Abstract
Before the 1990s it was widely believed that the adult brain was incapable of regenerating neurons. However, it is now established that new neurons are continuously produced in the dentate gyrus of the hippocampus and olfactory bulb throughout life. The functional significance of adult neurogenesis is still unclear, but it is widely believed that the new neurons contribute to learning and memory and/or maintenance of brain regions by replacing dead or dying cells. Many different factors are known to regulate adult neurogenesis including immune responses and signaling molecules released by immune cells in the brain. While immune activation (i.e., enlargement of microglia, release of cytokines) within the brain is commonly viewed as a harmful event, the impact of immune activation on neural function is highly dependent on the form of the immune response as microglia and other immune-reactive cells in the brain can support or disrupt brain function depending on the phenotype and behavior of the cells. For instance, microglia that express an inflammatory phenotype generally reduce cell proliferation, survival and function of new neurons whereas microglia displaying an alternative protective phenotype support adult neurogenesis. The present review summarizes current understanding of the role of new neurons in cognition and behavior, with an emphasis on the immune system’s ability to influence adult hippocampal neurogenesis during both an inflammatory episode and in the healthy uninjured brain. It has been proposed that some of the cognitive deficits associated with inflammation may in part be related to inflammation-induced reductions in adult hippocampal neurogenesis. Elucidating how the immune system contributes to the regulation of adult neurogenesis will help in predicting the impact of immune activation on neural plasticity and potentially facilitate the discovery of treatments to preserve neurogenesis in conditions characterized by chronic inflammation.
Keywords: microglia, stem cells, cytokines, adult neurogenesis, cognitive performance, hippocampus
Introduction to Adult Neurogenesis
Before the 1990’s, it was widely thought that neurons in the adult human brain did not regenerate. Consequently, it was thought that if neurons died during your adult life for any reason (e.g., oxidative stress, stroke, neurodegenerative disease, head trauma, normal aging), they would never be replaced. However, in the 1960s, Altman and colleagues challenged this idea, reporting that new neurons are continuously and spontaneously born in at least two regions of the adult rat brain: the subgranular zone of the hippocampus with cells migrating to the granule layer of the dentate gyrus (Altman and Das, 1965) and the subventricular zone with cells migrating to the olfactory bulb (Altman, 1969). Due to the longstanding dogma that postnatal neurogenesis is non-existent in mammals, it took more than three decades before the Altman and Das (1965) discovery was broadly accepted.
Multi-potent stem cells reside in the subgranular zone of the dentate gyrus and the subventricular zone. These stem cells divide asymmetrically producing one daughter progenitor cell and one stem cell. The progenitor cell can then divide asymmetrically producing daughter cells that differentiate into either astrocytes or neurons and one progenitor cell retaining the capacity to divide multiple times. Studying the growth, survival and differentiation of these new cells often involves exogenous administration of the thymidine analog, bromodeoxyuridine (BrdU) that labels dividing cells. The presence of new cells (i.e., BrdU positive cells) is then detected by an antibody conjugated to a fluorescent label, often in conjunction with other markers to distinguish the fate of the new cells. For instance, a BrdU positive cell that co-labels with neuronal nuclear protein (NeuN) would indicate a mature neuronal phenotype (see Figure 1). Whereas, a BrdU positive cell that co-labeled with S100β would indicate an astrocyte phenotype. One advantage of the double label technique as opposed to using gross morphology to identify whether new cells differentiated into neurons (Altman, 1969; Altman and Das, 1965) is that it does not rely on subjective assessment of the cellular phenotype. Additional modern immunohistochemical techniques (e.g., detection of the mitotic marker Ki67, and the marker of immature neurons, doublecortin [DCX]) in combination with double-labeling techniques have assisted in establishing that new neurons are continuously incorporated into the dentate gyrus and olfactory bulb throughout adulthood in most mammals (but perhaps not bats for hippocampal neurogenesis, Amrein et al., 2007).
Figure 1.
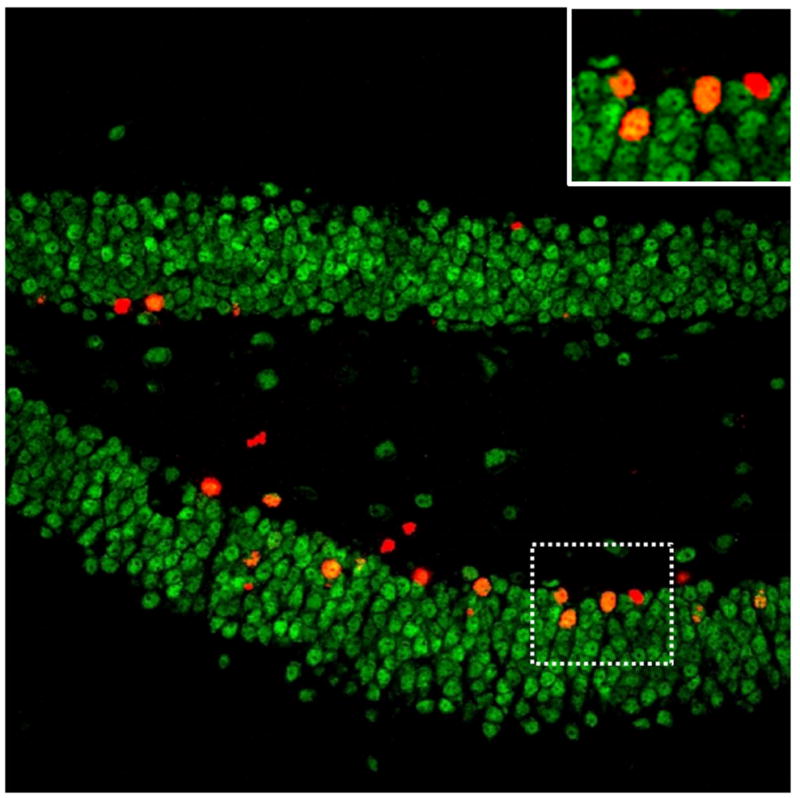
Image of a mouse dentate gyrus double-labeled with fluorescent antibodies against BrdU (new cells in red) and NeuN (mature neuronal marker in green). New cells that have differentiated into neurons are co-labeled with both BrdU and NeuN (new neurons in orange). The area within the dashed lines contains 3 new neurons and one new cell of unknown phenotype and is shown zoomed in at the top right.
Adult neurogenesis is thought to be an activity-dependent process (Deisseroth et al., 2004; Stone et al., 2011). Activation of the granule neurons generally leads to increases in neurogenesis, and inactivation leads to decreases. Whether neurogenesis can be regulated in an activity-independent manner is not known. The granule layer of the dentate gyrus can increase or decrease in volume by a relatively large magnitude, on order of 5–20% at a given age, depending on rates of neurogenesis that are largely determined by environmental factors such as stress, diet, environmental enrichment, and exercise (Clark et al., 2009; Coe et al., 2003; Mustroph et al., 2012; Pham et al., 2003; Rutten et al., 2010). Moreover, the increase and decrease in volume of the granule layer is attributed mostly to a change in the total numbers of new granule neurons as opposed to enlarged or shrinking neurons, or greater or lesser space between neurons (Rhodes et al., 2003).
