Abstract
Intensive scientific efforts in the past decades have helped shed light into the pathogenesis of endotoxin-induced inflammation. We have used multiplexing beadbased assays to characterize the responses in two models of in vivo LPS challenge. C57BL/6 mice were either injected intraperitoneally (endotoxemia) or intratracheally (acute lung injury; ALI) with lipopolysaccharide (LPS). The time courses (1h-24h) of the following 20 inflammatory mediators in plasma or broncho-alveolar lavages were simultaneously analyzed: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-12(p40), IL-13, Eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5) and TNF-α. While significant inductions of all mediators were found, substantial differences in their absolute concentrations, time points of maximal concentrations and clearances were observed. There were also notable variations in the patterns of several cytokines/chemokines when samples from endotoxemia and LPS-ALI were compared. These data may be helpful in defining analytic strategies including selection of optimal time points for studying the host immune response to endotoxin.
Keywords: Endotoxemia, shock, LPS, acute lung injury, bead-based assay
Introduction
The clinical picture (“sepsis”) of overwhelming infections leading to multi-organ failure and death was already known to physicians from ancient Greece more than 2,000 years ago. It took until the second half of the nineteenth century before Robert Koch, Louis Pasteur and others clearly proved that microbes account for infections (Beutler and Rietschel, 2003; Sakula, 1985).
The modern concept of “endotoxin” was propelled by work of Richard Pfeiffer (1858–1945) who discovered that the toxicity of Vibrio cholera was not dependent on its viability (Beutler and Rietschel, 2003; Pfeiffer, 1892). Subsequently, Pfeiffer identified a heat-stable toxic substance (endotoxin) that was an integral part of bacterial cell walls. Much later this endotoxin was chemically defined to be composed of lipid and polysaccharide components (lipopolysaccharides) (Raetz and Whitfield, 2002). Injection of LPS into laboratory animals or human volunteers was early known to cause typical signs of septic shock (fever, hypotension, tachycardia, consumptive coagulopathy) (Copeland et al., 2005; Suffredini et al., 1989). Tumor necrosis factor-α (TNFα) was found to be critically involved in the pathogenesis of the host responses to endotoxin (Michie et al., 1988).
It became clear that an LPS-binding protein (LBP) and the non-signaling membrane molecule, CD14, serve important functions in molecular recognition of LPS (Tobias et al., 1986; Wright et al., 1990). In 1998 the TLR4 receptor was cloned as the signaling receptor of LPS and point mutations in TLR4 rendered mice highly resistant to endotoxin (Poltorak et al., 1998). Today, TLR4 is appreciated as an important member of the pattern recognition receptors (PRRs) family of receptors and crucial to orchestrate innate immunity. Despite major advances in the field of endotoxin research in past decades, the complexity of the related molecular mechanisms remains to be fully understood.
Several hundred proteins with immune functions are regulated in response to endotoxin, thereby generating an urgent need for better analytic methodologies. In this report we take advantage of the use of multiplexing bead-based assays to profile mediator responses following activation of TLR4 by LPS in mice.
Materials and Methods
Mice
10–12 week old male C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA) and housed under pathogen-free conditions. All animal procedures were approved by the University Committee on Use and Care of Animals, University of Michigan in accordance with the U.S. National Institutes of Health guidelines.
Endotoxemia
Mice were injected i.p. with LPS (10 mg/kg bodyweight) from E. coli (O111:B4, Sigma-Aldrich, St. Louis, MO, USA) and EDTA-Plasma was collected and processed as described before (Bosmann et al., 2011).
LPS-induced acute lung injury
Mice were anaesthetized (ketamine/xylazine), 40 μg of LPS from E. coli (O111:B4, Sigma-Aldrich) slowly injected into the trachea. Broncho-alveolar lavage fluids were obtained at various time points as described elsewhere (Bosmann et al., 2012a).
Analysis of Mediators
The xMAP™/Bioplex-200 System was used for simultaneous quantification of mouse inflammatory mediators as described earlier (Bosmann et al., 2011). The analysis included the following cytokines and chemokines (Bioplex Pro mouse cytokine assay, BioRad, USA): IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-12(p40), IL-13, Eotaxin (CCL11), G-CSF, GM-CSF, IFN-γ, KC (CXCL1), MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5) and TNF-α.
Statistical analysis
All values are expressed as mean and error bars represent SEM. Data sets were analyzed by Student t test (GraphPad Prism 5.04 Software) and considered significant when P<0.05.
