Abstract
Antipsychotic and other medications used in the treatment of schizophrenia place a burden on the cholinergic subsystems of the brain, which have been associated with increased cognitive impairment in the disorder. This study sought to examine the neurobiologic correlates of the association between serum anticholinergic activity (SAA) and cognitive impairments in early schizophrenia. Neurocognitive performance on measures of memory and executive function, structural magnetic resonance imaging (MRI) scans, and SAA assays were collected from 47 early course, stabilized outpatients with schizophrenia or schizoaffective disorder. Voxel-based morphometry analyses employing general linear models, adjusting for demographic and illness-related confounds, were used to investigate the associations between SAA, gray matter morphology, and neurocognitive impairment. SAA was related to working memory and executive function impairments. Higher SAA was significantly associated with lower gray matter density in broad regions of the frontal and medial-temporal lobes, including the dorsolateral prefrontal cortex (DLPFC), hippocampus, and striatum. Lower gray matter volume in the left DLPFC was found to significantly mediate the association between SAA and working memory impairment. Disease and/or medication-related cholinergic dysfunction may be associated with brain volume abnormalities in early course schizophrenia, which may account for the association between SAA and cognitive dysfunction in the disorder.
Keywords: Anticholinergic activity, neurocognition, dorsolateral prefrontal cortex (DLPFC), schizophrenia, MRI
1. Introduction
Schizophrenia involves broad impairments in neurocognitive functioning (Saykin et al., 1994; Heinrichs and Zakzanis, 1998), and these deficits have been associated with poor functional outcome (Green et al., 2000). Evidence is emerging that at least some of the neurocognitive impairments individuals with schizophrenia experience are associated with increased anticholinergic activity, perhaps due to the antimuscarinic properties of different pharmacotherapies (Bymaster et al., 2003; Chew et al., 2008) or dysfunction in the cholinergic system associated with the disorder itself (Raedler et al., 2007). Antipsychotic and other supplemental pharmacological treatments for schizophrenia often produce anticholinergic-associated effects beyond their primary pharmacological targets (Raedler et al., 2000; Raedler et al., 2003), which have been repeatedly shown to be associated with exacerbation of neurocognitive impairment, particularly in memory (e.g., Tracy et al., 2001; Minzenberg et al., 2004). In addition, recent evidence has implicated the cholinergic system in the pathophysiology of schizophrenia (Raedler et al., 2007). However, surprisingly little is known about the underlying neurobiological mechanisms of the association between anticholinergic activity and cognitive impairment in schizophrenia. Investigation of the neurobiologic correlates of anticholinergic-associated neurocognitive deficits could have vital implications for the development of novel medications that do not burden the cholinergic subsystems of the brain and may further the understanding of the neuropathophysiology of schizophrenia.
There have been several investigations of the iatrogenic effects of anticholinergic activity, a broad term incorporating nonspecific muscarinic receptor antagonistic action, on neurocognitive functioning in schizophrenia. For instance, Minzenberg and colleagues (2004) measured anticholinergic activity with a pharmacological index formulated from a collection of in vitro studies of muscarinic receptor antagonism and a clinical index of anticholinergic side effects rated by clinicians. With these two measures of anticholinergicity, they found that impairment in memory, recall, and complex attention was related to higher anticholinergic activity. Tracy and colleagues (2001) measured serum anticholinergic activity (SAA) in patients with chronic schizophrenia and found that SAA was negatively correlated with measures of effortful memory and executive control. Other studies examining anticholinergic medication dosage and neurocognitive impairment in schizophrenia found that increased anticholinergic dosage was associated with deficits in verbal recall, verbal fluency, motor speed (Sweeney et al., 1991), and verbal memory (Brébion et al., 2004). Interestingly, Mori and colleagues (2002) found that withdrawing anticholinergic medications improved the performance of schizophrenia inpatients on measures of immediate and verbal memory.
It is well known that the cholinergic subsystems of the brain, notably the basal-forebrain cholinergic neurons projecting to regions such as the prefrontal cortex, regulate several fundamental cognitive functions such as memory, attention, and learning (Everitt and Robbins, 1997; Sarter and Bruno, 1997; Perry et al., 1999; Hasselmo and Sarter, 2011), and dysfunction to this acetylcholine regulated system can result in deficits in cognitive functioning (Sarter and Parikh, 2005). Recently, based on growing empirical evidence, Raedler et al., (2003; 2007) proposed that abnormalities of the muscarinic cholinergic system may be involved in the pathophysiology of schizophrenia. Indeed, decreased muscarinic receptor density has been identified in post-mortem brains of unmedicated and medicated patients with schizophrenia in several key brain regions implicated in neurocognitive impairment in the disorder, including the dorsolateral prefrontal cortex (DLPFC) (Crook et al., 2001; Dean et al., 2002), the superior temporal gyrus (Deng and Huang, 2005), the hippocampal formation (Crook et al., 2000; Scarr et al., 2007), the anterior cingulate cortex (Zavitsanou et al., 2004), and the striatum (Dean et al., 1996; Crook et al., 1999). In addition, evidence for a reduction in the density of cholinergic interneurons in the ventral striatum has been implicated in schizophrenia (Holt et al., 1999; Holt et al., 2005).