A highly active area of research focuses on the extent to which adult neurogenesis contributes to learning and memory (Gould et al., 1999; Kempermann, 2008) or whether it merely reflects development and maintenance of the brain regions where it occurs (Amrein et al., 2011). The learning and memory theory is in part based on the idea that new neurons which are not yet fully integrated into the circuitry could mold their connections to experiences more extensively as compared to older neurons that are already incorporated into the circuitry. Clearly old neurons can modify their synapses in response to experiences, but younger neurons may display a greater degree of malleability or plasticity. Electrophysiological evidence is consistent with the idea that young neurons are highly plastic units. For example, new neurons in the hippocampus and olfactory bulb display a lower threshold for long term potentiation (Nissant et al., 2009; Schmidt-Hieber et al., 2004; van Praag et al., 1999), and GABA acts as an excitatory neurotransmitter in young immature granule neurons of the dentate gyrus (Deisseroth and Malenka, 2005; Ge et al., 2006). New dentate gyrus granule neurons approximately 6–8 weeks old are also more likely than older neurons to display immediate early genes (IEG, e.g., c-Fos, Zif268, Arc) in response to a behavioral stimulus (Clark et al., 2012; Clark et al., 2011; Kee et al., 2007).
The assumption that new neurons are highly plastic units implies that there is a critical period in the development of new neurons where they display heightened plasticity, which is lost as they mature. However, the exact duration for this hypothetical critical period is not known, and may greatly vary between species. For example, in mice, it takes several days to weeks for a newborn cell in the granule layer of the dentate gyrus to display mature neuronal markers, and functionally integrate into hippocampal circuitry (Kempermann et al., 2003; van Praag et al., 2002) whereas in macaque monkeys it takes 6 months (Kohler et al., 2011). Similar analyses are not possible in humans for ethical reasons, but the macaque data suggest that if a critical period exists, it may be much longer in humans than in mice.
Many papers have reported positive correlations between levels of adult hippocampal neurogenesis and behavioral performance on hippocampus-dependent tasks (e.g., Gould, 1999; van Praag et al., 1999; Thuret et al., 2009), but correlation does not imply causation. Moreover, several articles have reported no correlation (Jaholkowski et al., 2009) or a negative correlation between levels of neurogenesis and learning (Ambrogini et al., 2004; Kerr et al., 2010; Rhodes et al., 2003). Many studies have attempted to test whether neurogenesis causally contributes to changes in behavioral performance by directly manipulating neurogenesis. Techniques to reduce neurogenesis that have been used include anti-mitotic chemical agents (Shors et al., 2001), focal irradiation (Clark et al., 2008; Meshi et al., 2006), and transgenic animals (e.g., Deng et al., 2009; Dupret et al., 2008; Saxe et al., 2006; Zhang et al., 2010), but most of these have various side effects that make interpretation difficult. Moreover, results often conflict. One example is determining the contribution of adult neurogenesis to pro-cognitive effects of exercise. Many studies have shown that exercise both increases adult hippocampal neurogenesis and enhances learning and memory (Mustroph et al., 2012; Trejo et al., 2008; van Praag et al., 1999). However, exercise produces many changes throughout the brain including modifying synapses (Dietrich et al., 2008; Eadie et al., 2005), increasing blood vessel density (Clark et al., 2009), increased release of trophic factors (Neeper et al., 1995), growth factors (Ding et al., 2006), neurotransmitter levels (Meeusen and De Meirleir, 1995). In one study, irradiation was used to ablate neurogenesis in runners and it was found that without the increase in neurogenesis from running, the behavioral enhancement on the water maze test of spatial learning was lost (Clark et al., 2008). However, an earlier study using a similar method in a different strain of mice found that neurogenesis was not required for environmental enrichment (that included running wheels) to improve water maze performance (Meshi et al., 2006). Hence, the extent to which new neurons generated from running contribute to pro-cognitive effects of exercise remains unclear.
Recent studies have suggested that new neurons in the adult hippocampus play a role in pattern separation, that is, the ability to form distinct representations of similar inputs (Clelland et al., 2009; Creer et al., 2010; Sahay et al., 2011). New neurons have also been suggested to play a role in cognitive flexibility (Burghardt et al., 2012). Others have proposed that new neurons play a specific role in the behavior that led to their survival and integration (Kee et al., 2007; Tashiro et al., 2007). In our opinion, new neurons in the granule layer of the dentate gyrus probably contribute to any and all the functions of the dentate gyrus, not in any one specific cognitive function such as pattern separation, or in one behavior that was coincident with their survival. New neurons may have some unique characteristics as compared to older neurons as discussed above but they eventually integrate into pre-existing hippocampal circuitry and therefore likely contribute to the general maintenance and function of the dentate gyrus. Evidence for the non-specificity of new neurons comes from a study showing that new neurons generated from running function in different behavioral tasks that do not involve running (Clark et al., 2012). In our opinion, the question of the functional significance of new neurons in the hippocampus is the same as the question of the functional significance of the granule layer of the dentate gyrus.
The microenvironment conducive for increasing net neurogenesis, i.e., proliferation, survival, differentiation, and network incorporation, is extremely complex and involves increases in vasculature (Palmer et al., 2000), growth factors (Anderson et al., 2002), trophic factors (Lee et al., 2002), changes in the chemical and electrical environment (Deisseroth et al., 2004), and glial support cells (Barkho et al., 2006; Morrens et al., 2012). The immune system contributes to the microenvironment through the cellular activity of immune cells in the brain (e.g., microglia) and from inflammatory signaling molecules entering from the periphery (Yirmiya and Goshen, 2011). Therefore activation of the immune system has the potential to influence the proliferation and survival of new neurons. In addition, many of the lifestyle factors that are known to regulate the proliferation, survival, differentiation and incorporation of new neurons into the circuitry, including, stress, diet and exercise, may exert some of their effects via interacting with the immune system and immune cells in the brain (Kohman et al., 2011; Kubera et al., 2011; Morgan et al., 2007; Wong et al., 2005; Wu et al., 2008). To the extent that adult neurogenesis is related to cognitive performance and behavior, immune-related changes in neurogenesis may have functional implications for cognition. Below we define neuroinflammation as it relates to neurogenesis, followed by a section focused on the impact of immune activation on neurogenesis, the contribution of age-related changes in immune function to neural plasticity, and the potential relevance of immune-induced alterations in hippocampal neurogenesis to cognitive function.
Neuroinflammation
The immune system can be divided into the innate and adaptive immune systems. While these divisions work together to orchestrate the immune response each serves distinct roles that facilitate recovery from an injury or infection. The innate immune system serves as the initial defensive to combat an infection. This response is rapid, but non-specific and is primarily mediated by cells of a myeloid origin (i.e., macrophages, neutrophils, and dendritic cells). In contrast the adaptive branch of the immune system is driven mainly by lymphocytes (i.e., T- and B-cells) which create an antigen specific immune response that takes time to develop, but provides lasting immunity. The combined efforts of the innate and adaptive branches of the immune system protect and facilitate recovery following infection or injury within the peripheral nervous system.
