Abstract
Previous in vitro studies have shown that CD4 T cells from old mice have defects in T cell receptor (TCR) signaling, immune synapse formation, activation, and proliferation. We have reported that removing a specific set of surface glycoproteins by ex vivo treatment with O-sialoglycoprotein endopeptidase (OSGE) can reverse many aspects of the age-related decline in CD4 T cell function. However, the specific mechanism by which this process occurs remains unclear, and it is unknown whether this enzymatic treatment can also restore important aspects of adaptive immunity in vivo. By using an in vivo model of the immune response based on adoptive transfer of CD4 T cells from pigeon cytochrome C (PCC)-specific transgenic H-2(k/k) TCR-Vα11Vβ3 CD4+ mice to syngeneic hosts, we now demonstrat that aging diminishes CD28 costimulatory signals in CD4 T cells. These age-associated defects include changes in phosphorylation of AKT and expression of glucose transporter type I, inducible T-cell costimulatory molecule, and CD40 ligand, suggesting that the lack of CD28 costimulation contributes to age-dependent loss of CD4 function. All of these deficits can be reversed by ex vivo OSGE treatment. Blocking B7-CD28 interactions on T cells prevents OSGE-mediated restoration of T cell function, suggesting that changes in surface glycosylation, including CD28, may be responsible for age-related costimulation decline. Finally, we showed that the age-related decline in CD4 cognate helper function for immunoglobin G production and long-term humoral immunity can also be restored by OSGE treatments of CD4 T cells prior to adoptive transfer.
Keywords: T cells, Aging, Lymphocytes, TCR signaling, Glycoproteins
Introduction
In vitro T cell activation involves the physical interaction of the T cell receptor (TCR)-CD3 chains with costimulatory molecules such as CD28 (1–4). These costimulatory molecules help to elicit a response leading to the formation of the TCR complex and reorganization of the cytoskeleton (5–7). The interaction between B-7 and the CD28 molecule expressed on the surface of T cells enhances the PI3K-AKT signaling pathway, including phosphorylation of AKT kinase at threonine 308 (pAKT308) (8–10). Activation of PI3K-AKT also leads to increased expression of several proteins, including glucose transporter type I (Glut-1) (11–15) and inducible T-cell costimulatory molecule (ICOS), involved in T cell-dendritic cell (DC) interactions (16) and follicular helper T cell function (17, 18). Progression of T cell activation also leads to expression of CD40 ligand (CD40L), which interacts with CD40 on B cells to trigger the antibody class switch from immunoglobin M to IgG (19). T cells also express negative regulators of activation such as programmed cell death-1 (16). The balance between interactions of these surface molecules and their targets on other immune cells determines the level of T cell response, thereby controlling the amount and diversity of the humoral response to an antigen (20).
In humans and mice, humoral immunity declines with age, resulting in limited IgG diversity and defects in long-term memory formation (21). It is likely that age-related defects in CD4 T cell function limit the production of a robust humoral response in elderly individuals (22–24). Using an adoptive transfer model, Haynes et al. found that CD4 cells from old donor mice did not proliferate well and had reduced CD40L expression (23). Furthermore, using CD4 T cells from transgenic AND mice, whose naïve CD4 cells express Vβ3-TCR+ recognizing amino acids 88 to 103 of pigeon cytochrome C (PCC), we have shown that age-related changes inhibit the early steps of T cell antigen-presenting cell interactions (T-APC), including immune synapse formation and TCR signaling (25). We hypothesized that changes in glycosylation of T cell proteins (26) might contribute to these defects in T cell-APC interactions and decline in downstream signaling, including that of CD28 (27–29). To test the functional implications of altered protein glycosylation, we evaluated the effects of removing specific sets of surface glycoproteins by using a bacterial enzyme, O-sialoglycoprotein endopeptidase (OSGE). OSGE digests segments of extracellular proteins that contain O-linked glycans bearing terminal sialic acid residues, including CD43, CD44, and CD45, but OSGE does not cleave the TCR chains and several costimulatory molecules (25). We found that OSGE treatment of CD4 T cells could reverse several age-related defects in T cell activation, including synapse formation, expression of CD25, cytokine production, and cytotoxic function (30–32). More recently, we have used an in vivo model of T cell activation based on adoptively transferring naïve CD4 T cells from young and old transgenic AND mice (Vβ3-TCR+) to syngeneic B10.BR hosts primed with PCC. This system documented age-related declines in early activation and proliferation that could be reversed by ex vivo OSGE treatment (33). Here we report experiments designed to elucidate the mechanism by which aging affects in vivo CD4 T cell cognate helper function, and shed light on its restoration by OSGE treatment. We also show that OSGE exposure of CD4 T cells leads to increased helper function for production of antigen-specific IgGs and enhanced long-term immunity after adoptive transfer into host mice.
Material and Methods
Animals and reagents
H-2(k/k) TCR-Vα11Vβ3 CD4+ mice (AND mice) and CD4 knock out (KO) mice on the B10.BR background were bred in our facilities from stock generously provided by Susan Swain and Laura Haynes (Trudeau Institute, NY). Specific-pathogen free B10.BR and [BALB/c × C57BL/6]F1 (CB6F1) mice were purchased from the Charles River Laboratories (Kingston, NJ) and from the National Institute of Aging contract colonies at Harlan (Indianapolis, IN), respectively. The mice were housed at the University of Michigan and were given free access to food and water. Sentinel animals were examined quarterly for serological evidence of viral infection; all tests were negative during the course of these studies. Mice found to have splenomegaly or macroscopically visible tumors at the time of sacrifice were not used for the experiments. AND mice used in the study were at 6–8 or 16–18 months of age, and the B10.BR or CD4KO adoptive host mice were 2–4 months of age. CB6F1 used in the study were at 6–8 or 20–22 (old mice) months of age.
