Summary
Primate evolution produced an increased capacity to respond flexibly to varying social contexts as well as expansion of the prefrontal cortex [1, 2]. Despite this association, how prefrontal neurons respond to social information remains virtually unknown. People with damage to orbitofrontal cortex (OFC) struggle to recognize facial expressions [3, 4], make poor social judgments [5] [6], and frequently make social faux pas [7, 8]. Here we test explicitly whether neurons in primate OFC signal social information and, if so, how such signals compare with responses to primary fluid rewards. We find OFC neurons distinguish images that belong to socially-defined categories, such as female perinea and faces, as well as the social dominance of those faces. These modulations signaled both how much monkeys valued these pictures and their interest in continuing to view them. Far more neurons signaled social category than signaled fluid value, despite the stronger impact of fluid rewards on monkeys’ choices. These findings indicate that OFC represents both the motivational value and attentional priority of other individuals, thus contributing to both the acquisition of information about others and subsequent social decisions. Our results betray a fundamental disconnect between preferences expressed through overt choice, which were primarily driven by the desire for more fluid, and preferential neuronal processing, which favored social computations.
Results
Monkeys differentially value socially distinct classes of images
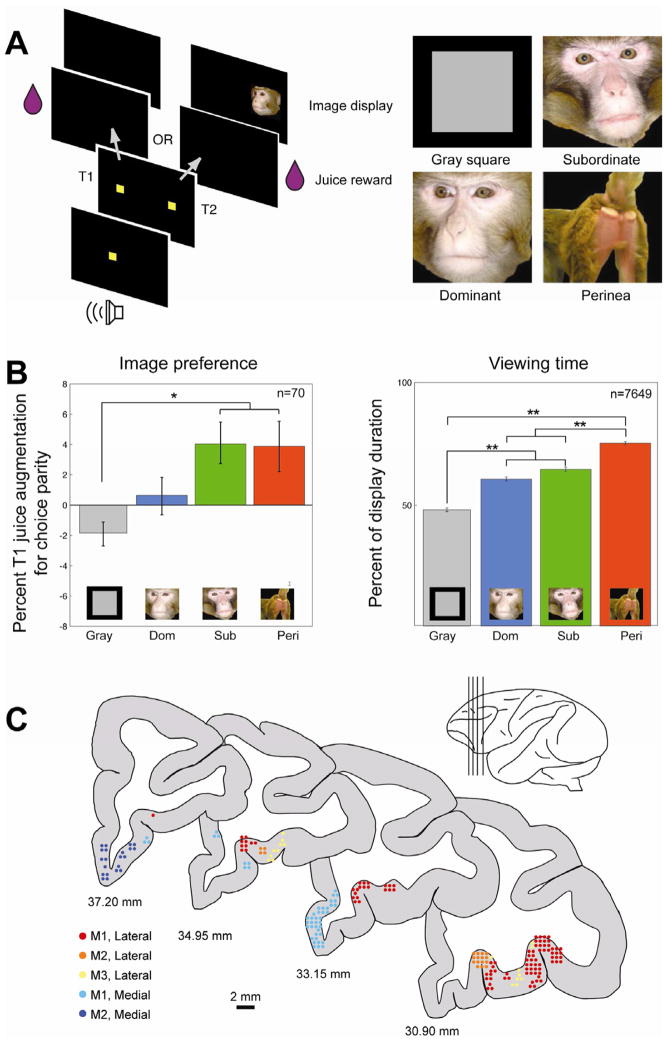
We studied the activity of 207 single OFC neurons in three male rhesus monkeys performing a choice task pitting fluid rewards against the opportunity to view socially-relevant pictures (Fig. 1a, b) [9, 10]. Monkeys chose between T1, resulting in fluid reward, and T2, resulting in fluid reward paired with an image from one of four social categories (nonsocial gray square, female perinea, dominant male faces, subordinate male faces), previously validated as motivationally relevant [9–11]. Importantly, access to fluid was controlled outside of experimental testing, thus motivating heightened sensitivity to fluid rewards (Figure S1). Nonetheless, multiple logistic regression of target choice as a function of fluid amount and image category revealed significant effects of both factors (odds of choosing T1, fluid Wald’s χ2 (df=1) = 6938.50, p<0.0000001; image Wald’s χ2 (df=3) = 12.30, p=0.006), confirming the intrinsic motivational significance of social stimuli [9].