While the brain is no longer considered to be an immune privileged site, as we now know that immune cells within the periphery communicate with the brain and immune cells actually reside within the brain differences exist between immune activation within the periphery and CNS. These immunological differences are likely related to the presence of the blood brain barrier (BBB) that separates the central and peripheral nervous systems. The BBB protects the brain by limiting what enters and leaves the brain creating distinct environments within the body. This boundary between the brain and periphery has direct effects on immune activity. While parallels between the type of cells that reside in and the activity they perform within the periphery and brain are evident, key differences exist. For instance, following a systemic infection cells of the innate immune system become activated and participate in initiating the adaptive immune response to aid in clearing the infection. Whereas cells in the brain that mediate the innate response cannot efficiently activate the adaptive branch of the immune system (Ransohoff and Brown, 2012). While cells from the periphery may be able to infiltrate the CNS and assist with clearing an infection, particularly when the BBB is compromised, majority of the burden of protecting the CNS from injury or infection falls on a select group of cells, namely, microglial cells, astrocytes, and mast cells. Mast cells reside within the brain and are important for attracting and potentially activating other immune cells within the brain via the secretion of proinflammatory cytokines and chemoattractants (Skaper et al., 2012). Astrocytes also contribute to the local immune response within the brain through the production of both pro- and anti- inflammatory cytokines, complements components, and chemokines (Ransohoff and Brown, 2012). Though a variety of cells contribute to the development of inflammation within the brain researchers have focused on microglial cells as the primary mediators of the neuroinflammatory response.
Microglia are myeloid cells derived from the embryonic yolk sac that migrate into the brain during early development and sustain a resident population through proliferation (Ajami et al., 2007; Alliot et al., 1999). Microglia are similar to peripheral macrophages as they can take on several phenotypes of activation that allows them to survey the environment, remove cellular debris or pathogens, and support regenerative processes. The induction of these varied phenotypes occurs in response to the signals from the surrounding environment. In response to an infection or injury microglia acquire a reactive inflammatory phenotype, also referred to as the classic phenotype. The classic inflammatory phenotype is characterized by increased microglia proliferation, morphological changes (e.g., swelling of the cell body and retraction of the processes), and the release of several inflammatory molecules including cytokines, chemokines, reactive oxygen species, and nitric oxide (Kettenmann et al., 2011). The induction of this classic inflammatory phenotype is at the heart of the neuroinflammatory response. For the sake of brevity we will use the term neuroinflammation interchangeably with activation of microglia towards the classic inflammatory phenotype, as the increased expression of pro-inflammatory cytokines and a host of inflammatory-related molecules associated with the classic phenotype defines the neuroinflammatory response for the purposes of this review.
Phenotype-specific effects of microglia on hippocampal neurogenesis
Microglia can have both supportive and harmful effects on adulthood neurogenesis depending on their state of activation. Microglia that express a proinflammatory phenotype generally impair neurogenesis whereas as microglia that express a neuroprotective phenotype can facilitate new cell survival. The present section will cover the differential phenotypes microglia express and the distinct effects each phenotype has on influencing hippocampal neurogenesis.
Resting microglia
In the healthy uninjured brain microglia typically reside in what’s been termed a resting state. Resting microglia display a ramified morphology, with many fine processes and a small cell body (Kettenmann et al., 2011). They use their fine cellular processes to vigilantly survey the surrounding area checking for evidence of damage or infection. In the hippocampus, resting microglia actively participate in adult neurogenesis through their phagocytic capacities (Sierra et al., 2010). Though thousands of new cells are born in the subgranular zone of the dentate gyrus every day, not all of these cells will survive and differentiate into neurons. At least half these new cells will die, likely through apoptosis, within the first few days to weeks after they are born (Biebl et al., 2000; Cameron and McKay, 2001; Dayer et al., 2003; Kempermann et al., 2003). Sierra et al. (2010) demonstrate that unchallenged microglia play a crucial role in neurogenesis by removing the apoptotic new cells through phagocytosis. Additionally, they report that phagocytosis of new cells does not activate microglia, indicating that phagocytosis is possible without complete activation of microglia. These findings show a novel role for microglia in supporting basal hippocampal neurogenesis in the absence of an activated phenotype.
Further evidence that microglia in the unchallenged state may influence hippocampal neurogenesis comes from the work of Walton et al. (2006) who found in culture that microglia release factors that rescue neuroblasts and instruct neuronal cell differentiation. Neural stem cells (NSCs) isolated from subventricular tissue of 8-day-old C57BL/6 mice were cultured and, as expected, the proportion of neuroblasts decreased the longer the cultured was maintained. This reduction in neuroblasts is reported not to result from depletion of NCSs, but rather appears to reflect a reduction in proliferation of non-neuronal cells, particularly microglia, as microglial cell loss was found to be correlated with the neurogenic potential of the culture. Additionally, the data indicate that microglia secrete unknown factors that prolong the culture’s neurogenic potential as conditioned medium from a microglia rich brain area enhanced the neurogenic capacity of cultured NCS. Interestingly, conditioned media derived from older mice was less effective than media derived from young mice in stimulating neurogenesis, indicating that age-related changes in microglia activity may contribute to the age-associated decline in neurogenesis (Walton et al., 2006). Collectively, the data indicate a role for microglia in supporting basal neurogenesis in the absence of microglia activation, broadening our understanding of the microglial cell’s role within the brain. Further work is needed to fully characterize the precise role microglia play in supporting neurogenesis within the hippocampus.
Classically activated microglia
Overwhelming evidence indicates that activation of microglia towards the classic inflammatory phenotype has negative effects on hippocampal neurogenesis. A common experimental method to simulate an inflammatory response within the brain and induce classic microglia activation is through administration, either centrally or systemically, of the bacterial endotoxin lipopolysaccharide (LPS). LPS activates microglia through binding to toll-like receptor-4 (TLR4) molecules and induces the release of several proinflammatory cytokines including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) among other inflammatory molecules.
The initial work demonstrating that classically activated microglia impair hippocampal neurogenesis was reported by Monje et al. (2003) and Ekdahl et al. (2003) following LPS administration. Ekdahl et al. (2003) report that 4 weeks intracortical infusion of LPS drastically decreased new neuron survival, whereas LPS had no effects of proliferation of new cells. Similar findings were observed by the independent report published by Monje et al. (2003) who found that an intraperitoneal injection of LPS significantly decreased new cell survival, but had no effect on proliferation. Additionally, LPS administration decreased the differentiation of new cells into neurons as evidenced by a reduction in expression of the early neuron marker doublecortin (DCX) in LPS treated rats. In both reports, the survival of new neurons was negatively correlated with the number of activated microglia. Further, administration of the microglia inhibitor, minocycline, blocked the LPS-induced decrease in new neuron survival, indicating microglia activation mediated the impaired neuron survival (Ekdahl et al., 2003). Collectively, these reports provided the foundational evidence, later replicated by other (Bastos et al., 2008, that neuroinflammation suppresses hippocampal neurogenesis through decreasing new neuron survival.
Though new cell survival is clearly impaired by neuroinflammation, additional papers have reported that inflammation also affects the proliferation component of hippocampal neurogenesis. For instance, Fujioka and Akema (2010) report that five hours after a single intraperitoneal (ip) injection of LPS, expression of the proliferation marker pHH3 (serine10 phosphorylated histone H3) was decreased in the granule layer. Further, they report the number of new cells was reduced in LPS-treated animals 24 hours after labeling cells with BrdU (Fujioka and Akema, 2010). Additionally, a transgenic mouse model that overexpresses the proinflammatory cytokine IL-6 from astrocytes shows reduced progenitor cell proliferation when samples were collected one day after BrdU administration (Vallieres et al., 2002). The reason for the discrepancy in the effect of LPS administration on proliferation is unclear as similar doses and routes of LPS were used as well as the species (i.e., rats), though differences in strain and the sex of the rats may alter the response to LPS in these studies (Fujioka and Akema, 2010, Monje et al., 2003).