All chemical reagents and Pigeon Cytochrome C (PCC) were purchased from Sigma (www.sigmaldrich.com). OSGE was obtained from Accurate Chemicals (www.accuratechemical.com). All flow cytometry antibodies were purchased from Biolegends (www.biolegends.com) or Becton Dickinson (www.bdbiosciences.com). Rabbit anti-Glut-1 was obtained from Epitomics (www.epitomics.com) and rabbit anti-pAKT(threonine 308) from Cell Signaling (www.cellsignal.com). CTLA4Ig was obtained from Pfizer Pharmaceutical (www.Pfizer.com). Anti-IgG isotypes coupled to alkaline phosphatase were purchased from Jackson Immunoresearch (www,jacksonimmuno.com). DNP-BSA was obtained from Biosearch Technologies (www.biosearchtech.com) while PCC-DNP was produced in our labs by coupling PCC and DNP-e-Aminocaproyl-OSu (PCC/DNP ratio 1:20). Naïve CD4 cells from the spleen and lymph nodes were obtained by negative selection using the Miltenyi CD4 purification kit II and CD62L positive selection, according to the manufacturer’s recommendations (www.miltenyibiotec.com). Analysis of a typical preparation showed the cells to be 90% positive for both CD3 and CD4.
In vitro T cell stimulation and detergent treatments
CD4 T cell stimulation and detergent treatments were performed as previously described (34). Briefly, CD4 T cells from young and old CB6F1 mice were purified by negative selection and 10 × 106 cells were incubated with CD3-FITC, CD28-PE, and CD4-PECy5 (10 μg/mL) in 1 mL of 5% BSA in HBSS for 45 min at 4°C. Cells were washed and TCR complex formation was initiated by crosslinking with 0.5 mL of goat anti-rat IgG (5 μg/mL) for 5 min at 37°C. Cells were washed and resuspended in 0.5 mL of PBS and 100-μl aliquots were incubated with 1 mL of 1% Brij-96 for 15 min at room temperature. The resulting cell suspensions were analyzed by flow cytometry.
Priming, adoptive transfer, blocking of CD28 signaling with a CTLA4 construct, and analysis of in vivo CD4 activation
Naive CD4 T cells from young and old AND mice were purified using the Miltenyi naïve CD4 purification kit, based on CD4 negative and CD62L positive selection. Then, each cell preparation was divided into 2 aliquots; one was left untreated and the other was treated with OSGE as described elsewhere (25). The cells were resuspended in HBSS and injected into the tail vein of host mice at numbers indicated below. For the CD28 blocking experiments, a CTLA4Ig construct was used as previously described (35, 36), with modifications. In some experiments, as indicated, a single dose of 500 μg of CTLA4Ig was injected intramuscularly prior to adoptive transfer CD4 T cells. In these experiments another dose of 500 μg of CTLA4Ig was injected intramuscularly, 24 hr later, followed immediately by adoptive transfer of the CD4 T cells. Then, at specific time points indicated in the text, lymph nodes (pooled cervical, axillary, brachial, and inguinal nodes), and spleens were harvested from each recipient. CD4 T cells were then purified by negative selection (37) and stained for with CD4 (PECy5), TCR-Vβ3 (PEclone KJ25), ICOS (biotin clone 7E), PD-1 (biotin clone RPM1–30), CD69 (biotin clone H1.2F3), and CD40L (biotin clone MR1) followed by Pacific Blue or alexa-700 streptavidin. Intracellular stains were performed as described (38) by permeabilization with 1% Triton in PBS followed by staining for Glut-1 or pAKT(308) using a FITC-coupled goat anti-rabbit secondary antibody. All analyses was performed using a Canto-II Flow Cytometer and FlowJo software, gating for CD4+ and TCR-Vβ3+ cells.
Immunization and antigen-specific IgG quantification by ELISA
Each experiment compared naïve CD4 T cells purified independently from 2 young and 2 old AND mice or from CB6F1 donor mice. Cells from each donor mouse were divided into 2 aliquots, of which one was treated with OSGE. Treated and control CD4 T cells from each donor mouse were then transferred to 3 individual host mice as described below. Host mice were primed with a single 100-μL injection of an emulsion containing 200 μg or 100 μg of DNP-PCC in incomplete Freund’s adjuvant in the left flank of the leg. Fifteen and 30 days later, blood was collected from the tail vein, and re-immunization was performed on day 45 for each host mouse by injecting 100 μg of DNP-PCC in incomplete Freund’s adjuvant. Blood was collected from the tail vein at days 60 and 75 after the first immunization, i.e., 15 or 30 days after the booster injection. For each serum sample, the amount of each IgG isotype (IgG1, IgG3, IgG2a, IgG2b, and IgG2c) against DNP hapten or PCC was measured by enzyme-linked immunosorbent assay using DNP-BSA and PCC (full protein) as targets.
Statistical analysis
Unless otherwise indicated, results are presented as means ± standard error of the mean (SEM). Statistical significance was assessed using a Mann-Whitney test, with the significance level set at p = 0.05.
Results
Aging reduces CD28 association to the TCR complex on CD4 T cells, but this defect can be reversed by OSGE treatment
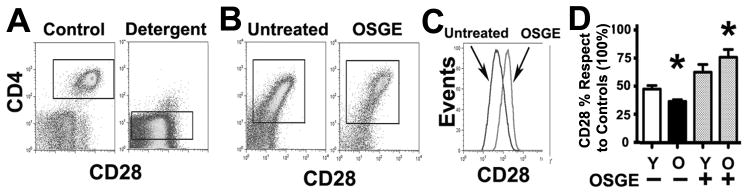
We have shown using in vitro assays that aging causes a significant decline in early TCR signals, cytoskeleton reorganization, and immune synapse formation (39, 40). We have also shown that this decline can be restored by treating CD4 T cells ex vivo with OSGE, an enzyme that removes a specific group of surface glycoproteins (25). We hypothesized that age-related defects in TCR signaling could be the result of decreased association between the CD28 costimulatory molecule and the TCR complex, and that OSGE treatments may restore CD28 association. During early CD4 activation, the TCR and costimulatory molecules form a complex that associates to the cytoskeleton and becomes resistant to lipid extraction by mild detergents such as Brij-96 (41–46). We used resistance to Brij-96 solubilization to test whether aging reduced CD28 association to the TCR complex. Purified CD4 T cells were treated with OSGE, stained for CD3, CD4, and CD28 and stimulated via the TCR. CD4 cells were then extracted with Brij-96 and evaluated by flow cytometry to measure the association of CD28 and CD4 to the detergent-resistant TCR complex, as described in Methods section. Figure 1A shows the levels of CD28 expression in resting CD4 T cells from young mice and the removal of CD28 after detergent treatment; these results implied that CD28 was not associated with the cytoskeleton or TCR before CD4 stimulation. After TCR stimulation, however, the TCR and CD28 formed a complex, rendering CD28 resistant to detergent extraction (Figure 1B, untreated cells). Pre-treating the cells with OSGE prior to TCR stimulation led to higher association of CD28 to the complex (Figure 1B, OSGE panel). The effect of OSGE treatment on detergent-resistant CD28 is shown in Figure 1C. To quantify these effects, we performed a series of 4 independent experiments (Figure 1D) using naïve CD4 T cells from young and old donors, normalizing the mean CD28 intensity for each group against that of the control young CD4 T cells (100%). We found no effect of stimulation, age, or OSGE treatment on the intensity of CD28, CD4, or CD3 for controls not exposed to detergent (data not shown), suggesting that neither OSGE nor aging affected antibody binding per se. OSGE treatment increased retention of CD28 in detergent-treated stimulated CD4 T cells from young donors, although this increase did not reach statistical significance (Figure 1D). In the absence of OSGE exposure, detergent-treated, stimulated CD4 T cells from old donors had a lower mean CD28 fluorescence than those from young donors (Young = 48%; Old = 36%; p = 0.009). These results suggest that age reduces the association of CD28 to the TCR complex following T cell stimulation. OSGE treatment of CD4 T cells from old donors led to significant enhancement of CD28 association to the TCR complex (untreated Old = 36%; OSGE-treated Old = 76%; p = 0.0003). These results suggest that OSGE treatment might enhance CD28 signaling in CD4 T cells from old mice by increasing recruitment of CD28 to the TCR complex.