Although the relationship between anticholinergic activity and neurocognitive impairment in schizophrenia has been repeatedly demonstrated, investigations of the neurobiologic correlates involved in this relationship have yet to be conducted in schizophrenia. However, while the pathophysiology is different from schizophrenia, studies of Alzheimer’s disorder have increasingly demonstrated the deleterious impact of antimuscarinic medications on Alzheimer-type pathology in Parkinson’s disease (Perry et al., 2003) and exacerbation of cognitive decline in both Alzheimer’s disease (Lu and Tune, 2003) and Parkinson’s disease (Ehrt et al., 2010). In addition, pharmacological investigations have indicated increased cognitive impairment in healthy older adults with the administration of antimuscarinic medications (Kay et al., 2006). As an example, a recent investigation found individuals with mild cognitive impairment at high risk for developing Alzheimer’s disease had significant gray matter volume loss in the basal forebrain cholinergic system, which was associated with cognitive impairment and increased gray matter loss in regions of the frontal and temporal lobes (Grothe et al., 2010). It is important to note that some evidence has indicated that anticholinergic-related cognitive impairments in schizophrenia maybe acute (More et al., 2002), which may reflect direct receptor effects rather than anticholinergic-related brain morphology. However, evidence from animal models also show the deleterious impact of continuous anticholinergic exposure on neurobiologic processes, such as long-term potentiation (Dorlap and Leung, 2008) and accelerated neurodegeneration (Yoshiyama et al., 2012), which may be related to gray matter atrophy. With regard to studies utilizing the specific measure of SAA,Nebes et al. (2005) found that healthy older individuals with higher SAA had greater cognitive decline associated with increasing white matter hyperintensity volumes.
With evidence for a link between cognitive decline, cholinergic system dysregulation, and gray matter atrophy in Alzheimer’s disease, and increasing evidence of cholinergic system dysfunction in schizophrenia (Raedler et al., 2003; Raedler et al., 2007) as well as anticholinergic-associated cognitive impairments, examination of the relationship between anticholinergic-associated neurocognitive impairment and the underlying neurobiologic morphology may be an important consideration in the understanding of the pathophysiology and treatment of schizophrenia. In this investigation, we examined associations between SAA, neurocognitive impairment, and gray matter density in a sample of early course, stabilized patients with schizophrenia. In addition, the mediating effects of gray matter morphology on the relationship between SAA and neurocognitive impairment were examined. We predicted that SAA would be associated with lower gray matter volume in broad regions of the frontal and medial-temporal lobes, particularly the DLPFC, hippocampus, and striatum, and that anticholinergic-associated lower gray matter volumes would mediate the relationship between SAA and neurocognitive impairment, particularly with regard to the identified relationship between DLPFC dysfunction and working memory impairments, based on previous findings (Glahn et al., 2005).
2. Methods
2.1. Participants
A total of 47 patients in the early course of schizophrenia (n = 32) or schizoaffective disorder (n = 15) participated in this research. All patient diagnoses were based on the Structural Clinical Interview for the DSM-IV (First et al., 2002). Study eligibility criteria included a diagnosis of schizophrenia, schizoaffective disorder, or schizophreniform disorder, the emergence of first psychotic symptoms (including duration of untreated psychosis) within the past 8 years, an IQ ≥ 80, a two-month period free of substance abuse prior to study enrollment, significant social-cognitive disability according to the Cognitive Style and Social Cognition Eligibility Interview (Hogarty et al., 2004), and the absence of MRI contraindications (e.g., neurological condition, ferromagnetic objects in the body).
Participants were mostly male (n = 31, 66%) and Caucasian (n = 31, 66%). Other race-ethnicity characteristics of participants included nine African Americans (19%), six Asian Americans (12.8%), and one of other race-ethnicity (2%). Overall, participants were young with an average age of 26.55 (SD = 6.71) years, had an average illness length fewer than five years (M = 3.16, SD = 2.23), and an average BPRS total score of 39.36 (SD = 9.66). Thirty-five of the participants were college educated (75%), although only 13 were employed (28%) at the time of this research. Participants in this study were part of a larger randomized-controlled trial of Cognitive Enhancement Therapy (CET; Eack et al., 2009). While 58 total participants were part of this larger study, baseline imaging and SAA data were only available for 47 participants. There were no significant differences between individuals with SAA and MRI data and the larger study sample with regard to most demographic characteristics, IQ, total brain volume, SAA, and neurocognitive functioning. However, individuals with SAA and MRI data were more likely to have attended college (n = 35, 75%), compared to those without MRI and SAA data (n = 4, 36%), χ2(1, N = 58) = 4.27, p = 0.039.
2.2. Medications
All participants were treated by a study psychiatrist with antipsychotic medications approved by the Food and Drug Administration for the treatment of schizophrenia. Only two participants were prescribed an antiparkinsonian agent. Medication characteristics of patients are presented in Table 1.
Table 1.
Baseline medication characteristics and serum anticholinergic activity of participants (N = 47)
| Medication | N (%) |
|---|---|
| Typical antipsychotica | 6 (13%) |
| Haloperidolb | 4 (9%) |
| Haloperidol decanoate | 1 (2%) |
| Perphenazine | 1 (2%) |
| Atypical antipsychotica | 46 (98%) |
| Aripiprazoleb | 11 (23%) |
| Clozapine | 7 (15%) |
| Ziprasidone | 4 (9%) |
| Olanzapine | 17 (36%) |
| Risperidone | 9 (19%) |
| Quetiapine | 3 (6%) |
| Antiparkinsonian | 2 (4%) |
| Benztropine | 1 (2%) |
| Biperiden | 1 (2%) |
| M(SD) | |
| CPZ Dosage | 434.50 (340.38) |
| BZT Dosagec | 1.50 (0.71) |
| Serum level (pmol/ml) | 6.88 (10.79) |
Percentage totals are more than 100% because several patients were taking more than one atypical antipsychotic or were taking both an atypical and typical antipsychotic medication.
Total number of medications is more than the sample size because of several patients taking more than one type of medication.
Conversion factors from Tran et al, 1997
CPZ = Chlorpromazine equivalent; BZT = Benztropine equivalent
2.3. Measures
2.3.1. SAA
SAA was measured using a competitive radioreceptor binding assay technique developed by Tune and Coyle (1980; 1981) and as described elsewhere (Mulsant et al., 2003; Chew et al., 2005). This assay accounts for all exogenous substances taken by a patient with anticholinergic effects, regardless of structure and muscarinic receptor subtype affinity. Only the free i.e., non-protein bound antimuscarinic substances in the serum were measured. Anticholinergic activity is expressed as picomoles of atropine equivalents per milliliter (pmol/ml).