The impact of inflammation on neurogenesis is not simply via effects on proliferation, survival and cell death, but also can affect the integration of new neurons into pre-existing neural networks and the cellular properties of the new cells that survive. For example, Jakbus et al. (2008) report that inflammation increases inhibitory signaling to new neurons. Specifically, Jakbus et al. (2008) examined changes in morphology and electrophysiological properties of new granule cells that were born in an environment characterized by chronic inflammation that was induced by an intra-hippocampal infusion of LPS and produced persistent microglia activation. Cells born one week after LPS infusion showed no changes in dendritic tree morphology, location within the granule layer, intrinsic membrane potential, or axon projection. However, the LPS-induced chronic neuroinflammation altered the synaptic input to both pre-existing and new neurons. Both new and pre-existing neurons showed an overall increase in the frequency of spontaneous excitatory postsynaptic current (EPSC) compared to granule cells in control animals, indicating that cells residing an inflammatory environment will receive an increase in excitatory activation. Though both new and pre-existing cells showed the increased excitatory input only the new cells born one week after LPS administration showed an enhance inhibitory input. The time between spontaneous inhibitory postsynaptic current (IPSC) was shorter in new neurons compared to pre-existing neurons. Additionally, new cells had a higher density of inhibitory synapses on their distal dendrites compared to pre-existing neurons. Collectively, these data indicate that neurons that mature in an inflammatory environment have increased inhibitory input from synapses that may potentially alter new neuron function.
Additional evidence of altered integration comes from the work of Belarbi et al. (2012) who demonstrated that chronic inflammation reduces new neuron participation in hippocampal processing of contextual information. In this study, rats received intracerebroventricular infusions of LPS or cerebral spinal fluid (CSF) for 28 days, rats then received five BrdU injections to label dividing cells. Two months after LPS or CSF treatment rats were placed in a novel environment and allowed to explore for 5 minutes and sacrificed 30 minutes later to assess differential expression of the plasticity-related immediate early gene Arc (activity regulated cytoskeleton-associated protein) in pre-existing and new neurons in the granule layer. As expected, the authors observed persistent microglia activation following chronic LPS administration, as evidenced by increased MHC II expression two months after treatment. Though microglia showed prolonged activation, the authors report no difference in the number of BrdU positive new cells in the granule layer. This lack of an effect on new cell survival may result from using a lower dose of LPS than prior studies. In agreement with past reports, 30 minutes after exploring a novel environment a greater proportion of new neurons expressed Arc compared to pre-existing neurons in the CSF-treated rats, indicating increased recruitment of new neurons. However, in the LPS-treated rats there was no difference in Arc expression between new and existing neurons, indicating that chronic inflammation attenuated the recruitment of new neurons into hippocampal networks (Belarbi et al., 2012). These findings in conjunction with the work of Jakbus et al. (2008) demonstrate that inflammation may alter the unique cellular processes that new neurons display during development potentially making them less able to fully integrate and function in pre-existing hippocampal networks.
The mechanisms through which inflammation reduces hippocampal neurogenesis is still not fully understood, but evidence indicates that activation of microglia and the subsequent release of inflammatory molecules creates an environment that does not support survival of new cells. Evidence suggests that the classic proinflammatory cytokines, IL-1β, IL-6, and TNF-α contribute to the inflammation-related decrease in neurogenesis (see Table 1). In vitro findings indicate that IL-6 is a key factor released by microglia that reduces neuron survival (Monje et al., 2003). Further overexpression of IL-6 within the brain reduces proliferation, survival, and neural differentiation of new cells (Vallieres et al., 2002).
Table 1.
Summary of the effects of select immune signaling molecules on proliferation, survival and neuronal differentiation of new cells in the hippocampus.
| Proliferation | Survival | Neuronal differentiation | Astrocyte differentiation | References | |
|---|---|---|---|---|---|
| IL-6 | ↓ | ↓ | ↓ |
Monje et al. (2003) Vallieres et al. (2002 |
|
| IL-1beta | ↓ | ↓ | ↑ |
Green et al. (2012) Koo and Duman (2008) |
|
| TNF-alpha | ↓ | ↓ | ↑ |
Keohane et al. (2010) Iosif et al. (2006) Johansson et al. (2008) Seguin et al. (2009) |
|
| Prostaglandins | ↓ |
Bastos et al. (2008) Keene et al. (2009) Monje et al. (2003) |
|||
| TGF-beta | ↑ | ↑ |
Battista et al. (2006) Mathieu et al. (2010) |
||
| IL-4 | ↑ | Butovsky et al. (2006) | |||
| IL-10 | ↑ |
Cacci et al. (2008) Kiyota et al. (2011) |
Note: ↓ indicates a reduction and ↑ indicates and increase. Cells that are left blank indicate that the effects are currently unknown.
Similar anti-neurogenic effects of TNF-α have also been reported. For instance, systemic administration of TNF-α was found to reduce cell proliferation within the granule layer (Seguin et al., 2009). Further, addition of TNF-α to an in vitro model of hippocampal neural differentiation led to increased apoptosis of hippocampal stem/progenitor cells (Hofer et al., 2011). Cultured hippocampal cells that undergo differentiation in the presence of TNF-α show a significant reduction in the proportion of cells that become neurons and an increase in the proportion of newly developed astrocytes (Keohane, et al., 2010). Exposing differentiating cells to TNF-α was found to increase expression of the anti-neurogenic gene hairy-enhancer-of-split 1 (Hes1) that decreases neuronal differentiation by suppressing expression of pro-neural genes (Keohane et al., 2010). These finding are in agreement with prior work that showed exposing new cell in culture to TNF-α increases the proportion of cells that differentiate into astrocytes (Johansson et al., 2008). Further, mice lacking the TNF-α type 1 receptor show increased basal levels of proliferation in the subgranular zone, indicating TNF-α acting through the type 1 receptor negatively regulates cell proliferation (Iosif et al., 2006). Collectively, the data indicate that elevated levels of TNF-α can reduce cell proliferation and impact differentiation of new cells by increasing the likelihood that these new cells will develop into astrocytes.