Figure 1. Flow cytometric analysis of CD28 association to the TCR complex in young and old CD4 T cells from CB6F1 mice.
A) Detergent treatment removes most of the CD28 found on the surface of unstimulated CD4 T cells. Resting CD4 T cells from CB6F1 mice were stained for CD3 (FITC), CD28 (PE), and CD4 (PECy5), treated with Brij-96 (Detergent) or vehicle (Controls), then analyzed by flow cytometry to evaluate CD28 fluorescence relative to CD4 fluorescence. B) TCR stimulation increases the resistance of CD28 to detergent extraction in CD4 T cells. Aliquots of untreated (left panel) or OSGE-treated (right panel) CD4 T cells were stained for CD28, stimulated by cross linking CD3, CD4, and CD28, exposed to detergent, and analyzed by flow cytometry for CD28 fluorescence. C) The histogram shows the difference between CD28 fluorescence of untreated cells (dark line) compared to OSGE-treated cells (light line). D) Quantification of CD28 associated to the detergent-resistant TCR complex. Each bar shows the relative mean CD28 retention ± SEM relative to controls (no detergent, 100%) in untreated and OSGE-treated CD4 T cells from 10 young (Y) and 10 old (O) CB6F1 mice. The asterisk (*) indicates statistical significance with respect to the young untreated group (p < 0.05).
Aging reduces CD28-dependent phosphorylation of AKT kinase and Glut-1 expression in vivo, and OSGE treatment restores both defects
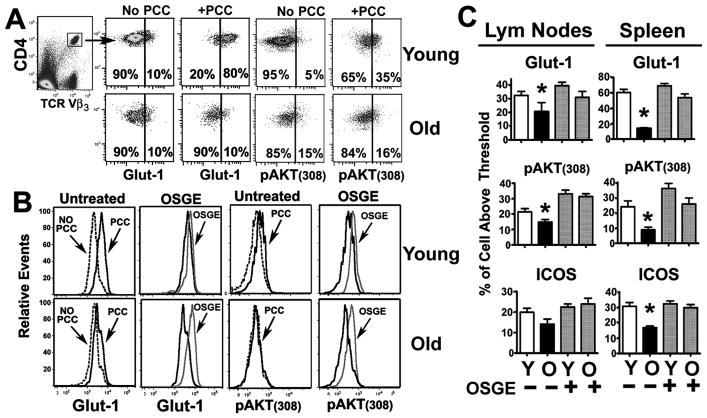
To test this hypothesis, we measured two CD28-dependent signaling events: phosphorylation of AKT at threonine 308 (pAKT(308)) and expression of Glut-1 (8–10), using young CD4KO host mice that had received 2 × 106 naïve CD4 T cells purified by positive selection as described in the Methods section (24). CD62L positive selection does not appear to affect naïve CD4 T cell activation, proliferation, or homing relative to CD4 T cells purified by negative selection (data not shown). For the adoptive transfer experiments, we used CD4KO mice as hosts, because these mice lack endogenous CD4 cells, which in turn facilitates the analysis of adoptively transferred CD4 T cells. In the absence of antigen (No PCC in Figure 2A), gated CD4+ TRC-VB3+ cells from young and old donors showed a bimodal distribution, with less than 15% of cells having high Glut-1 expression (Glut-1Hi) and high phosphorylation of AKT at threonine 308 (pAKT(308)Hi). The vertical line in the scatterplots of Figure 2 shows the threshold used to define cells as Glut-1Hi and pAKT(308)Hi. As expected, priming the adoptive hosts with PCC increased the expression of Glut-1 and pAKT(308) in CD4 T cells from young donors, with or without prior OSGE exposure (Figure 2B, top panels). CD4 T cells from aged mice did not show an increase in pAKT(308) and Glut-1 expression unless they had been treated with OSGE prior to the transfer (Figure 2B for comparison between OSGE treated and untreated). Figure 2C shows the mean values for the percentage of cellular expression of Glut-1Hi and pAKT(308)Hi in CD4 T cells, isolated from lymph nodes and spleen, thus illustrating the ability of OSGE to restore CD28 dependent signaling to aged CD4 T cells. Similar effects were also observed in the ICOS expression assays (Fig. 2C). We found significant age-related declines in the number of CD4 T cells scored as Glut-1Hi (2-fold in the lymph nodes, p = 0.03, and 4-fold in the spleen, p = 0.016). We found similar results in the case of pAKT(308)Hi levels (2-fold in the lymph nodes, p = 0.04, and 3-fold in the spleen, p = 0.03). OSGE treatments did not seem to enhance Glut-1 or pAKT(308) expression in CD4 T cells from young donors; however, OSGE treatment significantly increased both signals in CD4 T cells from old mice (lymph nodes: Glut-1, p = 0.04 and pAKT(308), p = 0.02; spleen: Glut-1, p = 0.02 and pAKT(308), p = 0.03). These results suggest that in vivo CD28 signaling declines with age and that ex vivo OSGE treatments can restore this costimulatory pathway. It has been suggested that de novo ICOS synthesis in CD4 T cells requires CD28 costimulation, and that ICOS can play a critical role in humoral immunity (2, 20). We found that aging resulted in a 1 to 2-fold decline in the numbers of CD4 T cells expressing ICOS after adoptive transfer; however, these differences only reached statistical significance in the spleen (p = 0.02). ICOS expression was also restored by OSGE treatment in CD4 T cells from old mice (lymph nodes, p =0.05; spleen, p = 0.02).