2.3.2. Neurocognition
In consideration of research indicating that anticholinergic activity is most frequently associated with memory and executive impairments (Tracy et al., 2001; Brébion et al., 2004; Minzenberg et al., 2004), specific measures of verbal memory, working memory, and executive function were extracted from a comprehensive neuropsychological battery. Verbal memory measures included immediate and delayed recall tests from Stories A and B of the Revised Wechsler Memory Scale (Wechsler, 1987), and list A total recall, short-term free recall, and long-term free recall measures from the California Verbal Learning Test (Delis et al., 1987). The digit span test from the Revised Wechsler Adult Intelligence Scale (Wechsler, 1981) was used to assess working memory. Finally, executive function was assessed using perseverative and non-perseverative errors from the Wisconsin Card Sorting Test (Heaton et al., 1993). Items from these domains were scaled to a common metric and averaged to produce indices of verbal memory, working memory, and executive function. Appropriate items were reverse-coded so that higher index scores were reflective of better cognitive functioning.
2.3.3. Image acquisition and processing
Structural magnetic resonance images (MRI) were obtained from participants using a 3-T Signa whole body scanner and head coil (GE Medical Systems, Milwaukee, WI). Whole brain volume was acquired in 124 1.5 mm-thick contiguous coronal slices with spoiled gradient recalled acquisition in steady state pulse sequence (TE = 5ms, TR = 25ms, acquisition matrix = 256 × 192, FOV = 24 cm). Images were manually examined for motion and quality by a masters-level imaging technician. Following acquisition and initial quality control, images were normalized to standard Montreal Neurological Institute (MNI) space and segmented using the unified segmentation algorithm (Ashburner and Friston, 2005) in Statistical Parametric Mapping Software, version 5 (SPM5) based on the adult MNI template supplied by the software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Using a 12 mm Gaussian kernel, the segmented images were then smoothed. The 12mm Gaussian kernel was selected to provide good balance between maximizing analytic sensitivity and minimizing intersubject variability. During image post-processing radio frequency inhomogeneity artifacts were corrected using a bias correction algorithm built into the segmentation procedure. A broad set of frontal (i.e., prefrontal cortex and anterior cingulate cortex), medial-temporal (i.e., parahippocampal gyrus, hippocampus, and striatum), and midbrain regions of interest (ROIs) were selected for this research, due to being frequently implicated in cognitive impairments early in the course of schizophrenia (Antonova et al., 2004) and the cholinergic neural pathway (Dutar et al., 1995). The Wake Forest University PickAtlas toolbox for SPM5 (Maldjian et al., 2003) was utilized to define image masks for region of interest analyses, with regional definitions delineated by Tzourio-Mazoyer et al., 2002.
2.4. Procedure
Participants were recruited from Western Psychiatric Institute and Clinic and community clinics in Pittsburgh, Pennsylvania from August 2001 to January 2006. Consensus conferences using video-taped interviews were utilized to determine patient eligibility for study participation, including psychiatric diagnosis. Eligible individuals were then assessed with the aforementioned measures of SAA, neurocognition, and structural MRI. This research was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent prior to their participation.
2.5. Data analysis
We began our analysis by examining the associations between SAA and neurocognitive performance using Pearson correlation coefficients executed in SPSS, version 19. In addition, to control for the possible influences of age, gender, illness length, and IQ on neurocognition, partial correlations were examined after adjusting for these confounders. Next, examination of the association between SAA and fronto-temporal gray matter density was investigated in SPM5 using ROI voxel-based morphometric (VBM) analyses employing general linear models, adjusting for the confounding effects of age, gender, illness length, and IQ. A description of ROIs mask selection and generation is outlined above. To maintain Type I error rates at acceptable levels in these analyses, a false discovery rate (FDR) significance threshold was set to p = 0.05, along with a minimum voxel-extent threshold of 30. After conducting VBM analyses, gray matter volumes of significant regional clusters (see Table 2) were then extracted from images modulated by their Jacobian determinants obtained during normalization. Associations between SAA and these volumetric data were then examined using partial correlation analysis after adjusting for age, gender, illness length, IQ, and total intracranial volume.
Table 2.
Partial correlations of areas of lower gray matter density, SAA, and neurocognitive functioning at baseline.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SAA | - | |||||||||||||
| Cognition | ||||||||||||||
| 2. Verbal memory | −0.21 | - | ||||||||||||
| 3. Working memory | −0.37* | 0.52** | - | |||||||||||
| 4. Executive function | −0.29† | 0.49** | 0.50** | - | ||||||||||
| Brain volume cluster | ||||||||||||||
| 5. Left hippocampus | −0.40** | 0.11 | 0.06 | −0.01 | - | |||||||||
| 6. Right hippocampus | −0.44** | −0.04 | 0.13 | 0.04 | 0.82** | - | ||||||||
| 7. Left superior frontal gyrus | −0.38* | 0.01 | 0.14 | 0.09 | 0.17 | 0.20 | - | |||||||
| 8. Right putamen | −0.41** | 0.05 | 0.10 | 0.05 | 0.40** | 0.47** | 0.28† | - | ||||||
| 9. Left putamen | −0.44** | 0.03 | 0.12 | 0.05 | 0.35* | 0.40** | 0.27† | 0.70** | - | |||||
| 10. Right middle frontal gyrus | −0.39* | 0.16 | 0.25 | 0.21 | 0.09 | 0.03 | 0.26† | 0.17 | 0.25 | - | ||||
| 11. Prefrontal cortex | −0.56** | 0.01 | 0.22 | 0.16 | 0.37* | 0.31* | 0.74** | 0.25 | 0.23 | 0.34* | - | |||
| 12. Left DLPFC | −0.55** | 0.05 | 0.40** | 0.18 | 0.41** | 0.44** | 0.48** | 0.26† | 0.23 | 0.35* | 0.79** | - | ||
| 13. Left motor cortex | −0.62** | 0.06 | 0.27† | 0.11 | 0.20 | 0.15 | 0.55** | 0.21 | 0.30* | 0.39** | 0.65** | 0.51** | - | |
| 14. Midbrain | −0.25 | −0.14 | 0.12 | 0.08 | 0.50* | 0.55** | 0.12 | 0.49** | 0.41** | 0.06 | 0.29† | 0.37* | 0.15 | - |
Note. Coefficients are from partial correlations analyses adjusting for the effects of age, gender, illness length, IQ, and total intracranial volume.