Figure 1.
Single unit recording from macaque orbitofrontal cortex during a social decision making task. (A) Left, trial structure. Monkeys fixated a central stimulus, then shifted gaze to T1, resulting in a fluid reward, or T2, resulting in a fluid reward and the subsequent presentation of an image. The volume of fluid reward and the image category displayed varied in a nested block design [9]. The social category of the image pool remained stationary for superblocks of approximately 60–100 trials, whereas the amount of fluid reward associated with the two targets remained stationary for blocks of ~20 trials. Right, exemplars of social image categories. (B) Monkeys differentially value distinct classes of social images. Left, image values for each image category as derived from session-by-session choice behavior. The y-axis represents the percent of juice supplement paired with T1 necessary to induce indifference between T1 and T2. The x-axis indicates the image category paired with T2. Positive values indicate that animals sacrifice juice to view an image category, whereas negative values indicate that monkeys must be “bribed” to view an image. Right, viewing time durations for each image category for the trials in which the monkey viewed an image display. Y-axis represents the percent of samples for which the monkey’s eye position fell within the confines of the presented image while that image was being displayed. *indicates p<0.05 ** indicates p<0.001. (C) Location of recording sites from all three monkeys, depicted on coronal cross sections from a macaque brain atlas [40]. Each dot represents one neuron. Cool-colored dots represent those neurons grouped into Areas 14/25 (medial OFC), and hot-colored dots represent neurons grouped into Areas 11/13 (lateral/orbital OFC). Different colors within each shad represent individual monkeys.
In order to directly compare the effects of fluid and image class on monkeys’ choices, we calculated the value of each image set using the sign-reversed point of subjective equivalence (PSE) [9] during each session (see Experimental Procedures). A one way ANOVA revealed the value of each image class differed based on its social content (F(3,276)=4.84, p=0.003; Figure 1b). Specifically, the gray square had lower value than perinea and subordinate faces (Tukey HSD, p<0.05). Perinea and subordinate faces both had positive values (3.88 ±1.66 and 4.11 ± 1.37, respectively), dominant faces were neutral (0.5953 ± 1.23 SEM), and the gray square had negative value (−1.88 ± 0.7916). These results confirm that socially defined pools of images are meaningful to monkeys.
OFC neurons transiently respond to fluid and image rewards
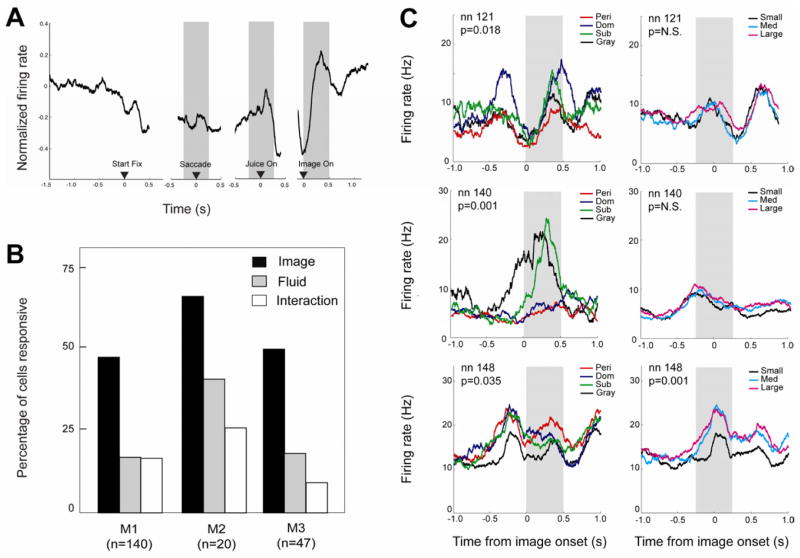
A normalized population firing rate histogram (Fig. 2a) showed transient modulations in activity following juice delivery and picture onset. Based on this temporal profile and task structure, we identified three epochs of neuronal activity for analysis (Fig. 2a; see also Experimental Procedures): a peri-saccade epoch, a peri-juice epoch, and an image epoch. In each, a significant number of neurons [n=63 of 207 (30.4%) or more in each of three epochs, p(n>62)<0.0001, binomial test; based on paired