Figure 2. Age impairs, and OSGE improves, induction of Glut-1, pAKT, and ICOS in adoptively transferred CD4 T cells.
A) pAKT(308) levels and Glut-1 expression in resting and activated CD4 T cells. CD4 cells from AND mice were adoptively transferred to non-primed (no PCC) or PCC-primed CD4KO mice, and spleens and lymph nodes were collected 16 hours later. T cells were purified and stained for CD4/VB3-TRC, Glut-1, and pAKT(308). Gated CD4+VB3+ cells were used to evaluate the expression of Glut-1 or pAKT(308) in resting or activated CD4 T cells. The vertical line shows the threshold used to distinguish Glut-1 and pAKT(308)low from Glut-1 and pAKT(308)hi cells for the calculations illustrated in panel (C). B) Effect of aging and OSGE treatments on Glut-1 expression and pAKT(308). Representative analysis of the effects of aging and OSGE on Glut-1 expression and pAKT(308) in CD4 T cells. Untreated or OSGE-treated CD4 T cells from young (top histograms) or old (bottom histograms) AND mice were adoptively transferred to CD4KO hosts primed with PCC (dark lines) or left unprimed (dot lines). Glut-1 expression was analyzed as described in panel A above. Priming with PCC increased Glut-1 expression and pAKT on gated CD4VB3+ cells from young, but not from old, donor mice. Pre-treatment with OSGE (light lines) enhanced Glut-1 and pAKT in primed CD4 T cells from old donors. C) Quantification of the effects of aging and OSGE on Glut-1, pAKT, and ICOS. Each bar represents mean percentage (± SEM) of CD4 T cells from 8 young and 4 old AND donor mice with enhanced expression of Glut-1 or ICOS and increased AKT phosphorylation. The asterisk (*) indicates statistical significance with respect to untreated young cells in primed hosts.
To test whether OSGE restoration of CD4 T function from old micewas CD28 dependent, B10.BR host mice were treated with a CTLA4Ig construct known to block the CD28/B7 interaction in vivo (35, 36). We treated B10.BR host mice with CTLA4Ig or vehicle prior to adoptive transfers, as described in the Methods section. Then, purified naïve CD4 T cells from young and old AND mice were labeled with CFSE, treated with OSGE or vehicle as previously described (47), and 2 × 106 CD4 cells were adoptively transferred to each B10.BR untreated or CTLA-treated mouse. Eighteen hours later, the lymph nodes and spleen were harvested and stained as described in the Methods section and as shown in Figure 2 (with modifications), with gating for CFSE+, VB3+, and CD4+ cells to discriminate between the adoptively transferred and endogenous CD4 T cells. Then, gated CD4 T cells were analyzed for Glut-1, pAKT(308), and ICOS expression, as well as CD69 expression as described elsewhere (33). The scatterplots obtained for each stain were similar to those shown in Figure 2A (data not shown). Figure 3 shows the mean ± SEM from 3 different experiments. As expected, and similar to the CD4KO model, we found significant age-related declines in the expression of Glut-1, ICOS, CD69, and pAKT(308), while OSGE treatments restored their expression. However, host mice treated with CTLA4Ig (Figure 3, CTLA4+ bars), without prior treatment by OSGE, showed significantly inhibited signaling and activation of CD4 T cells from young donors, although little effect was noted in OSGE-untreated CD4 T cells from old donors. When CD4 T cells were treated with OSGE, we found that CTLA4Ig treatment could significantly inhibit OSGE-mediated restoration of CD4 T cell activation in cells from old donors (indicated by significantly decreased Glut-1, pAKT(308), ICOS, and CD69 staining in OSGE-treated cells). These findings suggest that the effects of OSGE on CD4 T cell function in old mice are driven by restoration of CD28 signaling.
Figure 3. Inhibition of B7-CD28 interaction can prevent OSGE-mediated restoration of Glut-1, pAKT, CD69, and ICOS in adoptively transferred CD4 T cells from old mice.
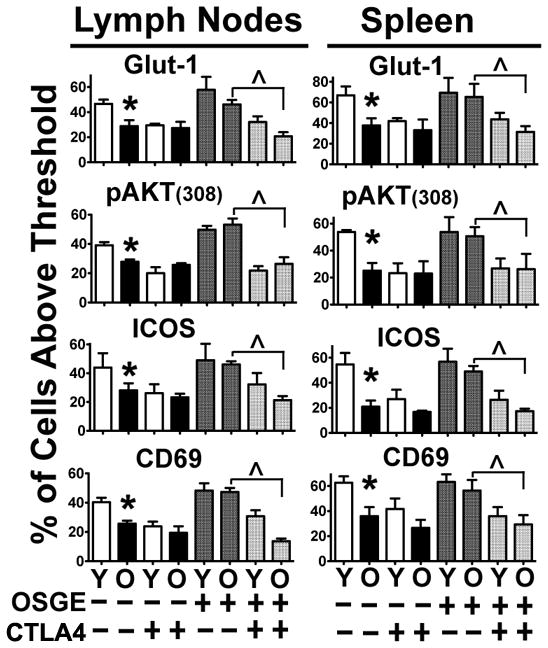
B10.BR host mice were untreated or treated with CTLA4Ig prior the adoptive transfer of CD4 T cells from young and old AND mice treated with OSGE or vehicle. Glut-1, pAKT(308), CD69, and ICOS were analyzed 18 hours later by flow cytometry as described in Figure 2. Each bar represents the mean percentage (± SEM) of CD4 T cells from 3 independent experiments, with a total of 3 young and 3 old AND donor mice. The asterisk (*) indicates statistically significant declines in the CD4 response from old mice, while the (^) represents statistically significant inhibition of the OSGE-mediated restoration of these processes by CTLA4Ig treatment of CD4 T cells from old mice.
