):759. doi: 10.1037/0033-295x.108.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. A reconsideration of the brain areas involved in the disruption of morphosyntactic comprehension. Brain and Language. 1994;47(3):461–463. [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5(2):79–83. [PubMed] [Google Scholar]
- Freedman ML, Martin RC. Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology. 2001;18(3):193–226. doi: 10.1080/02643290126002. [DOI] [PubMed] [Google Scholar]
- Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38(5):831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from fMRI adaptation. Cerebral Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Grewe T, Bornkessel I, Zysset S, Wiese R, Yves von Cramon D, Schlesewsky M. Linguistic prominence and Broca’s area: the influence of animacy as a linearization principle. NeuroImage. 2006;32(3):1395–1402. doi: 10.1016/j.neuroimage.2006.04.213. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Martin R, Burton P. Converging functional magnetic resonance imaging evidence for a role of the left inferior frontal lobe in semantic retention during language comprehension. Cognitive Neuropsychology. 2010;26(8):685–704. doi: 10.1080/02643291003665688. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery test manual. Psychological Corp; 1982. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in cognitive sciences. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Lepage M, Richer F. Inter-response interference contributes to the sequencing deficit in frontal lobe lesions. Brain. 1996;119(4):1289. doi: 10.1093/brain/119.4.1289. [DOI] [PubMed] [Google Scholar]
- Luria AR. Traumatic aphasia. The Hague; Mouton: 1970. [Google Scholar]
- Martin RC, He T. Semantic short-term memory and its role in sentence processing: A replication. Brain and Language. 2004;89(1):76–82. doi: 10.1016/S0093-934X(03)00300-6. [DOI] [PubMed] [Google Scholar]
- Martin RC, Romani C. Verbal working memory and sentence comprehension: A multiple-components view. Neuropsychology. 1994;8(4):506. [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41(3):293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Newman SD, Ikuta T, Burns T. The Effect of Semantic Relatedness on Syntactic Analysis: An fMRI Study. Brain and Language. 2010;113(2):51–58. doi: 10.1016/j.bandl.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(3):263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biology. 2009;7(8):e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional Specialization for Semantic and Phonological Processing in the Left Inferior Prefrontal Cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: scoring and rationale. Clinical Aphasiology. 1996;24:121–33. [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cognitive Neuropsychology. 2005;22:661–694. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of experimental psychology: Human learning and memory. 1980;6(2):174. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Thothathiri M, Schwartz MF, Thompson-Schill SL. Selection for position: The role of left ventrolateral prefrontal cortex in sequencing language. Brain and Language. 2010;113(1):28–38. doi: 10.1016/j.bandl.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]