Elevated levels of IL-1β are implicated in altering neurogenesis by affecting cell proliferation and differentiation. NPCs have been shown to express the type 1 IL-1 receptor (IL-1R1), indicating that IL-1β may act directly on NPCs (Green et al., 2012). Culturing embryonic rat hippocampal NPCs in the presence of IL-1β significantly reduces NPC proliferation and the growth of neurospheres. Additionally, in a differentiation assay the presence of IL-1β increased the proportion of cells that became astrocytes and reduced the percentage of new neurons (Green et al., 2012). To investigate the effects of chronic neuroinflammation on neurogenesis, IL-1β was overexpressed in the adult hippocampus using a Cre-lox conditional transgenic mouse model. Chronic IL-1β expression decreased the number of DCX+ cells in the granular layer of the hippocampus at both 1 and 3 months after IL-1β over-expression (Wu et al., 2012). The reduction in new neurons (i.e., DCX+ cells) is mediated through activation of the IL-1R1, as overexpression of IL-1β in the hippocampus of IL-1R1 knockout mice had no effect on DCX+ cells. Further, chronic expression of IL-1β reduced the proportion of new cells that differentiated into new neurons and increased the proportion of cells that became astrocytes, indicating that IL-1β shifts the fate of new cells towards astrocytes (Wu et al., 2012). IL-1β has also been implicated in mediating stress-induced reductions in hippocampal neurogenesis. Koo and Duman (2008) showed that acute or chronic stress decreases cell proliferation and that the reduction could be blocked by administration of the IL-1 receptor antagonist (IL-1Ra) as well as mice that lacked the IL-R1 showed no effect on cell proliferation following stress exposure. Additionally, they report that chronic stress decreased the number of new neurons, as measured by DCX expression in BrdU positive cells, and that this reduction could be prevented by central infusion of IL-1Ra (Koo and Duman, 2008). To identify the mechanism through which IL-1β reduced proliferation, adult neural progenitor cells were cultured in the presence or absence of IL-1β. Results showed that the addition of IL-1β did not increase cell death, but rather levels of cyclin D1, a protein involved in regulating mitotic events, was reduced in the presence of IL-1β and again this effect could be blocked by IL-1Ra (Koo and Duman, 2008). Taken together, the data indicate that IL-1β can suppress progenitor cell proliferation by slowing the cell cycle and that IL-1β alters rates of differentiation by increasing the probability that a new cell will differentiate into an astrocyte rather than a neuron.
In addition to proinflammatory cytokines, prostaglandins are released in response to an immune challenge which may mediate aspects of the inflammation-induce reduction in hippocampal neurogenesis (Table 1). Monje et al. (2003) report that administration of indomethacin, a non-selective inhibitor of cyclooxygenase (COX), an enzyme that allows for conversion of arachidonic acid to prostaglandins, attenuates microglia activation and prevented the LPS-induced decrease in new cell survival. Later work demonstrated that the ability of indomethacin to prevent the reduced neuron survival following LPS administration is likely mediated by COX-2, as a selective inhibitor of COX-2 blocked the LPS-induced decrease in new neuron survival, whereas a selective COX-1 inhibitor did not rescues cell survival (Bastos et al., 2008). Further work indicates that signaling through prostaglandin E2 receptor subtypes EP1 and EP2 are required for LPS-induced suppression of hippocampal neurogenesis (Keene et al., 2009). Collectively, these data indicate that the decreased survival associated with an immune challenge is mediated in part by the production of prostaglandins.
Another major component of the immune response that can alter neurogenesis is activation of hypothalamic-pituitary adrenal (HPA) axis. Evidence suggests that the increases in glucocorticoid levels resulting from activation of the HPA axis contribute to the inflammation-induced decreases in hippocampal neurogenesis (Wolf et al., 2009). Immune stimulants, such as LPS, that induce a large increase in corticosterone levels in the brain of rodents are associated with reduced neurogenesis whereas administration of a T-cell specific stimulant, staphylococcus enterotoxin B (SEB), which produces a smaller increase in corticosterone levels in the brain compared to LPS actually enhances hippocampal neurogenesis (Wolf et al., 2009). These data indicate that moderate increases in corticosterone support hippocampal neurogenesis whereas substantial increases may inhibit neurogenesis, potentially indicating that elevated corticosterone levels induced by activation of the immune system may contribute to the reduction in neurogenesis.
Activation of the immune system induces a broad response that leads to release of a host of immune molecules, including cytokines, prostaglandins, and chemokines, and activation of the HPA neuroendocrine system among others. Generally, the transient activation of the immune system is beneficial and aids the body in recovering from an infection, but the induction of a neuroinflammatory response has detrimental effects on neurogenesis which may be of particular importance when the immune response becomes chronic or is exaggerated. Together, the evidence indicates that a variety of immune molecules can negatively affect neurogenesis and often have overlapping effects. Proinflammatory cytokines generally decrease cell survival and shift the balance away from neurogenesis and towards astrogliogenesis. Further, elevated levels of prostaglandins and glucocorticoids are implicated in inflammation-associated reductions in new cells survival. Cooperatively, the secretion of inflammatory molecules within the brain likely modifies the neurogenic niche, creating an environment in which new neurons cannot prosper. Interestingly, astrocyte production is favored during times of inflammation. Whether the enhancement of astrocyte differentiation has any functional significance in facilitating the neuroinflammatory response or subsequent recovery from has not been determined.
Alternatively activated microglia
In contrast to the classic inflammatory phenotype, microglia can acquire what’s been termed the alternative neuroprotective or M2 phenotype that plays a central role in regenerative processes (for review see Colton, 2009). The alternative phenotype is characterized by expression of MHC II, arginase 1 (AG1), mannose receptor (MRC1), peroxisome proliferation activation receptor gamma (PPAR-γ), Ym1 (Chitinase 3-like 3), and found in inflammatory zone 1 (FIZZ1) (Colton, 2009). Further, the alternatively activated microglia often show increased expression of the anti-inflammatory cytokines interleukin-10 (IL-10), transforming growth factor-β (TGF-β), and growth factors such as insulin-like growth factor (IGF), neural growth factor (NGF), and brain derived neural growth factor (BDNF). The expression of the alternative phenotype often follows an inflammatory response, potentially making the alternative phenotype a compensatory response to aid in healing (Cacci et al., 2008; Thored et al., 2009). Anti-inflammatory cytokines, such as IL-4 and IL-13 can induce expression of the alternative phenotype (Butovsky et al., 2006; Colton, 2009). Given the protective properties associated with the alternative phenotype it has been suggested that microglia expressing the alternative phenotype contribute to the maintenance of neurogenesis.
Evidence suggests that anti-inflammatory cytokines may support neurogenesis (see Table 1). For example, Mathieu et al. (2010) report that chronic expression of the anti-inflammatory cytokine TGF-β, induced by administration of an adenoviral vector expressing TGF-β into the SVZ increased survival of BrdU positive cells. Further, Battista et al. (2006) found that TGF-β plays a role in the increase in hippocampal neurogenesis following adrenalectomy (ADX). ADX has been reported to increase proliferation of new cells as well as increase neuronal differentiation. They found that inhibiting TGF-β with a blocking antibody had no effect on the ADX-induced increase in proliferation, but reduced neuronal differentiation, indicating the TGF-β may encourage new cells to differentiate into neurons (Battista et al., 2006).
The notion that microglia have differential effects on neurogenesis depending on the phenotype they express is supported by work by Butovsky et al. (2006). They found that neural progenitor cells (NPCs) cultured with microglia that were stimulated with LPS showed low levels of neuronal differentiation, whereas NPCs cultures with IL-4 stimulated microglia showed an increased the proportion of new neurons. In agreement, more cells were found to be DCX+ when co-cultured with microglia stimulated with IL-4 compared to control cells that were cultured with un-stimulated microglia (Butovsky et al., 2006). Additionally, stimulating microglial cells with IL-4 increased microglia expression of IGF-1 that is known to support neurogenesis (Annenkov, 2009). In agreement, a more recent paper reported that co-culturing NPCs with IL-4 stimulated microglia enhanced neuronal differentiation. Additionally, it was observed that IL-10 stimulated microglia enhance NPC proliferation, but have no effect on differentiation in culture (Kiyota et al., 2011). Further, Cacci et al. (2008) reported that in culture microglia releasing IL-10 were supportive of neuronal differentiation and new cell survival. Collectively these data indicate that expression of the alternative phenotype in microglia supports different aspects of neurogenesis and that depending on the activating stimulus can enhance proliferation or the production of new neurons.