Results and Discussion
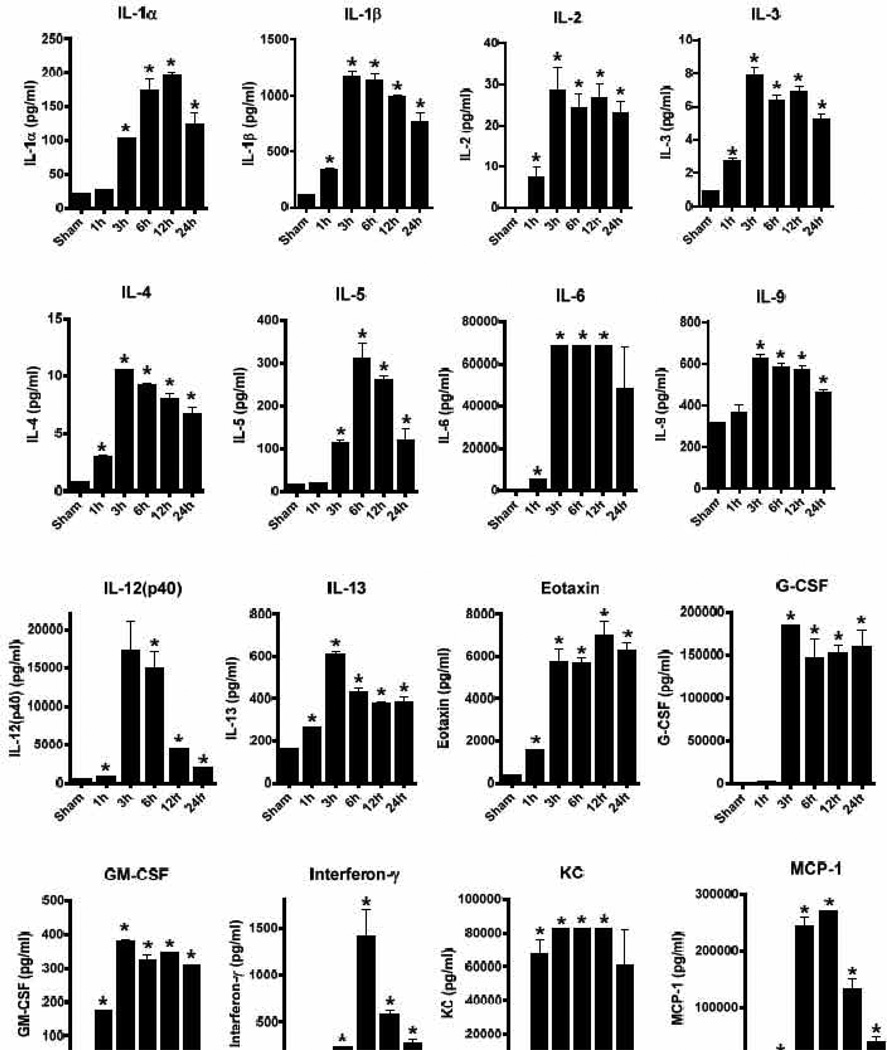
To characterize the acute inflammatory response following activation of the TLR4 receptor by LPS, we sought to simultaneously analyze the appearance of multiple proinflammatory cytokines and chemokines. A well-established model of LPS challenge by injection into the peritoneum (endotoxemia, endotoxic shock) was used to generate plasma samples from C57BL6/J mice (Fig. 1). The dose of LPS (10 mg/kg body weight) used in these studies has been described by us to cause mortality rates around 80%, with the first fatalities usually occurring 24 hours or later after injection (Bosmann et al., 2012b). The plasma samples from time points 1h – 24h after endotoxemia were analyzed for the concentrations of 20 mediators by multiplexing bead-based assays (Fig. 1). The values were compared to measurements in untreated normal (sham) C57BL/6J mice as base lines. Clearly, there was a broad upregulation of all 20 mediators analyzed by LPS compared to sham values. However, substantial differences in the absolute concentrations, time courses and clearance where noted among distinct mediators. TLR4-activation in the endotoxemia model resulted in moderate concentrations (8–600 pg/ml) of Th2 signature cytokines (IL-3, IL4, IL-5, IL-13). Much higher concentrations (circa 1400 pg/ml) were observed for the Th1 signature cytokine interferon-γ (Fig. 1). Extraordinary high amounts of proinflammatory IL-6 (> 60 ng/ml) and G-CSF (>150 ng/ml) as well as several chemokines such as KC (>80 ng/ml), MCP-1 (>250 ng/ml) and MIP-1 (~40 ng/ml) were detected (Fig. 1). Most mediators reached their maximum around 6–12 h, with the exception of a rapid surge of TNFα after only 1 h followed by a rapid fall off (Fig. 1). The half-life (clearance rates) of mediators clearly differed. For example, the 1 h surge of TNFα was reduced by around 95% already at 3 h. Similarly, the concentrations of IL-12(p40), interferon-γ, MIP-1α and MIP-1β rapidly declined over time (Fig. 1). These findings demonstrate that a careful selection of the optimal time points is important for all experiments where immune activation in response to TLR4-activation is to be investigated. The rapid decline of TNFα in endotoxic shock may also help to explain why numerous clinical trials with TNFα neutralizing agents have failed to demonstrate convincing efficacy (Abraham et al., 1998; Abraham et al., 1995; Fisher et al., 1996). In the clinical management of patients with developing sepsis it is unrealistic to initiate anti-TNFα therapy within 1 h, since commonly more time is required to establish the diagnosis. It would be tempting to speculate if anti-TNFα strategies would convey better survival benefits when used as a prophylactic treatment in high-risk patients. Interestingly, meta-analysis from different anti-TNFα trials show a small significant trend towards favorable effects of these substances in sepsis (Reinhart and Karzai, 2001). The caveat in all of these studies is that use of a TLR4 antagonist (eritoran) in septic humans recently resulted in cessation of the clinical trials because of lack of efficacy (Matsuyama, 2011).