t-test, Bonferroni corrected for n=3 epochs] showed significant modulation in firing rate from baseline (last 500 ms of ITI). We next explored whether these response patterns varied across subregions of OFC. Neurons were identified as either medial OFC (Walker’s areas 14 and 25; [12]) or lateral OFC (areas 11 and 13) based on MRI reconstruction of electrode placement and recording depth (Fig. 1c). In all three epochs, a significantly greater number of neurons in lateral OFC than medial OFC showed significant firing rate modulations [χ2 =11.26–18.28 in each of three epochs, all p<0.001; 87/126 (69.1%) lateral and 22/76 (30.0%) medial OFC neurons had at least one significantly modulated epoch].
Figure 2.
OFC neurons respond to both fluid and visual social rewards. (A). Average population response. Note transient increases in firing rate time locked to the onset of juice delivery and the onset of image. PSTH represents population average response of all neurons aligned to task events. Left panel, aligned to initial fixation (trial start); middle panels, aligned to saccade and juice reward onset; right panel, aligned to image onset. Shaded gray boxes indicate the three epochs (saccade, juice delivery, image display) used for subsequent firing rate analysis. Firing rate was normalized by subtracting baseline (ITI) firing rate on each trial. PSTH is collapsed across all juice reward sizes and all image categories. Only trials in which the monkey chose to view the image are included. (B) Percentage of recorded neurons with firing rates significantly modulated by social image category (black bar), fluid amount (gray bar), or their interaction (white bar) for three different monkeys. The number of neurons modulated by image is significantly larger than that modulated by fluid or their interaction for all three monkeys. (C) Top and middle panel: representative neurons with firing significantly modulated by social image category (left), but not by fluid reward magnitude (right). Bottom panel displays an example neuron whose firing was significantly modulated by both image category (left) and fluid amount (right). For all example neurons, time zero is aligned to image reward onset in left panel and juice reward onset in right panel. Gray shaded boxes indicate the epoch relevant to the depicted statistical results. For illustration purposes PSTHs depict non-normalized firing rates smoothed with a 200 ms boxcar during trials for which the monkey chose to view an image.
OFC neurons differentiate outcomes but not decisions
We next explored whether responses of OFC neurons depend on active choice by comparing two-target free-choice trials with single target forced choice trials (see Experimental Details). A three-way ANOVA with image category, juice amount, and trial type (free choice or forced choice) as factors revealed that, in both the juice and image epochs, fewer neurons than expected by chance conveyed information about trial type (p>0.13 for both). This suggests OFC neurons respond similarly regardless of whether or not the monkey actively makes a choice. Based on this analysis, all subsequent analyses included both forced and free choice trials in order to increase statistical power.