The pro-neurogenic effects of microglia have also been reported in an ischemic injury model. Initially, following injury, microglia express the inflammatory phenotype. However, with time a portion of the cells begin to express the alternative phenotype. Thored et al. (2009) showed that following an ischemia-induced injury in the striatum microglia acquired the alternative phenotype which persisted for 16 weeks post-injury. Additionally the injury increased new cell production within the SVZ and many of these new cells migrated to the injury site within the striatum. Alterations in microglial cell activation have been suggested to support the production and migration of new cells to the site of injury by acquiring the alternative neuroprotective phenotype. Microglia within the SVZ showed increased expression of IGF-1 after stroke that corresponded to the increases in neurogenesis (Thored et al., 2009). Collectively these data indicate that alternatively activated microglia facilitate neuronal differentiation, migration and integration into neuronal networks following an injury.
Research on an animal model of Alzheimer’s disease has shown that neuroinflammation may contribute to the depression of neurogenesis and that converting microglia to the alternative phenotype may alleviate the decrease in neurogenesis. The transgenic mouse APP+PS1 model of Alzheimer’s disease that expresses both the human amyloid precursor protein (APP) and human presenilin-1 (PS1) show a reduced number of new neurons compared to non-transgenic mice (Biscaro et al., 2012; Kiyota et al., 2011). Chronic administration of minocycline has been reported to increase new neuron survival in APP+PS1 mice, indicating that microglia activation contributes to the decrease in hippocampal neurogenesis (Biscaro et al., 2012). Additionally, work by Kiyota et al. (2011) showed that overexpression of the anti-inflammatory cytokine IL-10 attenuates the reduction in neurogenesis in the Alzheimer mouse model. They report that APP+PS1 mice that overexpressed IL-10 within the hippocampus increased the number of new cells and the number DCX+ cells and BrdU+ NeuN+ co-labeled cells. Increased IL-10 expression did not reduce the total amyloid beta load within the hippocampus, indicating that reductions in Aβ cannot explain the enhancement of hippocampal neurogenesis. One possibility, though not directly assessed in the study, is that elevated IL-10 levels within the hippocampus reduced microglia expression of the inflammatory phenotype, which is known to reduce neurogenesis, and/or increased expression of the alternative phenotype in microglia, which is associated with enhanced neurogenesis. Collectively, the data potentially indicate that balancing the pro- and anti-inflammatory activity within the brain may have beneficial effects on measures of neural plasticity in the diseased brain.
Role of microglia in activity-induced increases in neurogenesis
As discussed, neurogenesis is regulated by a variety of factors. Exercise has been consistently shown to enhance hippocampal neurogenesis by increasing neuronal differentiation and new cell survival. Though the data are limited, there is reason to speculate that immune cells may participate in the enhancement of neurogenesis following exercise and environmental enrichment. For example, Ziv et al. (2006) report that interactions between T-cells and microglia participate in the environmental enrichment-induced increase in neurogenesis. They found that SCID mice, which lack functional B- and T-cells, fail to show the enrichment-induced increase in neurogenesis. However, the enhancement of neurogenesis can be recovered if T-cells that are specific to a neural antigen are replaced. The authors suggest that following environmental enrichment T-cells may shift microglia towards the alternative M2 phenotype, as microglia showed an increase in MHC II expression and many of them appeared to co-label with the neuroprotective molecule, IGF-1 (Ziv et al., 2006). Additionally, the beneficial effects of environmental enrichment in T-cell replaced SCID mice were blocked if minocycline was administered, indicating that microglia are involved in this response.
The involvement of T-cells and microglia was called into question by the work of Olah et al. (2009) who found that 10 days of wheel running increases neurogenesis, but found no evidence that microglial cells were activated from exercise, as measured by MHC II expression. Additionally, they found no evidence of T-cell infiltration within the brain. However, the authors did observe an increase in microglia proliferation from exercise that occurred in several areas and therefore was not specific to neurogenic regions. The differences between these reports may result from methodological differences as Ziv et al. (2006) employed environmental enrichment that included running wheels, whereas Olah et al. (2009) used only running wheels. Though discrepancies exist, both studies provide evidence that microglial cells are responsive to the environment and/or physical activity. Further support, comes from work by Kohman et al. (2011) who found that voluntary wheel running significantly increases the proportion of microglia that co-label with IGF-1 in both adult and aged mice. These data are in agreement with the findings of Ziv et al. (2006). Olah et al. (2009) failed to observe an increase in IGF-1 expression following exercise, but this difference likely reflects the shorter running periods (i.e., 10 days versus 8 weeks) and possibly a difference in assessment of gene expression versus protein levels. Collectively, these data indicate that microglia are responsive to environmental factors, such as engaging in exercise, and that these differences are correlated with levels of neurogenesis. However, future work is needed to establish whether the relationship is causal, i.e. that microglia directly contribute to changes in neurogenesis.
A recent study by Vukovic et al. (2012) supports a role for microglia in exercise-induced increases in neurogenesis. In agreement with prior findings, two weeks of wheel running was found to increase the proportion of DCX+ cells, BrdU+ cells and increase the percentage of neurospheres observed. The authors created single cell suspensions of neural precursor cells (NPCs) isolated from the hippocampus of exercising or sedentary mice and then either added microglial cells or left them without microglia and assessed the proportion of neurospheres 14 days later. The absence of microglia had no effect on neurosphere formation in sedentary animals. However, the absence of microglia significantly reduced the proportion of neurospheres in runners. Further, if microglia from exercising mice were added to a NPC culture from a sedentary mouse there was a significant increase in the percent of neurospheres compared to NPCs that received microglia from a sedentary animal, indicating that exercise-induced changes in microglia may facilitate the increase in neurogenesis. The authors propose a role for the chemokine CX3CL1 for mediating the exercise-induced changes in microglia that contribute to increased NPC activity (Vukovic et al., 2012). Exercise significantly increased CX3CL1 levels in the hippocampus and two weeks of central administration of a CX3CL1 blocking anti-body prevented the beneficial effects of exercise on neurosphere formation. Additionally, when recombinant CX3CL1 was added to NPC culture there was a significant increase in the proportion of neurospheres, but this effect was attenuated if microglia were absent from the culture, indicating that microglia are required to observe the beneficial effects of CX3CL1 (Vukovic et al., 2012). Collectively, the data indicate that microglia activity is altered by lifestyle factors, such as exercise and environmental enrichment, and that these changes may support the pro-neurogenic response, potentially through altering the environment in which new cells are born.