Of note, CTLA4Ig treatment did not appear to deplete the populations of antigen-presenting cells such as macrophages (data not shown, CD11b marker in spleen =1.44% and lymph nodes =0.54% versus CTLA4-treated spleen =1.23% and lymph nodes =0.41%), dendritic cells (CD11c in spleen = 2.3% and lymph nodes =0.8% versus CTLA4-treated spleen =2.03% and lymph nodes = 0.63%), or B cells (B220 in the spleen = 58% and lymph nodes = 36% versus CTLA4-treated spleen = 58% and lymph nodes = 32%).
Aging reduces CD40L expression, but not PD-1 expression, of CD4 T cells in vivo, and OSGE treatments can reverse this defect
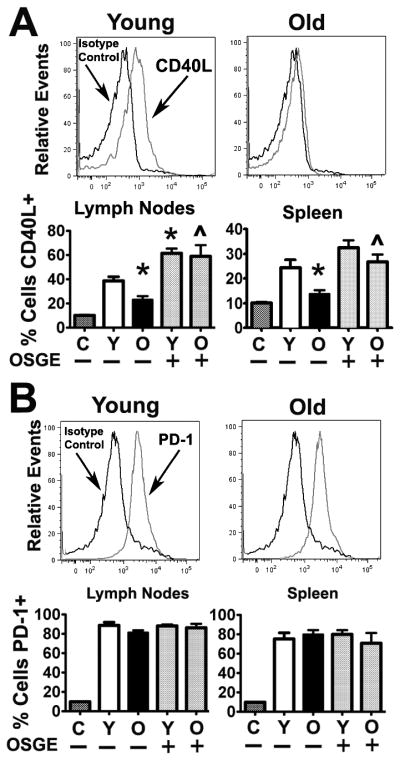
To test whether OSGE treatments affected the expression of CD40L and programmed cell death 1 (PD-1) expression, which are important factors in the regulation of humoral immunity and T cell expansion, respectively (48–50), we performed an adoptive transfer experiment similar to those shown in Figure 2, and evaluated CD4 T cells 5 days after cell transfer. As shown in Figure 4A, CD4 T cells derived from old mice expressed very low levels of CD40L following adoptive transfer, suggesting defects in cognate helper function relative to CD4 T cells from young mice. However, as shown in Figure 4B, PD-1 was expressed in CD4 T cells from both young and old donors. Quantification based on 3 independent experiments (a total of 8 young and 4 old AND donors) shows a significant 2-fold age-related decrease in CD40L expression (Figure 4A histograms; lymph nodes, p = 0.009; spleen, p= 0.009) but no effect of aging on PD-1 expression. OSGE treatments enhanced CD40L expression in CD4 T cells from young mice, although this difference was only statistically significant in the lymph nodes (p = 0.02). On the other hand, OSGE treatments significantly enhanced CD40L expression in CD4 T cells from old mice in the lymph nodes (p = 0.01) and spleen (p = 0.03), but did not affect PD-1 expression.
Figure 4. Aging impairs CD40L expression, but not PD-1, in adoptively transferred CD4 T cells, while OSGE improves CD40L expression.
A) Analysis of CD40L expression. Untreated CD4 T cells from young (left histograms) or old (right histograms) AND mice were adoptively transferred to CD4KO hosts. PCC priming of the host increased the expression of CD40L (light lines) compared to isotype controls (dark lines) on gated CD4VB3+ cells from young, but not from old, donor mice. The histogram bars represent the mean percentage (± SEM) of CD40L(+) of untreated and OSGE-treated cells with respect to isotype controls (C) from 8 young and 4 old AND donor mice. The asterisk (*) indicates statistical significance with respect to untreated young cells in primed hosts, while the (^) represents statistical significance relative to untreated old cells. B) Analysis of PD-1 expression. CD4 cells from the CD40L analysis were further stained for PD-1, showing that PCC priming increased PD-1 expression (light lines) compared to isotype controls (dark lines) on gated CD4VB3+ cells from young and old donor mice. Quantitative analysis from 8 young and 4 old AND mice demonstrated that PCC increased PD-1 expression in all groups, with no significant effect of age or OSGE noted.
OSGE can reverse the age-related decline of IgG isotype production and recall responses
To test whether adoptive transfer of OSGE-treated CD4 T cells could affect humoral immunity, we performed a pilot experiment that evaluated whether different numbers of OSGE-treated and untreated CD4 T cells from young AND mice affected antibody production in response to PCC protein in CD4KO host mice. The results from these experiments are shown in Supplemental Figure 2. From these experiments, we concluded that transfer of approximately 2 × 104 OSGE-treated CD4 T cells appeared to the best for enhancing the production of IgG1 and IgG2b in response to PCC protein. However, since the diversity of antigens present in the PCC protein can mask the effects of OSGE or aging, we tested whether OSGE treatment could improve T cell support for humoral immunity in response to a specific hapten by immunizing with DNP-PCC, and evaluating the production of various isotypes of DNP-specific IgG. We transferred 2 × 104 untreated or OSGE-treated CD4 T cells from young and old AND mice into CD4KO host mice and analyzed the anti-DNP humoral response as described in the Methods section.