More neurons signaled social image category than fluid amount
We next determined whether OFC neurons differentiated between distinct classes of social information (gray square, dominant faces, subordinate faces, and perinea), and compared that with differentiation of fluid rewards (small, medium, large). We first analyzed firing rates of each neuron on trials monkeys saw an image (ANOVA, factors = image category and received fluid amount). The number of neurons showing significant effects did not differ between monkeys (observed vs. expected, χ2 =2.33, df=2, p>0.31), so data were collapsed. A greater number of neurons (105 of 207, 50.24%) showed a main effect of image category (χ2 = 46.69, df=1, p<0.0001) than fluid reward size (38 of 207, 18.4%, Figure 2) during the saccade, juice and/or image epoch. Notably, during the juice epoch, 64 of 207 (30.9%) neurons showed a main effect of image category, and, during the image epoch, 15 of 207 (7.25%) showed a main effect of juice amount. This implies OFC neurons respond in a context-dependent manner, and do not simply convey direct experience of reward or visual experience. Relatedly, whether or not monkeys chose the image (T1 vs. T2) did not significantly alter the number of neurons responsive to image category or fluid (all p>0.16, see Table S1).
We next performed post-hoc t-tests to determine how much of image coding could be attributed to social images vs. the gray square. Among neurons discriminating between image classes, an equal number of neurons had significantly different firing rates for the gray square vs. the other categories (Tukey’s HSD test, Bonferroni corrected, n=61 for gray square vs. the three social categories in one or more task epochs; n=61 within the three social categories; See also Table S2). An equal number of neurons in medial and lateral OFC signaled fluid size, social image class, or their interaction (χ2 <1.88, p>0.11). We next analyzed whether the number of neurons modulated by image category was affected by target choice, which determined whether the monkey actually saw an image. Using a more lenient significance threshold (p<0.05), 31 of 207 cells had a main effect of image category during the image epoch during T2 (image) trials. Of these, 17 (54.8%) were also modulated by image category during T1 (blank) trials. This suggests that at least half of the neurons responsive to social category are encoding current social context, rather than simply signaling visual properties of the stimuli. Moreover, there were no correlations between firing rate and with image-based measures of image similarity or redness [13, 14], nor did neurons discriminate between dominant faces and their phase-scrambled counterparts (see Supplementary Experimental Procedures). Overall, these observations demonstrate that a large proportion of OFC neurons convey socially relevant information, even when other factors, such as gustatory rewards, dominate choice.
OFC neuron activity reflects the value of social outcomes
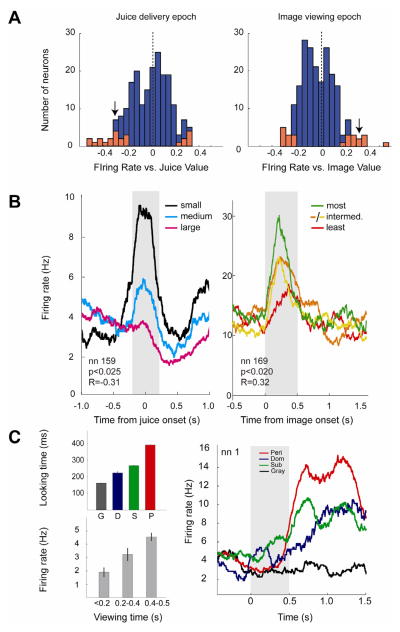
The above analyses do not reveal whether social signals in OFC are motivational in nature. To address this question, we used linear regression to analyze firing rates of each neuron as a function of fluid or social value on each trial while holding the other outcome constant. By determining the indifference point (PSE) based on choices made in the decision task, we established social image value in terms of fluid amount (percent of average fluid size) for each category of image (see above Results for more detail). During the image epoch, a significant number of neurons [n=24 of 207, 11.6%, p(n>23)<0.001, binomial test] showed significant correlations between firing rate and social image value (Fig. 3). During the juice epoch, an equivalent number of neurons [n=24 of 207, 11.6%, p(n>23)<0.001, binomial test] showed significant correlations between firing rate and fluid reward value (Fig. 3), as expected from prior studies [15]. Although the number of neurons encoding social image value and juice value were the same, these two populations of neurons were distinct (overlap = 2 neurons). However, motivational value signals were not temporally constrained to receipt of juice reward or visual social experience: the activity of 26 (12.6%) neurons was correlated with image value during the juice epoch, and the activity of 27 (13.0%) neurons was correlated with juice value during the image epoch. The number of neurons representing image value and juice value in medial OFC vs. lateral OFC did not differ (χ2 <0.57, p>0.45). Overall, these findings suggest OFC neurons carry information about social value as inferred from choice behavior, but these signals may be maintained separately from representations of gustatory value.