Role of microglia in age-related decline in neurogenesis
Hippocampal neurogenesis persists throughout an individual’s life, as centurions show evidence of neurogenesis (Knoth et al., 2010). However, there is a substantial decline in neurogenesis with normal aging. Aging is associated with a decrease in both proliferation and survival of new cells (Blackmore et al., 2009; Kohman et al., 2011; van Praag et al., 2005). Several factors likely contribute to the age-related decrease in neurogenesis. For instance, neural progenitor cells have been reported to proliferate at a slower rate in middle-aged rats compared to adults (Walter et al., 2011) and there is evidence that the number of viable stem cells is reduced in aged animals (Rao et al., 2005; Seki and Arai, 1995). An additional contributing factor may be age-related changes in the neuroendocrine system, as prolonged reductions in corticosterone levels have been reported to attenuate the age-associated reduction in hippocampal neurogenesis (Montaron et al., 2006). Age-related changes in microglial cell activity and elevated basal levels of neuroinflammatory molecules may further contribute to suppression of neurogenesis.
Normal aging, in the absence of an infection or injury, is associated with increased microglia activity and development of low-grade chronic inflammation (for review see Dilger and Johnson, 2008). Microglia in the aged brain are primed towards the classic inflammatory phenotype, as microglia in aged animals show increased expression of MHC II, CD86 and moderately elevated levels of IL-1β and IL-6 (Frank et al., 2006; Godbout et al., 2005; Griffin et al., 2006; Perry et al., 1993; Sierra et al., 2007; Tarr et al., 2011; Ye and Johnson, 1999). Additionally, in vivo and in vitro models have shown an age-related increase in the proportion of dividing microglia and that aged female, but not male, mice show an increase in the total number of microglia in the hippocampus compared to adults (Kohman et al., 2011; Mouton et al., 2002; Rozovsky et al., 1998). Presently, the factors or events that induce these age-related changes microglia have yet to be fully elucidated. However, an age-related decrease in neuroimmune regulatory molecules (e.g., CD200 and fractalkine), an increase in irregular protein folding, and microglia senescence have been proposed to contribute to the development of primed microglia within the aged brain (Dilger and Johnson, 2008; Frank et al., 2006; Streit and Xue, 2009).
The age-related decrease in hippocampal neurogenesis is correlated with microglia activation and levels of proinflammatory cytokines within the brain (Gemma et al., 2007; Kuzumaki et al., 2010). As noted, conditioned medium from microglial cells from aged mice was less effective in stimulating neurogenesis in culture compared to medium from adult mice, potentially indicating that microglia from aged animals may release factors that are detrimental to neurogenesis or have decreased expression of factors that support neurogenesis (Walton et al., 2006). Additionally, Gemma et al. (2007) report that administration of the capase-1 inhibitor, which blocks the conversion of IL-1β to the mature form, significantly increases the number of new cells in the granular cell layer in aged rats. Similar increases in neurogenesis were obtained when aged rats were treated with fractalkine (Bachstetter et al., 2011). The chemokine, fractalkine, is a neuroimmune regulatory molecule released by neurons that acts on CX3CL1 receptors (CX3CR1) localized on microglial cells and maintains microglia in their resting phenotype (Cardona et al., 2006; Harrison et al., 1998). Aged rats show reduced protein levels of fractalkine within the hippocampus relative to adults (Bachstetter et al., 2011). Restoring fractalkine levels in aged rats by administrating fractalkine for seven days was found to increases the number of new cells within the granule cell layer. The ability of fractalkine to increase neurogenesis in aged rats is proposed to results from a reduction in IL-1β levels. Chronically blocking the CX3CR1 receptor in adults is reported to increase IL-1β levels and decrease proliferation. Further, the anti-proliferative effects of inhibiting the CX3CL1 receptor can be blocked if an IL-1 receptor antagonist is administered (Bachstetter et al., 2011). These findings indicate that decreased levels of fractalkine reduces regulatory control over microglia and subsequently results in an increase in IL-1β that has been shown to inhibit neurogenesis. Collectively, the data indicate that age-related changes in neuroinflammatory molecules contribute to the decline in hippocampal neurogenesis with aging. However, neuroinflammation is clearly not the only factor regulating neurogenesis during aging. Decreases in neurogenesis occur much sooner in life than neuroinflammatory changes. Moreover, while attenuating inflammation within the aged brain increases neurogenesis, the recovery is not complete. Hence, attenuation of neuroinflammation can only modify the naturally occurring downward trajectory of adult neurogenesis, it cannot reverse it. Inflammation can further suppress neurogenesis during aging, but others factors such as loss of stem cells, elevated corticosterone level, or senescence of cells produce the major declines in hippocampal neurogenesis with aging.
Potential role of neurogenesis in inflammation-induced cognitive deficits
Activation of the immune system leads to a host of well characterized behavioral changes, some of which are adaptive responses that facilitate recovery and others less so. Sickness behavior is a constellation of behavioral alterations that include a decrease in locomotion, social and sexual behavior, development of anorexia and a fever response (Dantzer, 2004). These behavioral changes are thought to reflect an altered motivation state rather than a physical disability and expedite recovery through energy conservation among other mechanisms. In addition to the expression of sickness behaviors, activation of the immune system has been shown to impair aspect of cognitive function. These inflammation-induced cognitive deficits may simply reflect unwelcomed side effects of immune activation that can persist beyond the expression of sickness behaviors (Kohman et al., 2007). While it is beyond the scope of the present review to discuss all of the research that demonstrates inflammation can impair cognitive function we intend to highlight central findings and the potential connections between the alterations in behavior and hippocampal neurogenesis.
Though exceptions can be found, the cognitive deficits induced by inflammation are typically observed in hippocampus-dependent tasks, such as contextual fear conditioning, the Morris water maze, or tasks that have a hippocampal component such as two-way active avoidance (Hein et al., 2010; Kohman et al., 2007; Kranjac et al., 2012; Pugh et al., 1998; Sparkman et al., 2005; Yirmiya and Goshen, 2011) whereas tasks such as auditory fear conditioning that are independent of hippocampal activity are unaffected (Pugh et al., 1998). Inflammation has been shown to affect various stages of memory formation from impairing acquisition to disrupting consolidation and reconsolidation broadening the potential scope of processes that may be affected by inflammation (Kohman et al., 2007; Kranjac et al., 2012; Pugh et al., 1998; Yirmiya and Goshen, 2011). Generally, immune activation is a transient response and with it the deficits in cognition recede as the immune response terminates. However, certain factors such as normal aging or the presence of a chronic inflammatory disease may make individuals more susceptible to persistent inflammation-induced cognitive deficits. Therefore identifying the mechanisms through which inflammation impairs cognitive processes may have particular benefit for individuals suffering from conditions characterized by chronic inflammation.
Currently, the mechanisms through which inflammation impairs cognitive function remain unknown. However, several processes known to play a role in learning and memory are impaired following immune activation. For instance, evidence indicates that inflammation may impair cognitive processes through disrupting aspects of long-term potentiation (LTP), one of the most widely recognized cellular mechanisms for learning and memory (Cunningham et al., 1996; Murray and Lynch, 1998). An additional mechanism through which inflammation could disrupt cognitive function is through altering levels of neurotrophic factors in the brain such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), and insulin-like growth factor (IGF), which are known to be critically involved in supporting memory formation, neurogenesis and LTP (Fan et al., 1995; Guan and Fang, 2006; Kranjac et al., 2012; Park et al., 2011).