p<0.10
p<0.05
p<0.01
Finally, mediator analyses were conducted, utilizing the volumetric data, to examine the indirect effects of SAA on impaired neurocognitive functioning, through gray matter morphology. This was accomplished by calculating the association between (1) SAA and lower gray matter volumes in fronto-temporal regions, (2) lower fronto-temporal gray matter volumes and neurocognitive functioning, and (3) SAA and neurocognitive functioning based on the mediator-analytic framework of Baron and Kenny (1986). Within this framework, mediation depends, in part, on the demonstration of significant shared associations between gray matter volume (the mediator), SAA (the predictor), and neurocognitive functioning (the outcome). If such relationships exist, the well-documented direct association between SAA with neurocognitive functioning (Tracy et al., 2001; Minzenberg et al., 2004) will be reduced when accounting for the mediator, gray matter volume, due to the mediating effect of lower gray matter volume on the relationship between SAA and cognitive performance. The size and significance of the indirect effects of SAA on neurocognitive functioning through lower gray matter volume was quantified using an asymptotic test of the distribution of indirect effects developed by MacKinnon et al., 2007. All mediator analyses accounted for the potential confounding effects of age, gender, illness length, IQ, and total intracranial volume. To conserve power, only significant confounding covariates showing an association with SAA and/or neurocognitive performance were retained in mediator models (e.g., age, gender, illness length, IQ). Level of psychopathology and global illness impairment were considered as potential confounders, however neither of these measures (e.g., BPRS total scores, GAS) were related to SAA or neurocognitive performance. The duration (and nature) of previous treatment was also considered a potential confounder. While comprehensive information on treatment exposure prior to study enrollment was not available, these data are broadly represented by our inclusion of illness duration in the analysis models. Additionally, because antipsychotic medications have different known cholinergic potency (Bymaster et al., 2003; Chew et al, 2008), secondary analyses were conducted after adjusting for variability among participants receiving high-loading anticholinergic antipsychotic medications. Adjusting for variability in receipt of high-loading anticholinergic medications did not alter relations between SAA, brain morphology, and cognition; and thus was not included as a confounding covariate. The results of this investigation were also reanalyzed in SPM8, which did not significantly change the findings (see supplementary table). As such, we have continued to present the original analyses. Only SAA displayed a significantly skewed distribution, which was corrected using logarithmic transformations.
3. Results
3.1. Relationship between SAA and neurocognitive performance
Investigation of the association between SAA and neurocognitive performance indicated that increased SAA was significantly associated with poorer performance on measures of working memory (r = −0.31, p = 0.033). In addition, increased SAA had a trend level association with poorer executive function (r = −0.27, p = 0.058) performance. There was no association observed for SAA and verbal memory performance (r = −0.20, p = 0.160). As can be seen in Table 2, the association between SAA and neurocognitive functioning persisted after controlling for the confounding effects of age, gender, illness length, and IQ, such that increased SAA was significantly associated with poorer working memory (pr = −0.37, p = 0.013). This negative relationship remained at trend level once confounding variables were controlled for with regard to executive function (pr = −0.29, p = 0.057) performance.
3.2. Areas of lower gray matter density associated with SAA
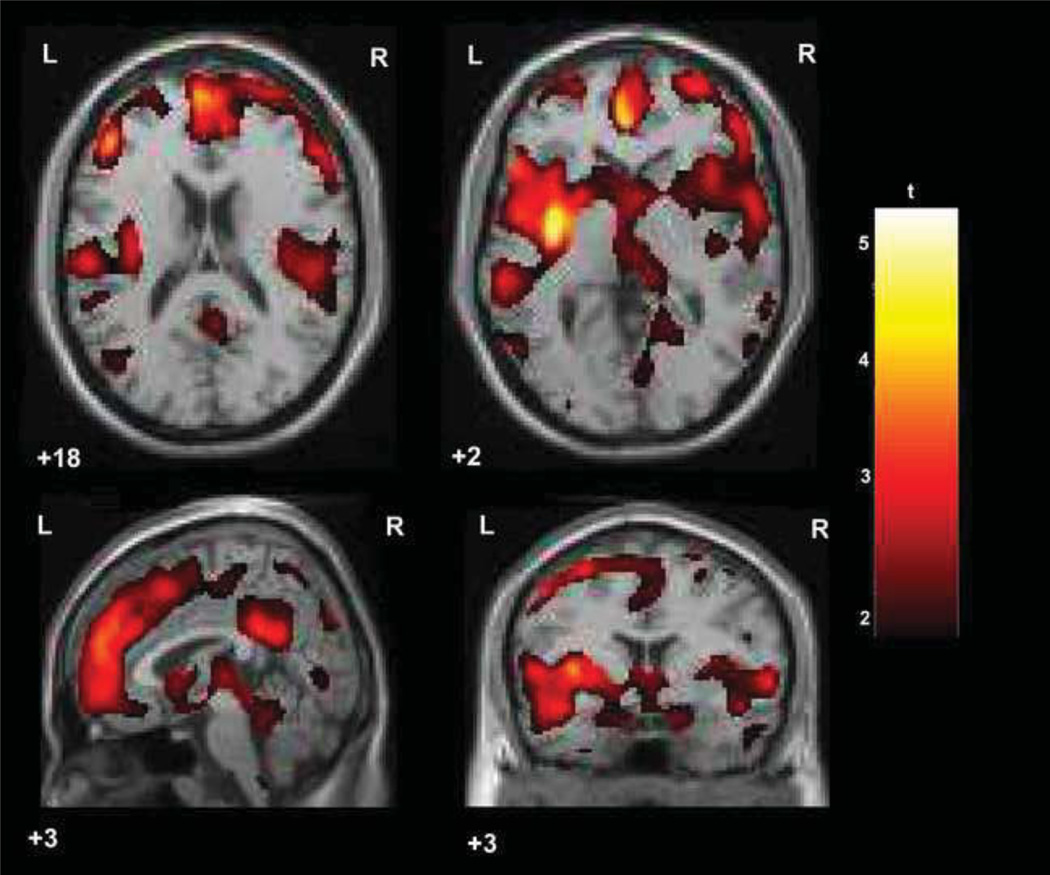