Figure 3.
OFC neurons signal fluid and social image value. (A) Correlation coefficients between normalized firing rates and fluid rewards (left) and image rewards (right) for the juice delivery epoch and image epoch, respectively. Significant neurons are highlighted in red. Black arrowheads designate the r-values of the two neurons depicted in (B). Social r-values were calculated using firing rates measured while fluid reward was held constant (i.e. only trials yielding the medium-sized fluid reward), and juice r-values were calculated using normalized firing rates measured while image was held constant (i.e. only trials displaying the gray square). Both forced choice and free choice trials resulting in image display were included. (B) Representative neurons with firing modulated by fluid reward magnitude (left) and social image value (right). Black, cyan and magenta represent small, medium, and large fluid amounts, respectively; Green, orange, yellow, and red represent different image categories ordered by value (as derived from choice behavior), ranked in the order listed. Time zero is aligned to juice reward onset (left) and image reward onset (right). Gray shaded boxes indicate the epoch relevant to the depicted statistical results. Histograms are smoothed with a 200 ms boxcar. For illustration purposes, plots depicted in (B) are derived from non-normalized firing rates during trials for which the monkey chose to view an image, and are collapsed across all image categories (left) or juice amounts (right). (C) OFC neurons signal social interest. Left top panel, mean looking durations for the four image categories during a sample session. G, gray square; D, dominant faces; S, subordinate faces; P, perinea. Left bottom panel, mean firing rates of the example neuron as a function of looking duration. Right panel, non-normalized firing rate of a neuron recorded during the same session, segregated by image category. PSTH is aligned to image onset; black bar indicates the period during which the image was displayed. Histogram is smoothed with a 200 ms boxcar.
Our findings suggest an approximately equal proportion of neurons in OFC encode image value and juice value. This is surprising since juice value strongly determined choices. Although distinct image classes tended to measurably bias choices (Figure 1), these effects were subtle. Surprisingly, the number of neurons that discriminated between social image categories during image viewing was significantly greater than the number of neurons that signaled image value (60 vs. 24; χ2 =19.36, p<0.0001). Further, the number of neurons that discriminated between social image categories during the juice delivery epoch was significantly greater than the number of neurons with firing rates correlated with juice value (64 vs 24; χ2 =23.09, p<0.00001) during the same epoch. Weak encoding of value but robust encoding of categorical social information suggest OFC neurons may contribute to more than simple binary choice.
To test this idea, we next asked whether firing rates of OFC neurons varied with how long monkeys viewed each class of images once they chose them. Viewing time is often used as a measure of interest in both nonhuman primates and human infants [16, 17]. As in previous studies [9], viewing time and choice preferences in our data were not strictly correlated, suggesting these two metrics index distinct processes. We explored whether firing rates were correlated with how long monkeys chose to view particular social images in the subset of neurons for which we had viewing time data (n=71, 23 of which had transient firing rate changes during the image epoch). Based on the temporal profile of cell activity, we analyzed two epochs, during image display and immediately following image offset. We found that firing rates of a small but significant number of neurons were correlated with viewing time in the latter epoch [n=7, p(n>6)<0.025, binomial test, Figure 3], but not the former [n=3, p(n>2)=0.47]. We next performed a two-way ANCOVA on these neurons using viewing time data and social image value as covariates (n=71). This revealed that viewing time, but not choice-based image value, was significantly associated with firing rate [n=13 for viewing time, p(n>12)<0.0001; n=3 for image value, p(>2)>0.4]. These findings suggest OFC neurons also convey information about interest or attention as indexed by prolonged gaze [18].