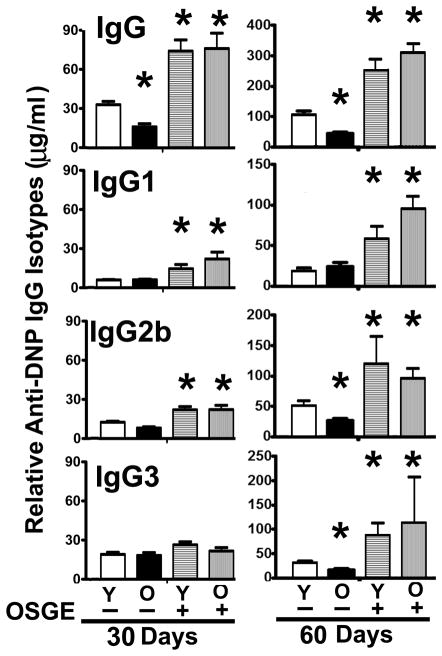
As expected (23), controls that received CD4 T cells but no immunization did not show detectable levels of anti-DNP IgG or anti-DNP IgM (data not shown). There were no effects of aging or OSGE on anti-DNP IgM production at 15 days (for anti-PCC response see Supplementary Figure 2), consistent with the relative T cell independence of IgM. By 30 days, however, we found IgG1, IgG2b, and IgG3 anti-DNP antibodies in all groups. Anti-DNP IgG2a and IgG2c were not detected. Figure 5 shows a summary of the results, based on 3 independent experiments with a total of 9 young and 3 old donor AND mice. In the absence of OSGE exposure, CD4 T cells from old donors stimulated 50% less total anti-DNP IgG than those from the young donor group (p = 0.03). Each of the individual anti-DNP IgG isotypes also showed a slight effect of donor age, although the differences did not reach statistical significance. Treating young CD4 T cells with OSGE led to a significant 3-fold increase in production of total anti-DNP IgG. A similar pattern was observed for each isotype, with significant OSGE effects on IgG1 and IgG2b production. OSGE also improved the function of old CD4 T cells, producing a significant 4 to 5-fold increase in total anti-DNP IgG and in IgG1 and IgG2b production. OSGE-treated old CD4 T cells were at least as effective in supporting antibody production as untreated CD4 T cells from young mice, demonstrating that OSGE treatment of CD4 T cells could restore the age-related decline of T cell support for the primary humoral response.
Figure 5. Analysis of anti-DNP specific IgG isotype response in CD40KO hosts after adoptive transfer of CD4 T cells from young and old AND mice.
Untreated (−) or OSGE-treated (+) naive CD4 T cells from young (Y) or old (O) AND mice were adoptively transferred to CD4KO hosts primed with DNP-PCC. Blood was collected 30 days later and the anti-DNP-specific total IgG and IgG1, IgG2, and IgG3 isotypes were measured in the serum. Each bar represents the mean concentration (± SEM) of each anti-DNP IgG from 8 young or 3 old AND donor mice. The asterisk (*) indicates statistical significance with respect to untreated (−) young CD4 cells T (open bars). Right panel: IgG levels in sera 60 days after initial immunization, i.e., 15 days after re-immunization. The asterisk (*) indicates statistical significance with respect to untreated young CD4 T cells (open bars) at the corresponding time point.
Since CD4 T cell memory formation and antibody recall also declines with age (51, 52), we re-immunized the mice with 200 μg of DNP-PCC, and again measured total anti-DNP IgG and specific isotypes 60 days after the initial immunization, i.e., at 15 days after boosting. As expected, re-immunization enhanced the production of each IgG isotype relative to the primary response (note changes in Y-axis between 30 and 60 days in Figure 5). The effect of donor age on the performance of untreated T cells was significant at this 60-day time point for IgG2b, IgG3, and total IgG. OSGE treatment resulted in a statistically significant, approximately 5-fold, enhancement in total anti-DNP IgG production with a similar pattern noted for each IgG isotype. There was no effect of donor age on T cell help after OSGE treatment. Thus, ex vivo OSGE treatments enhanced the T cell helper function for secondary humoral antibody responses, and reversed the age-related decline of CD4 memory function.
CD4KO mice are lymphopenic, and their use as adoptive hosts may distort some features of the normal physiological immune response. Therefore, we performed a similar set of experiments using normal young B10.BR mice as hosts (Figure 6A). As expected, control B10.BR host mice that had not received any transferred cells (“C” lanes of Figure 6A) produced low levels of anti-DNP IgG in the primary response at 30 days, and somewhat higher levels during the secondary response at 75 days. Transferring untreated young or old CD4 T cells did not lead to a statistically significant increase in the levels of primary or secondary anti-DNP IgG production with respect to the controls, suggesting that endogenous host CD4 T cells provide ample cognate cell function and that untreated CD4 cells have little additional effect after transfer. In contrast, OSGE-treated CD4 T cells significantly enhanced the production of total anti-DNP IgG 2 to 6-fold at 30 days, with a similar result for each isotype (except for IgG3 in the primary response). These effects were independent of the age of CD4 T cells transferred. These results suggest that transferred OSGE-treated CD4 T cells provide enhanced cognate cell function, even in the presence of a simultaneous endogenous immune response.
Figure 6. Anti-DNP-specific IgG response in B10.BR and CB6F1 hosts after adoptive transfer of CD4 T cells.
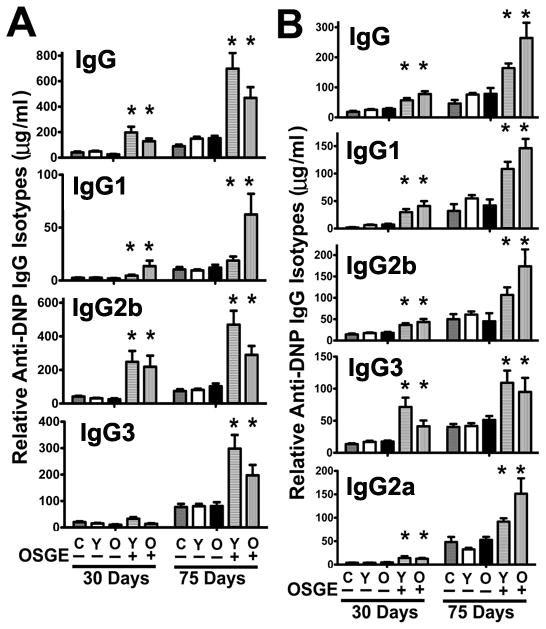
A) Analysis using the B10.BR model. Untreated (−) or OSGE-treated (+) naive CD4 T cells from young (Y) or old (O) AND mice were adoptively transferred to B10.BR hosts primed with DNP-PCC. Control mice (C), which had not received transferred cells, are shown in parallel. Anti-DNP concentrations were evaluated 30 days after priming, and at 75 days after initial immunization, i.e., 30 days after re-immunization at day 45. Bars represent the mean percentage (± SEM) of each anti-DNP IgG from 8 young and 3 old donor mice. The asterisk (*) indicates statistical significance with respect to untreated (−) young CD4 T cells (open bars). B) Analysis of the CB6F1 model; details are the same as those of panel A.