As can be seen in Table 3 and Figure 1, increased SAA was significantly associated with lower gray matter density in broad regions of the prefrontal cortex, with a large cluster of voxel relations covering the bilateral DLPFC, bilateral anterior cingulate cortex, bilateral orbitofrontal cortex, and the left ventrolateral prefrontal cortex. A smaller, yet considerably sized set of voxel cluster relations were also apparent in the striatum, specifically the bilateral putamen, such that increased SAA was associated with lower gray matter density in these areas. In addition, higher anticholinergicity was associated with lower gray matter density in the bilateral hippocampal complex, the left premotor and secondary motor cortices, and the midbrain. No significant positive associations were found between SAA and gray matter density. Subsequent volumetric analyses confirmed associations between SAA and lower gray matter volume in all clusters previously observed in VBM analyses, with the exception of the midbrain cluster (see Table 2). All significant volumetric associations were medium to large in magnitude, with the strongest associations with SAA occurring in the left motor cortex, prefrontal cortex, and left DLPFC clusters.
Table 3.
Voxel-based morphometric analyses examining the relationship between serum anticholinergicity and gray matter morphology in early course schizophrenia patients
| MNI coordinates (x,y,z) | Cluster size | Location | BA | z | p | pFDR | Direction |
|---|---|---|---|---|---|---|---|
| −4, 44, 4 | 6637 | Bilateral medial prefrontal cortex, bilateral anterior cingulate cortex, bilateral orbitofrontal cortex, right dorsolateral prefrontal cortex | 8/9/10/11/32/46 | 4.67 | <0.001 | 0.004 | Negative |
| −46, 36, 16 | 1050 | Left ventromedial prefrontal cortex, left dorsolateral prefrontal cortex | 10/46/47 | 4.47 | <0.001 | 0.004 | Negative |
| −32, −6, −2 | 477 | Left putamen | - | 4.56 | <0.001 | 0.004 | Negative |
| −46, 6, 52 | 440 | Left premotor and secondary motor cortex, left middle frontal gyrus | 6 | 3.37 | <0.001 | 0.009 | Negative |
| −12, −14, −18 | 271 | Left hippocampal complex | 28/34 | 3.58 | <0.001 | 0.006 | Negative |
| −2, −20, −4 | 248 | Midbrain | - | 2.98 | 0.001 | 0.016 | Negative |
| 12, −14, −18 | 154 | Right hippocampal complex | 28/34 | 3.74 | <0.001 | 0.005 | Negative |
| 34, 12, 40 | 100 | Right middle frontal gyrus | 8/9 | 3.24 | 0.001 | 0.011 | Negative |
| 26, 6, 8 | 98 | Right putamen | - | 2.91 | 0.002 | 0.017 | Negative |
| −18, 38, 52 | 44 | Left superior frontal gyrus | 8 | 2.77 | 0.003 | 0.021 | Negative |
Note. In the above analysis, p is the uncorrected significance level and pFDR is the voxel-wise corrected significance threshold value.
BA = Brodmann’s Area
Figure 1.
Broad regions of fronto-temporal gray matter loss associated with high anticholinergic activity in early course schizophrenia patients.
3.3. Mediating effects of anticholinergic-associated gray matter morphology on neurocognitive impairment
After finding that increased SAA was associated with impairments in working memory and executive functioning, as well as lower gray matter in broad fronto-temporal regions, exploratory mediator analyses were conducted for completeness, with the exception of the expected association between the DLPFC and working memory impairment (Glahn et al., 2005), to examine the degree to which brain regions with lower gray matter volume demonstrating significant associations with SAA mediated the effects of anticholinergicity on neurocognitive performance. This was accomplished by investigating the associations between SAA, gray matter volume, and neurocognitive functioning (see Table 2). Interestingly, poorer neurocognitive functioning was only significantly associated with lower gray matter volume in the left DLPFC, with patients showing lower gray matter volumes in the left DLPFC performing worse on measures of working memory (pr= 0.40, p =0.007). Noteworthy, other cluster regions that also include the DLPFC (e.g., medial prefrontal cortex, left superior frontal gyrus) were not significantly correlated with poorer working memory performance. This may be possible because these anticholinergic-associated lower gray matter volume clusters involve other anatomical regions not strongly associated with working memory impairment in schizophrenia, most likely distilling any contribution of the DLPFC. Subsequent mediator analyses indicated that lower gray matter volume in the left DLPFC significantly mediated the association between SAA and working memory impairment (indirect effect = −0.34, z’ = −1.75, p = 0.026). No other mediating effects were observed between SAA, gray matter volume, and cognitive function.