Discussion
Our findings demonstrate that OFC neurons signal socially relevant information derived from visual images. OFC neurons respond transiently to social visual stimuli, differentiate between socially distinct classes of images, weakly signal the value of these images to the monkey, and convey interest in continuing to view a particular image. These results complement recent findings that OFC neurons track fluid reward value when it is altered by social context [19]. However, in our study, more than twice as many neurons were sensitive to image category than fluid amount, despite monkeys’ choices being dominated by fluid rewards. This finding suggests decision value is not the sole determinant of firing rate changes in OFC [20]. Different types of social stimuli are capable of inducing a vast array of cognitive and physiological states that may not be observable in simple binary choices. For example, approach behavior can be elicited by both negatively valenced states, such as the desire to seek more information in the case of a threatening stimulus (e.g. a dominant male face), or by positively valenced states, as is the case for sexual imagery (e.g. female perinea). Avoidance behavior can arise from disgust, fear, or mere indifference. This suggests OFC neurons may contribute to other processes beyond the calculation of value.
OFC neurons also signaled the length of time monkeys looked at an image. Looking time represents an alternative and complementary measure of interest or attention that differs qualitatively from preferences expressed though choice, and is often used in developmental psychology and comparative cognition studies [21]. We found OFC neurons with firing rates unambiguously modulated by looking time, which suggests that qualities associated with this metric, such as salience or emotional responses, may be represented in OFC.
Our findings indicate that socially relevant information may have privileged access to OFC, which is well positioned anatomically to bind visual social and affective information, given abundant connections with both sensory and limbic regions [22–24]. Visual inputs from face-sensitive areas in temporal cortex may explain the presence of face-sensitive patches in OFC revealed by functional imaging [25, 26]. Importantly, social actors are rarely static, and the meaning of their actions must be constantly tracked and updated. Adaptive social behavior depends on the location, affective state, social status, reproductive state and social context of both oneself and others.
Our findings are also consistent with work showing OFC lesions disrupt the ability to respond appropriately to shifting environmental conditions, such as altered reward contingencies in reversal-learning tasks [27–29]. Prior studies documenting recruitment of OFC neurons by choosing between food or fluid rewards [15, 19, 30, 31] may also reflect similar processes; adaptive foraging demands behavioral flexibility to respond to resource depletion, prey dispersion, and other factors [32, 33]. For social animals like monkeys, foraging for food or fluid rarely occurs in isolation [34, 35]. The trade-off between foraging and social vigilance resonates with our finding that more OFC neurons signaled social information than gustatory information. Among socially sophisticated animals like macaques, social interaction requires dynamic behavioral flexibility that may place proportionately greater demands on neural circuitry that updates real-time estimates of the locations, identity, affective states, and intentions of others. We speculate that OFC, along with other regions of frontal cortex [36, 37], is part of a specialized neural circuit that evolved concomitantly with increasingly sophisticated social behavior, a conjecture consistent with the direct relationship between OFC size and social network size in humans [38], as well as group size across primates [39].
Experimental Procedures
All procedures were designed and conducted in compliance with the Public Health Service’s Guide for the Care and Use of Animals and were approved by the Duke University Institutional Animal Care and Use Committee. Individual neurons were recorded from orbitofrontal cortex of three adult male rhesus macaques (Macaca mulatta) while they performed a modified pay-per-view decision task ([19, 20, [11]] (see Figure 1 and Supplementary Experimental Procedures). The amount of fluid reward and the set of images paired with each of two targets varied in blocks. Image sets consisted of either a uniform gray square or dominant faces (n=60), subordinate faces (n=60), and female perinea images (n=60), all presented randomly with replacement. Image value was defined as the magnitude of fluid required to equilibrate the frequency of choosing each of the two options for each set of images, as estimated by the mean of a cumulative normal function fit to choice frequencies as a function of fluid magnitude for choosing to view an image [19]. Viewing time was recorded for a subset of neurons (n=70). Horizontal and vertical eye positions were sampled at 1000 Hz by an infrared camera (Eyelink 1000, Kanata, Ontario, Canada). Neuronal waveforms were discriminated online (Plexon: Dallas, TX, USA) and firing rates analyzed off-line (Matlab: Natick, MA, USA). For all analyses, normalized neuronal response was calculated by subtracting baseline activity (last 500 ms of ITI) from firing rates calculated for 500 ms epochs (typically peri-saccade, peri-juice, and image display durations).