To examine whether OSGE treatment could enhance humoral responses using a non-transgenic model, we performed a similar experiment in which 2 × 106 naïve CD4 T cells from young and old CB6F1 (non-transgenic) donor mice were transferred to young CB6F1 recipients. In this model, the frequency of responding CD4 T cells would initially be quite low, and TCR affinity and diversity would be higher than in the previous models based on TCR-specific transgenic mice. The results are shown in Figure 6B. As expected, adoptive transfer of untreated CD4 T cells, regardless of donor age, did not significantly alter anti-DNP IgG production with respect to control host mice that had not received CD4 cells. However, OSGE-treated CD4 T cells from young and old donors significantly increased all anti-DNP-IgG isotypes tested in both primary and secondary responses, typically by a factor of 2–3 relative to those of control mice.
Discussion
Loss of CD28 expression may be involved in the age-related decline of human T cell function (53, 54). In mice, aging does not reduce overall CD28 expression (not shown); however, our data now suggest that age may block association of CD28 to the TCR complex, with a consequent decline in CD28 costimulation during the activation process. In addition, our new results suggest that the ability of OSGE treatment to enhance T cell responses may be due to correction of the age-related loss in CD28 participation in T cell activation. The results shown in Figures 1 and 3 support a model in which surface proteins (possibly CD45, CD44, and CD43) block CD28 translocation to the TCR complex and (by extension) to the immune synapse. OSGE can cleave a diverse array of surface glycoproteins bearing O-linked, sialylated glycan chains, and it is not yet clear which of these limit T cell activation; aging may block other costimulatory signals besides CD28 (i.e., CD4, CD45, LFA-1), contributing to defects in multiple signaling pathways (55–57).
Supporting the idea that restoration of CD28 function may contribute to the effects of OSGE on T cell activation, we found that age reduces, and OSGE restores, CD28 dependent signals, including phosphorylation of pAKT(308) and Glut-1 expression. These data correspond well to our previous in vitro results (47, 58), demonstrating that aging decreases the occurrence of early events dependent on CD28 signals, such as translocation and phosphorylation of Grb-2, Vav, and PLCγ, and that OSGE is capable of restoring these processes. In addition, the effects of OSGE on CD4 T cell function when CD28/B-7 interactions are blocked by CTLA4Ig treatment (Figure 3), suggest that OSGE modulates CD28 signaling. However, these results do not exclude the possibility that the effects of age and OSGE on Glut-1 and pAKT may reflect the outcome of multiple OSGE-sensitive costimulatory signals, perhaps including activities of CD4, LFA-1, and CD45 (55–57). Nevertheless, our results are the first to suggest that lack of CD28 signaling is involved in the age-related decline of CD4 T cell function in vitro (Figure 1) and in vivo (Figures 2 and 3). Interestingly, these results correlate with some of the early reports in humans in which defects in CD4 function have been linked to a loss of CD28 expression (54).
The induced expression of surface receptors and ligands on the surface of CD4 T cells plays an important role in the activation of other immune cells, such as dendritic cells and B cells. Since aging can affect CD4 activation, it is not surprising that CD4 T cells from old donors fail to express ICOS (Figure 2). The lack of ICOS is a novel and interesting result due to the involvement of ICOS in dendritic cell activation. Mice lacking ICOS expression on T cells show defects in macrophage and dendritic cell opsonization of IgM-coated antigens and cytokine secretion (59, 60), both essential for induction of B cell function in humoral immunity (61). Our data suggest that the age-related decline of T cell ICOS expression could explain some of these defects in dendritic function and opsonization (61); therefore, it would be interesting to test whether OSGE-treated CD4 T cells can improve dendritic cell function in old mice.
Age-related defects in CD4 T cell cognate helper function contribute to poor B cell function and decreased humoral responses in the elderly. These defects have been attributed to decreased CD40L expression (52). Our data confirm an age-associated reduction in CD40L expression, and show that ex vivo OSGE treatments can restore this aspect of CD4 T cell function. Previous reports (33, (51) and our data shown in Figures 1– 4 suggest that CD4 T cells from old mice do not proliferate due to defective activation and expression of proliferation-related surface proteins. We found that naïve CD4 T cells from old mice do not proliferate (see Supplemental Figure 1). The data from PD-1 expression (Figure 4), however, show that these cells do respond by increased expression of PD-1, suggesting that the pathways required for PD-1 expression may not be age sensitive. It is possible that PD-1 expression on CD4 T cells from old mice leads to impairments in proliferation and in differentiation to a memory phenotype. In this context, naïve CD4 T cells from old mice are known to differentiate into defective memory cells (51); thus, it would be interesting to determine whether PD-1+ CD4 T cells from old mice develop into a defective memory population.
Our results also show that OSGE-treated cells provide helper function in 3 models of adoptive immunity, using TCR-transgenic CD4 T cells transferred into either lymphopenic CD4KO mice or into intact B10.BR mice, or using non-transgenic T cells transferred into syngeneic CB6F1 hosts. Aging impairs function only in the first of these systems, and T cells from both young and old mice show substantial OSGE-dependent improvements in helper function for primary and secondary responses in all 3 transfer systems. Similar conclusions emerge when sera are evaluated for antibodies to the PCC carrier protein (Supplemental Figures 2 and 3), instead of the DNP hapten featured in Figures 5 and 6. Our results suggest that the age-related decline of CD4 T cell function and humoral responses can be restored by OSGE, and that one mechanism for this restoration is the improvement of CD28 recruitment to the TCR and CD28 signaling. Furthermore, the ability of OSGE to improve helper function of CD4 T cells from young mice suggests that OSGE-sensitive targets, perhaps related to CD28 signals, may be limiting for some aspects of T cell function in mice of any age. In addition, our data do not rule out the idea that the improvement of humoral responses after OSGE treatment of CD4 T cells may reflect not only enhanced activation, proliferation and CD40L expression in CD4 T cells (see Figure 3, Supplemental Figure 1), but also enhanced cytokine production. We have previously shown augmented cytokine production, in vitro, as a consequence of OSGE treatment of CD4 T cells (31).
It is possible that the ability of OSGE to enhance support of CD4 T cells for humoral immune responses might someday prove useful in specific clinical settings, such as those in which T cells are activated or expanded in culture prior to re-introduction into patients (62), or in other situations where a decline in the humoral immunity results from poor CD4 T cell responses (21, 22).
Supplementary Material
Acknowledgments
Support: This work was supported by an NIH grant (AG019619) and the University of Michigan Nathan Shock Center for the Biology of Aging (AG024824).