4. Discussion
Increased anticholinergic activity has been linked to the exacerbation of neurocognitive impairment in schizophrenia (Sweeney et al., 1991; Tracy et al., 2001; Brébion et al., 2004; Minzenberg et al., 2004). In addition, dysfunction in the cholinergic system has been proposed to have a role in the pathophysiology of schizophrenia (Raedler et al., 2007) with several regions important for neurocognition showing decreased muscarinic receptor availability (Crook et al., 2000; Crook et al., 2001; Dean et al., 2002; Raedler et al., 2003; Zavitsanou et al., 2004). Despite evidence for cholinergic system dysfunction and replicated findings of neurocognitive deficits associated with antimuscarinic burden, no study has investigated the neurobiologic correlates that may be involved in anticholinergic-associated neurocognitive impairment in individuals with early course schizophrenia. This investigation examined the relationships among gray matter morphology, SAA, and neurocognitive functioning in stabilized, early course patients with schizophrenia. In addition, the mediating relationship between SAA and neurocognitive functioning through gray matter morphology was examined in these patients with early schizophrenia.
Results from this cross-sectional investigation indicated impairments in working memory and executive function were associated with increased SAA. No association between increased SAA and verbal memory performance was observed, in contrast to other studies, which could reflect a unique pattern of associations in the early course of schizophrenia or variability across measurement strategies for assessing verbal memory. Significant negative associations between increased SAA and lower gray matter densities were indicated in the frontal and temporal lobes, with no observed positive relations. Specifically, lower gray matter density related to increased SAA was found broadly in the prefrontal cortex, notably the bilateral DLPFC, anterior cingulate cortex, orbitofrontal cortex, and the left ventrolateral prefrontal cortex. Additionally, lower gray matter density in the bilateral putamen and hippocampal complex, and the left premotor and secondary motor cortices were related to increased levels of SAA. Interestingly, lower gray matter volume in the left DLPFC mediated the relationship between SAA and working memory dysfunction.
These findings present potential implications for further understanding the pathophysiology and treatment of schizophrenia. With an accumulation of evidence for muscarinic dysfunction in schizophrenia, a cholinergic muscarinic hypothesis of the disease has been proposed (Raedler et al., 2007). Patients with schizophrenia have decreased density of M1 and M4 type muscarinic receptors in the prefrontal cortex, specifically the DLPFC (Crook et al., 2001; Dean et al., 2002), the hippocampal formation (Crook et al., 2000; Scarr et al., 2007), the superior temporal gyrus (Deng and Huang, 2005), the anterior cingulate cortex (Zavitsanou et al., 2004), and the caudate-putamen (Dean et al., 1996; Crook et al., 1999). That this study found anticholinergic-associated lower gray matter densities in the very regions shown to have decreased muscarinic receptor availability supports the pathophysiologic involvement of cholinergic dysfunction in cognitive impairment early in the disorder.
Additionally, these findings have implications regarding the use of medications with high anticholinergic burden in the treatment of schizophrenia, especially early in the disease process. There have been varied results with regard to the effects of antipsychotic medications on brain structure in schizophrenia (Scherk and Falkai, 2006; Navari and Dazzan, 2009), and findings from this literature are not entirely consistent with the results of this study (e.g., an enlarged basil ganglia is often observed with prolonged antipsychotic treatment [Scherk and Falkai, 2006; Navari and Dazzan, 2009]). However, there have been several investigations of the cholinergic mechanisms of antipsychotic medications. Clozapine and olanzapine are the two antipsychotic medications associated with high anticholinergic activity (Bymaster et al., 2003; Chew et al., 2008). In fact,Raedler et al. (2000) found that the anticholinergic property of olanzapine at low (5mg/dy) and high (20 mg/dy) doses reduced muscarinic binding in regions of the cortex and striatum. Clozapine has also been found to decrease muscarinic receptor availability in the cortex and basal ganglia of patients with schizophrenia (Raedler et al., 2003). Furthermore, clinical data, although limited, suggests that exposure to anticholinergic medications increases Alzheimer-type pathology in patients with Parkinson’s disease (Perry et al., 2003) and adversely affects the course of Alzheimer’s disease by increasing neurodegeneration (Lu and Tune, 2003). Although more research is required, this may be the case for schizophrenia patients treated with anticholinergic-associated medications. These findings may also reflect the inability of patients with lower gray matter and associated neurocognitive impairment to resist the antimuscarinic burden of some medications. Nonetheless, animal studies (Dorph-Peterson et al., 2005) and a recent investigation with schizophrenia patients (Ho et al., 2011) show decreases in brain volumes and astrocyte numbers (Konopaske et al., 2008) associated with chronic exposure to antipsychotic medications. As such, the findings of this investigation may reflect pathophysiologic dysfunction in the cholinergic system, in addition to the relationships between antipsychotic-associated anticholinergic activity on lower gray matter and cognitive impairment in patients early in the course of schizophrenia. With the advent of drug development for the amelioration of cognitive impairment in schizophrenia, these results have important implications regarding the development of novel medications that do not burden the cholinergic subsystems of the brain.