Supplementary Material
Acknowledgments
The authors thank Rebecca Watson for helpful comments. The research was funded by a Cure Autism Now Young Investigator Award (K.K.W.), a Hilda and Preston Davis Foundation Postdoctoral Fellowship Program in Eating Disorders Research Award (K.K.W.), NIH RO1-EY013496 (M.L.P.), and NIH RO1-MH-0867712 (M.L.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Preuss TM. Do rats have prefrontal cortex? the Rose-Woolsey-Akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends in Neurosciences. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 4.Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- 5.Willis ML, Palermo R, Burke D, McGrillen K, Miller L. Orbitofrontal cortex lesions result in abnormal social judgements to emotional faces. Neuropsychologia. 2010;48:2182–2187. doi: 10.1016/j.neuropsychologia.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 7.Cicerone KD, Tanenbaum LN. Disturbance of social cognition after traumatic orbitofrontal brain injury. Archives of Clinical Neuropsychology. 1997;12:173– 188. [PubMed] [Google Scholar]
- 8.Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2006;18:871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- 9.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 10.Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Current Biology. 2008;18:419–424. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE. 2009:4. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. The Journal of Comparative Neurology. 1940;73:59–86. [Google Scholar]
- 13.Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. Sexual skin color contains information about the timing of the fertile phase in free-ranging Macaca mulatta. Int J Primatol. 2009;30:777–789. [Google Scholar]
- 14.Waitt C, Gerald M, Little A, Kraiselburd E. Selective attention toward female secondary sexual color in male rhesus macaques. American Journal of Primatology. 2006;68:738–744. doi: 10.1002/ajp.20264. [DOI] [PubMed] [Google Scholar]
- 15.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushnell I, Sai F, Mullin J. Neonatal recognition of the mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- 17.Demaria C, Thierry B. Responses to animal stimulus photographs in stumptailed macaques (Macaca arctoides) Primates. 1988;29:237–244. [Google Scholar]
- 18.Shimojo S, Simion C, Shimojo E, Scheier C. Gaze bias both reflects and influences preference. Nature Neuroscience. 2003;6:1317–1322. doi: 10.1038/nn1150. [DOI] [PubMed] [Google Scholar]
- 19.Azzi JCB, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann NY Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez J. Species comparative studies and cognitive development. Trends Cogn Sci. 2005;9:118–125. doi: 10.1016/j.tics.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 23.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363:642– 664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 24.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann NY Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 25.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of faceselective cortex in the macaque frontal lobe. Nature Neuroscience. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadj-Bouziane F, Bell AH, Knusten TA, Ungerleider LG, Tootell RBH. Perception of emotional expressions is independent of face selectivity in monkey inferior temporal cortex. Proceedings of the National Academy of Sciences. 2008;105:5591. doi: 10.1073/pnas.0800489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 28.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. The Journal of Neuroscience. 2004;24:7540. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton ME, Behrens TEJ, Buckley MJ, Rudebeck PH, Rushworth MFS. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 31.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 32.Charnov EL. Optimal Foraging, Marginal Value Theorem. Theoretical Population Biology. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 33.Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- 35.Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- 36.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MFS. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 37.Sallet J, Mars R, Noonan M, Andersson J, O’Reilly J, Jbabdi S, Croxson P, Jenkinson M, Miller K, Rushworth M. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 38.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RIM. Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunbar RIM. Why are apes so smart? In: Kappeler PM, Pereira ME, editors. Primate life histories and socioecology. Chicago: University Of Chicago Press; 2003. pp. 285–298. [Google Scholar]
- 40.Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.