Figure 1.
Release of inflammatory mediators following intraperitoneal challenge with LPS (endotoxemia). Male C57BL/6J mice (n=4–6 mice per group and at each time point) were injected with LPS and plasma at different time points analyzed for the concentrations (expressed as pg/ml) of 20 cytokines/chemokines by multiplexing bead-based assays. Sham indicates normal C57BL/6J mice injected with PBS i.p. only. Student t-test, * P < 0.05 as compared to sham.
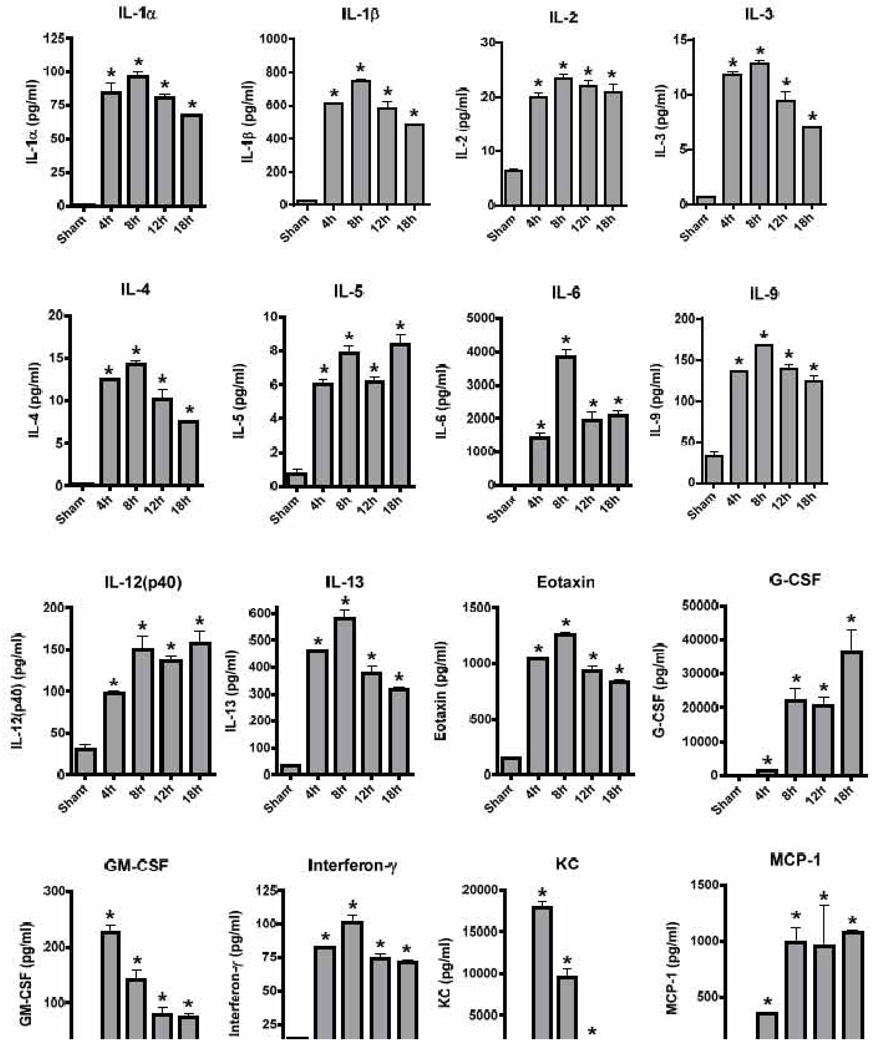
Next, we studied the response to LPS following intratracheal deposition in mouse lungs. Using this model of LPS-induced acute lung injury (ALI), there was overt upregulation of all 20 mediators analyzed (Fig. 2). In absolute numbers, lower concentrations of Th2-related mediators were found with TLR4-activation in the lungs. G-CSF (> 30 ng/ml), MIP-1α (> 15 ng/ml) and TNFα (~100 ng/ml) were the mediators produced in highest quantities (Fig. 2). The time course of KC indicated a more rapid disappearance from the alveolar compartment following ALI as compared to a constant plasma elevation of KC in endotoxemia (Fig. 1, 2). The release patterns of RANTES also differed greatly in the two in vivo models of LPS-challenge. Unlike in endotoxemia, the presence of TNFα in BALF after LPS-ALI was more constant over time. Collectively, substantial differences in the quantities and time curves of the analyzed proinflammatory mediators were seen as a function of the application site of LPS (i.v. versus i.t.). Such differences may be related to variations of numbers and phenotypes of toll like receptor-expressing immune cells in lungs compared to the peritoneal compartment (spleen, liver) and elsewhere (Chen et al., 2006). Several novel regulator genes to convey responsiveness towards LPS have been identified recently and are termed E2F1 and Ptch (Yang et al., 2011). These regulators of the TLR4-pathway may also be involved in organ-specific immune response characteristics. Additional regulatory elements in TLR4-signaling have been identified as microRNAs such as miR-142-3p (Sun et al., 2011). Notably, the sensitivity to LPS is also highly variable among several commonly used inbred mice strains. The situation becomes even more complex, when human subjects are studied. Humans (and rabbits) are much more sensitive to LPS as compared to rodents. This may be partly explained by higher levels of circulating antibodies to LPS in rodents (Reid et al., 1997). Recent advances in the field of sequencing methodologies have allowed the identification of several genetic polymorphisms of the host which account for differences in the responses to endotoxin (Agbeko et al., 2010; Marsik et al., 2005). One has to admit that more than a century after the concept of “endotoxin” was introduced to modern science many aspects of the underlying molecular mechanisms remain poorly understood. Future efforts will be necessary to find more effective modalities for treatment of the complications of gram-negative infections (sepsis, ALI).
Figure 2.
Release of inflammatory mediators following intrapulmonary challenge with LPS (LPS-ALI). Male C57BL/6J mice (n=5 per group and at each time point) were injected with LPS intratracheally. Broncho-alveolar lavage fluids were collected at different time points and the concentrations (expressed as pg/ml) of 20 mediators detected by multiplexing bead-based assays. Sham mice received PBS intratracheally. Student t-test, * P < 0.05 as compared to sham.