Although SAA was broadly associated with lower gray matter in fronto-temporal regions, these preliminary results should be interpreted within the context of some limitations. It is important to first remember that this was a cross-sectional investigation, and because SAA was not observed over time, inferences regarding the causality of the association between SAA and gray matter morphology cannot be made. It will be essential for future research to examine the effects of longitudinal, cumulative exposure to anticholinergic medications on gray matter morphology and assess long-term changes in SAA to understand the degree to which the link between anticholinergicity and gray matter morphology is causal in nature. This investigation was also characterized by a relatively small sample size (N = 47). Despite generally large voxel cluster relations and sufficient power to detect associations of SAA with brain morphology, this small sample size may have inhibited our ability to detect smaller relations between SAA and gray matter volume. In addition, the type of medications used to treat participants was not controlled in this study based on known anticholinergic properties of certain antipsychotic medications. The radioreceptor binding assay technique utilized is not a specific measure of antipsychotic anticholinergic activity, but rather measures all exogenous substances taken by a patient with an antimuscarinic burden and possible basal anticholinergic activity (Tune and Coyle, 1980). Further, given the confirmatory nature of volumetric correlations and stringent FDR correction during VBM analyses, we did not use a correction for multiple inference tests during correlation and mediator analyses. However, the FDR approach is less conservative than traditional Bonferroni-based methods, and while widely used in the neuroimaging literature (Genovese et al., 2002), smaller voxel cluster relations need to be interpreted with some caution. Lastly, we did not characterize a healthy control group limiting our ability to make inferences about the degree to which lower gray matter is abnormal in patients with schizophrenia in relation to levels of SAA. Together, these limitations precluded our ability to make strong inferences about the associations between SAA, neurocognition, and gray matter morphology in early course schizophrenia. It is possible that chronic denervation of cholinergic neurons in the basal nuclei may lead to regional cortical atrophy as has been reported in Alzheimer’s disease (Cullen et al., 1997); such secondary effects may be related to the effects of cholinergic mechanisms on long-term potentiation (Doralp and Leung, 2008). Alternatively, it is also possible that the neurobiology of schizophrenia involves central cholinergic alterations that may be reflected in peripheral cholinergic tone (Berman et al., 2007). Future studies of CNS cholinergic function in antipsychotic naïve schizophrenia patients are likely to shed light in this regard. Future longitudinal studies will also be needed to examine the temporal contribution of anticholinergic activity to brain loss and cognitive impairment in early schizophrenia in comparison to healthy controls in order to further delineate the clinical implications for the use of medications with high antimuscarinic properties. Given recent meta-analytic and review evidence for significant frontal-medial and cingulate cortex gray matter loss in patients at high risk for developing schizophrenia (Smieskova et al., 2010; Fusar-Poli et al., 2011a; Fusar-Poli et al., 2011b), an additional future direction would be investigate of cholinergic system dysfunction in patients during this prodromal phase.
In summary, impairments in working memory and executive function, but not verbal memory, were related to increased SAA with concomitant lower gray matter volume in the left DLPFC mediating the relationship between working memory deficits and increased SAA. In addition, increased SAA was associated with lower gray matter in broad regions of the prefrontal cortex, the bilateral putamen, and the left premotor and secondary motor cortices. The elucidation of anticholinergic-associated lower gray matter as a mechanism of the disease process or pharmacotherapy, as well as the development of antipsychotic compounds without an anticholinergic burden are critical directions to further enhance the understanding of the neuropathophysiology and treatment of cognitive impairment early in the course of schizophrenia.
Supplementary Material
Acknowledgements
This work was supported by NIMH grants MH 60902 (MSK) and MH 79537 (SME). We thank the late Gerard E. Hogarty, M.S.W. for his leadership and direction as Co-Principal Investigator of this study, and Susan Cooley M.N.Ed., Anne Louise DiBarry, M.S.N., Konasale Prasad, M.D., Haranath Parepally, M.D., Debra Montrose, Ph.D., Diana Dworakowski, M.S., Mary Carter, Ph.D., Sara Fleet, M.S., and Denise Soriso for their help in various aspects of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
In the past year, Shaun M. Eack, Ph.D. has received consulting fees from Abbott Laboratories. Bruce G. Pollock, M.D., Ph.D. receives research support from the National Institute of Health and the Canadian Institutes of Health Research. Within the past five years he has been a member of the advisory board of Lundbeck Canada (final meeting was May 2009) and Forest Laboratories (final meeting was March 2008). Dr. Pollock has served one time as a consultant for Wyeth (October 2008) and Takeda (July 2007). He was also a faculty member of the Lundbeck International Neuroscience Foundation (LINF) (final meeting was April 2010). In the past year, Matcheri S. Keshavan, M.D. has received a Glaxo Smith Kline grant and a Sunovion grant for cognitive remediation studies.