Inflammation is also associated with reduced basal and event-induced neural activation as measured by expression of the immediate early gene Arc (Frank et al., 2010; Hein et al., 2010), indicating that neuroinflammation blunts the brain response to a learning experience as compared with a healthy brain. As described, inflammation decreases neurogenesis which has been correlated with performance in several of the behavioral tasks that show deficits following an immune challenge. These data potentially indicate that the inflammation-induced deficits in cognitive performance may be related to the reductions in neurogenesis, particularly when the inflammatory response persists for extended periods of time. However, presently there is no direct evidence to indicate that inflammation-induced suppression of hippocampal neurogenesis mediates the cognitive deficits associated with immune activity. Future work is needed to address the extent to which reductions in hippocampal neurogenesis directly contribute to the cognitive deficits following immune activation.
Conclusion
The evidence clearly indicates that neuroinflammation can suppress hippocampal neurogenesis. However, we are just beginning to understand the role microglia play in regulating neurogenesis under basal conditions in the absence of an infection or injury. Clearly, microglia are not simply waiting around in the brain for an infection to occur, but rather are participating in basal neural processes, such as neurogenesis. Progress in research has created a broader view of microglia’s role within the brain. Potentially through understanding the phenotypes microglial cells can acquire, and the factors and circumstances that induce the varied states of activation, we can identify means of regulating microglia in ways that promote brain health and cognitive function. Further, a more complete understanding of the immune system’s interaction with new cells born in the adult brain and how immune activation contributes to or modifies the neurogenic niche has vast potential to aid in development of novel therapies to facilitate regenerative processes within the brain. New strategies that target microglia cells and/or neuroinflammatory signaling in the brain could potentially improve the success of neural replacement therapy, and help to preserve the rate of neurogenesis throughout life.
Highlights.
New neurons are continuously produced in two regions of the adult brain, the hippocampus and the olfactory bulb. This review discusses current understanding of how the immune system affects adult neurogenesis and the implications for cognitive function.
Acknowledgments
Funding: Supported by grants from National Institute of Health, MH083807 and DA027487 to J.S.R and from National Institute on Aging K99AG0404184 to R.A.K.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–52. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci Lett. 2004;359:13–6. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Amrein I, Isler K, Lipp HP. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci. 2011;34:978–87. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–22. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Annenkov A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis. Mol Neurobiol. 2009;40:195–215. doi: 10.1007/s12035-009-8081-0. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–44. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–21. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos GN, Moriya T, Inui F, Katura T, Nakahata N. Involvement of cyclooxygenase-2 in lipopolysaccharide-induced impairment of the newborn cell survival in the adult mouse dentate gyrus. Neuroscience. 2008;155:454–62. doi: 10.1016/j.neuroscience.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Arellano C, Ferguson R, Jopson T, Rosi S. Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav Immun. 2012;26:18–23. doi: 10.1016/j.bbi.2011.07.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis. 2012;9:187–98. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells. 2009;27:2044–52. doi: 10.1002/stem.120. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012 doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–25. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, Deyoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012 doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation- neurogenesis coupling. Neuron. 2005;47:775–7. doi: 10.1016/j.neuron.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008;28:10766–71. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Char D, Bagby GJ, Gelato MC, Lang CH. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204–12. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–62. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Akema T. Lipopolysaccharide acutely inhibits proliferation of neural precursor cells in the dentate gyrus in adult rats. Brain Res. 2010;1352:35–42. doi: 10.1016/j.brainres.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O’Keeffe GW, Nolan YM. A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311–21. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–72. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–53. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Grandgirard D, Burri D, Frohlich TK, Leib SL. Bacterial meningitis impairs hippocampal neurogenesis. J Neuropathol Exp Neurol. 2011;70:890–9. doi: 10.1097/NEN.0b013e3182303f31. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16:439–51. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–88. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–54. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Keene CD, Chang R, Stephen C, Nivison M, Nutt SE, Look A, Breyer RM, Horner PJ, Hevner R, Montine TJ. Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol. 2009;174:2300–9. doi: 10.2353/ajpath.2009.081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–9. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43:127–35. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kerr AL, Steuer EL, Pochtarev V, Swain RA. Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition. Neuroscience. 2010;171:214–26. doi: 10.1016/j.neuroscience.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2011 doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci U S A. 2011;108:10326–31. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Deyoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Sparkman NL, Day CE, Paquet A, Akkaraju GR, Boehm GW. Alleviation of the effects of endotoxin exposure on behavior and hippocampal IL-1beta by a selective non-peptide antagonist of corticotropin-releasing factor receptors. Brain Behav Immun. 2007;21:824–35. doi: 10.1016/j.bbi.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranjac D, McLinden KA, Deodati LE, Papini MR, Chumley MJ, Boehm GW. Peripheral bacterial endotoxin administration triggers both memory consolidation and reconsolidation deficits in mice. Brain Behav Immun. 2012;26:109–21. doi: 10.1016/j.bbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–59. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–8. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Piantanida AP, Pitossi F. Chronic expression of transforming growth factor-beta enhances adult neurogenesis. Neuroimmunomodulation. 2010;17:200–1. doi: 10.1159/000258723. [DOI] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–88. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- Morrens J, Van Den Broeck W, Kempermann G. Glial cells in adult neurogenesis. Glia. 2012;60:159–74. doi: 10.1002/glia.21247. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–5. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–30. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- Olah M, Ping G, De Haas AH, Brouwer N, Meerlo P, Van Der Zee EA, Biber K, Boddeke HW. Enhanced hippocampal neurogenesis in the absence of microglia T cell interaction and microglia activation in the murine running wheel model. Glia. 2009;57:1046–61. doi: 10.1002/glia.20828. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park SE, Lawson M, Dantzer R, Kelley KW, McCusker RH. Insulin-like growth factor-I peptides act centrally to decrease depression-like behavior of mice treated intraperitoneally with lipopolysaccharide. J Neuroinflammation. 2011;8:179. doi: 10.1186/1742-2094-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–7. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–29. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–71. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–76. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–16. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE. Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging. 1998;19:97–103. doi: 10.1016/s0197-4580(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Rutten BP, Brasnjevic I, Steinbusch HW, Schmitz C. Caloric restriction and aging but not overexpression of SOD1 affect hippocampal volumes in mice. Mech Ageing Dev. 2010;131:574–9. doi: 10.1016/j.mad.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–8. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Seguin JA, Brennan J, Mangano E, Hayley S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat. 2009;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–82. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012 doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005;86:244–51. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–9. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, McLinden KA, Kranjac D, Kohman RA, Amaral W, Boehm GW. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1beta expression. Behav Brain Res. 2011;217:481–5. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Thuret S, Toni N, Aigner S, Yeo GW, Gage FH. Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus. 2009;19:658–69. doi: 10.1002/hipo.20550. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–11. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–92. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–43. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32:1906–14. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23:3121–8. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJ, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci Lett. 2005;390:76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Wu MD, Hein AM, Moravan MJ, Shaftel SS, Olschowka JA, O’Banion MK. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1beta and not rescued by voluntary running. Brain Behav Immun. 2012;26:292–300. doi: 10.1016/j.bbi.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Shen Q, Dong S, Xu Z, Tsien JZ, Hu Y. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol Aging. 2008;29:1502–11. doi: 10.1016/j.neurobiolaging.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang J, Giesert F, Kloos K, Vogt Weisenhorn DM, Aigner L, Wurst W, Couillard-Despres S. A powerful transgenic tool for fate mapping and functional analysis of newly generated neurons. BMC Neurosci. 2010;11:158. doi: 10.1186/1471-2202-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]