Acknowledgements
This research was supported by grants from the National Institutes of Health, USA (GM-29507, GM-61656 to P.A.W.), the Deutsche Forschungsgemeinschaft (Project 571701, BO 3482/1-1 to M.B.) and by the Center of Thrombosis and Hemostasis (CTH) funded by the Federal Ministry of Education and Research (BMBF, module A/JG to M.B.). The authors appreciate the excellent staff support of Sue Scott, Beverly Schumann and Robin Kunkel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Abraham E, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- Abraham E, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- Agbeko RS, et al. Genetic polymorphisms in the endotoxin receptor may influence platelet count as part of the acute phase response in critically ill children. Intensive Care Med. 2010;36:1023–1032. doi: 10.1007/s00134-010-1857-x. [DOI] [PubMed] [Google Scholar]
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nature reviews Immunology. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- Bosmann M, et al. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012a doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M, et al. Complement Activation Product C5a Is a Selective Suppressor of TLR4- Induced, but Not TLR3-Induced, Production of IL-27(p28) from Macrophages. Journal of immunology (Baltimore, Md; 1950) 2012b;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M, et al. The Outcome of Polymicrobial Sepsis is Independent of T and B Cells. Shock. 2011 doi: 10.1097/SHK.0b013e3182295f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide. Journal of immunology (Baltimore, Md; 1950) 2006;177:2373–2383. doi: 10.4049/jimmunol.177.4.2373. [DOI] [PubMed] [Google Scholar]
- Copeland S, et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CJ, Jr, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- Marsik C, et al. The Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms influence the late inflammatory response in human endotoxemia. Clin Chem. 2005;51:2178–2180. doi: 10.1373/clinchem.2005.051649. [DOI] [PubMed] [Google Scholar]
- Matsuyama K. Eisai's sepsis drug eritoran fails to save more lives in final-stage study. 2011 [Google Scholar]
- Michie HR, et al. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R. Untersuchungen über das Choleragift. Medical Microbiology and Immunology. 1892;11:393–412. [Google Scholar]
- Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annual review of biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RR, et al. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. Journal of immunology (Baltimore, Md; 1950) 1997;159:970–975. [PubMed] [Google Scholar]
- Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:S121–S125. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- Sakula A. Robert Koch: the story of his discoveries in tuberculosis. Ir J Med Sci. 1985;154:3–9. doi: 10.1007/BF02938285. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117:6172–6183. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias PS, et al. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. The Journal of experimental medicine. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yang IV, et al. Novel regulators of the systemic response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2011;45:393–402. doi: 10.1165/rcmb.2010-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]