References
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: A selective review. Schizophrenia Research. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berman JA, Talmage DA, Role LW. Cholinergic circuits and signaling in the pathophysiology of schizophrenia. International Review of Neurobiology. 2007;78:193–223. doi: 10.1016/S0074-7742(06)78007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Brébion G, Bressan RA, Amador X, Malaspina D, Gorman JM. Medications and verbal memory impairment in schizophrenia: The role of anticholinergic drugs. Psychological Medicine. 2004;34:369–374. doi: 10.1017/s0033291703008900. [DOI] [PubMed] [Google Scholar]
- Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, Kirshner MA, Sorisio DA, Bies RR, Gharabawi G. Anticholinergic activity of 107 medications commonly used by older adults. Journal of the American Geriatrics Society. 2008;56(7):1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Chew ML, Mulsant BH, Pollock BG. Serum anticholinergic activity and cognition in patients with moderate-to-severe dementia. America Journal Geriatric Psychiatry. 2005;13:535–538. doi: 10.1176/appi.ajgp.13.6.535. [DOI] [PubMed] [Google Scholar]
- Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sciences. 1999;64:1761–1771. doi: 10.1016/s0024-3205(99)00114-9. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: A study of the human hippocampal formation. Biological Psychiatry. 2000;48:381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: A study of Brodmann’s Areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. American Journal of Psychiatry. 2001;158:918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM, Double KL, Brooks WS, Creasey H, Broe GA. Cell loss in the nucleus basalis is related to regional cortical atrophy in Alzheimer’s disease. Neuroscience. 1997;78:641–652. doi: 10.1016/s0306-4522(96)00569-6. [DOI] [PubMed] [Google Scholar]
- Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Molecular Psychiatry. 1996;1:54–58. [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Deng C, Huang X. Decreased density of muscarinic receptors in the superior temporal gyrus in schizophrenia. Journal of Neuroscience Research. 2005;81:883–890. doi: 10.1002/jnr.20600. [DOI] [PubMed] [Google Scholar]
- Doralp S, Leung LS. Cholinergic modulation of hippocampal CA1 basal-dendritic long-term potentiation. Neurobiology of Learning and Memory. 2008;90:382–388. doi: 10.1016/j.nlm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant M, Senut M, Lamour Y. The septohippocampal pathway: Structure and function of a central cholinergic system. Physiological Reviews. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive enhancement therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60:1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: A cohort study. Journal of Neurology, Neurosurgery, & Psychiatry. 2010;81:160–165. doi: 10.1136/jnnp.2009.186239. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, McGuire P, Sacchetti E. Neuroanatomy of vulnerability to psychosis: A voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews. 2011a;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: A critical review of neuroimaging studies. European Psychiatry. 2011b doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, Amunts K, Suarez-Gonzalez A, Cantero JL. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cerebral Cortex. 2010;20:1685–1695. doi: 10.1093/cercor/bhp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology REVIEWS. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes. Archives of General Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Keshava M, Cooley S, DiBarry AL, Garrett A, Parepally H, Zoretich R. Cognitive enhancement therapy for schizophrenia: Effects of a 2-year randomized trial on cognition and behavior. Archivesof General Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Herman MM, Hyde TM, Kleinman JE, Sinton CM, German DC, Hersh LB, Graybiel AM, Saper CB. Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience. 1999;94:21–32. doi: 10.1016/s0306-4522(99)00279-1. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Bachus SE, Hyde TM, Wittie M, Herman MM, Vangel M, Saper CB, Kleinman JE. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: An in situ hybridization study. Biological Psychiatry. 2005;58:408–416. doi: 10.1016/j.biopsych.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kay G, Crook T, Rekeda L, Lima R, Ebinger U, Arguinzoniz M, Steel Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. European Urology. 2006;50:317–326. doi: 10.1016/j.eururo.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biological Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CJ, Tune LE. Chronic exposure to anticholinergic medications adversely affect the course of Alzheimer Disease. American Journal of Geriatric Psychiatry. 2003;11:458–461. [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CC. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behavioral Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. American Journal of Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Mori K, Yamashita H, Nagao M, Horiguchi J, Yamawaki S. Effects of anticholinergic drug withdrawal on memory, regional cerebral blood flow and extrapyramidal side effects in schizophrenic patients. Pharmacopsychiatry. 2002;35:6–11. doi: 10.1055/s-2002-19831. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults. Archives of General Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Meltzer CC, Saxton JA, Houck PR, Halligan EM, DeKosky ST. Serum anticholinergic activity, white matter hyperintensities, and cognitive performance. Neurology. 2005;65:1487–1489. doi: 10.1212/01.wnl.0000183152.16690.26. [DOI] [PubMed] [Google Scholar]
- Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Annals of Neurology. 2003;54:235–238. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends in Neuroscience. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Molecular Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Lafargue T, Urbina RA, Egan MF, Pickar D, Weinberger DR. In vivo olanzapine occupancy of muscarinic acetylcholine receptors in patients with schizophrenia. Neuropsychopharmacology. 2000;23:56–68. doi: 10.1016/S0893-133X(99)00162-1. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Egan MF, Weinberger DR. Central muscarinic acetylcholine receptor availability in patients treated with clozapine. Neuropsychopharmacology. 2003;28:1531–1537. doi: 10.1038/sj.npp.1300210. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, Egan MF, Coppola R, Weinberger DR. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. American Journal of Psychiatry. 2003;160:118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research Reviews. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nature Reviews Neuroscience. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naïve patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Scarr E, Sundram S, Keriakous D, Dean B. Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biological Psychiatry. 2007;61:1161–1170. doi: 10.1016/j.biopsych.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Current Opinion in Psychiatry. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rössler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis – A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Keilp JG, Haas GL, Hill J, Weiden PJ. Relationships between medication treatments and neuropsychological test performance in schizophrenia. Psychiatry Research. 1991;37:297–308. doi: 10.1016/0165-1781(91)90065-w. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, Giovannetti T, Abraham G, Josiassen RC. Anticholinergicity and cognitive processing in chronic schizophrenia. Biological Psychology. 2001;56:1–22. doi: 10.1016/s0301-0511(00)00083-1. [DOI] [PubMed] [Google Scholar]
- Tran PV, Dellva MA, Tollefson GD, Beasley CM, Potvin JH, Kiesler GM. Extrapyramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. Journal of Clinical Psychiatry. 1997;58:205–211. doi: 10.4088/jcp.v58n0505. [DOI] [PubMed] [Google Scholar]
- Tune L, Coyle JT. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Archives of General Psychiatry. 1980;37:293–297. doi: 10.1001/archpsyc.1980.01780160063007. [DOI] [PubMed] [Google Scholar]
- Tune L, Coyle JT. Acute extrapyramidal side effects: Serum levels of neuroleptics and anticholinergic. Psychopharmacology. 1981;75:9–15. doi: 10.1007/BF00433493. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papthanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- Yoshiyama Y, Kojima A, Itoh K, Uchiyama T, Arai K. Anticholinergics boost the pathological process of neurodegeneration with increased inflammation in a tauopathy mouse model. Neurobiology of Disease. 2012;45(1):329–336. doi: 10.1016/j.nbd.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Katsifis A, Mattner F, Haung X. Investigation of M1/M4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29:619–625. doi: 10.1038/sj.npp.1300367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.