We wish to thank Lynn Winkelman, Jessica Sewald, Samuel Rosenbaum, Lisa Burmeister, and Sabrina Friedline for technical assistance, and Sara Fischer for editorial assistance.
Abbreviations
- Vβ3+
Vβ3-TCR+ transgenic CD4+ cells
- CFSE
carboxyfluerescein diacetate, succinimidyl ester
- OSGE
O-sialoglycoprotein endopeptidase. OSGE treated, ex vivo treatments with OSGE
- Glut-1
glucose transporter type I
Footnotes
Conflict of Interest: None of the authors has any financial or commercial conflicts of interest to declare.
References
- 1.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109:295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Berkel ME, Oosterwegel MA. CD28 and ICOS: similar or separate costimulators of T cells? Immunol Lett. 2006;105:115–122. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Lockhart M, Marin E, Graf B, Abe R, Harada Y, Sedwick CE, Miller J. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Lockhart M, Kim M, Miller J. Cutting edge: A role for inside-out signaling in TCR regulation of CD28 ligand binding. J Immunol. 2011;187:5515–5519. doi: 10.4049/jimmunol.1102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 6.Yokosuka T, Saito T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol Rev. 2009;229:27–40. doi: 10.1111/j.1600-065X.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 9.Dodson LF, Boomer JS, Deppong CM, Shah DD, Sim J, Bricker TL, Russell JH, Green JM. Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol Cell Biol. 2009;29:3710–3721. doi: 10.1128/MCB.01869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti R, Jung CY, Lee TP, Liu H, Mookerjee BK. Changes in glucose transport and transporter isoforms during the activation of human peripheral blood lymphocytes by phytohemagglutinin. J Immunol. 1994;152:2660–2668. [PubMed] [Google Scholar]
- 12.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis. 2004;32:182–190. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Farese RV. Insulin-sensitive phospholipid signaling systems and glucose transport. Update II. Exp Biol Med (Maywood) 2001;226:283–295. doi: 10.1177/153537020122600404. [DOI] [PubMed] [Google Scholar]
- 14.Kim DI, Lim SK, Park MJ, Han HJ, Kim GY, Park SH. The involvement of phosphatidylinositol 3-kinase/Akt signaling in high glucose-induced downregulation of GLUT-1 expression in ARPE cells. Life Sci. 2007;80:626–632. doi: 10.1016/j.lfs.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Somwar R, Sumitani S, Taha C, Sweeney G, Klip A. Temporal activation of p70 S6 kinase and Akt1 by insulin: PI 3-kinase-dependent and -independent mechanisms. Am J Physiol. 1998;275:E618–625. doi: 10.1152/ajpendo.1998.275.4.E618. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 17.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 18.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7:467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- 22.Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17:476–479. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–3472. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 26.Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–631. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- 27.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 28.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 29.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 30.Berger SB, Sadighi Akha AA, Miller RA, Garcia GG. CD43-independent augmentation of mouse T-cell function by glycoprotein cleaving enzymes. Immunology. 2006;119:178–186. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger SB, Sadighi Akha AA, Miller RA. A glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17:983–991. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- 32.Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119:187–194. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia GG, Miller RA. Ex vivo enzymatic treatment of aged CD4 T cells restores antigen-driven CD69 expression and proliferation in mice. Immunobiology. 2011;216:66–71. doi: 10.1016/j.imbio.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 35.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 36.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 37.Garcia GG, Miller RA. Increased Zap-70 association with CD3zeta in CD4 T cells from old mice. Cell Immunol. 1998;190:91–100. doi: 10.1006/cimm.1998.1394. [DOI] [PubMed] [Google Scholar]
- 38.Garcia GG, Sadighi Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. J Immunol. 2007;179:6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev. 2011;10:26–34. doi: 10.1016/j.arr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 41.Marano N, Holowka D, Baird B. Bivalent binding of an anti-CD3 antibody to Jurkat cells induces association of the T cell receptor complex with the cytoskeleton. J Immunol. 1989;143:931–938. [PubMed] [Google Scholar]
- 42.Geppert TD, Lipsky PE. Association of various T cell-surface molecules with the cytoskeleton. Effect of cross-linking and activation. J Immunol. 1991;146:3298–3305. [PubMed] [Google Scholar]
- 43.Rozdzial MM, Malissen B, Finkel TH. Tyrosine-phosphorylated T cell receptor zeta chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3:623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 44.Peck MD, Li Z, Jy W, Chu AJ, Bourguignon LY. Association of murine splenocyte CD3 complex to the cytoskeleton: absence of modulation by exogenous fatty acids. Cell Biol Int. 1996;20:531–537. doi: 10.1006/cbir.1996.0069. [DOI] [PubMed] [Google Scholar]
- 45.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol. 1997;175:51–57. doi: 10.1006/cimm.1996.1040. [DOI] [PubMed] [Google Scholar]
- 46.Rozdzial MM, Pleiman CM, Cambier JC, Finkel TH. pp56Lck mediates TCR zeta-chain binding to the microfilament cytoskeleton. J Immunol. 1998;161:5491–5499. [PubMed] [Google Scholar]
- 47.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–3472. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 48.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 49.Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–414. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 50.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 51.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci USA. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirk CJ, Freilich AM, Miller RA. Age-related decline in activation of JNK by TCR- and CD28-mediated signals in murine T-lymphocytes. Cell Immunol. 1999;197:75–82. doi: 10.1006/cimm.1999.1567. [DOI] [PubMed] [Google Scholar]
- 54.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tinkle CW, Lipschitz D, Ponnappan U. Decreased association of p56lck with CD4 may account for lowered tyrosine kinase activity in mitogen-activated human T lymphocytes during aging. Cell Immunol. 1998;186:154–160. doi: 10.1006/cimm.1998.1313. [DOI] [PubMed] [Google Scholar]
- 56.Dornan S, Sebestyen Z, Gamble J, Nagy P, Bodnar A, Alldridge L, Doe S, Holmes N, Goff LK, Beverley P, Szollosi J, Alexander DR. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277:1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 57.Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, Sperling AI. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med. 2004;199:1277–1283. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 59.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 60.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 61.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grupp SA, June CH. Adoptive cellular therapy. Curr Top Microbiol Immunol. 2011;344:149–172. doi: 10.1007/82_2010_94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.