Abstract
Evidence suggests that the small chloroplast heat-shock protein (Hsp) is involved in plant thermotolerance but its site of action is unknown. Functional disruption of this Hsp using anti-Hsp antibodies or addition of purified Hsp to chloroplasts indicated that (a) this Hsp protects thermolabile photosystem II and, consequently, whole-chain electron transport during heat stress; and (b) this Hsp completely accounted for heat acclimation of electron transport in pre-heat-stressed plants. Therefore, this Hsp is a major adaptation to acute heat stress in plants.
PSII (the H2O-oxidizing, quinone-reducing complex) is usually the most heat sensitive of the chloroplast thylakoid-membrane protein complexes involved in photosynthetic electron transfer and ATP synthesis and is one of the most thermolabile photosynthetic processes in general (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux, 1993). Within PSII, the O2-evolving-complex proteins are frequently the most susceptible to heat stress, although both the reaction center and the light-harvesting complexes can be disrupted by high temperatures as well (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux, 1993). Thermotolerance of PSII varies widely among species and there is also variation in the extent of acclimation of PSII to heat stress (Berry and Björkman, 1980; Weis and Berry, 1988). With the exception of the probable protective effect of xanthophyll-cycle carotenoids (Havaux et al., 1996) and isoprene (Sharkey and Singaas, 1995) on membrane stability and PSII function, little is known about the protective adaptations of PSII to heat stress. However, accumulating evidence suggests that chloroplast Hsps are involved in photosynthetic and PSII thermotolerance.
For example, when phenotypic variation in the production of the major chloroplast lmw Hsp is induced (e.g. by manipulating N availability), increased levels of the lmw Hsp are positively correlated with increased thermotolerance of PSII (Stapel et al., 1993; Clarke and Critchley, 1994; Heckathorn et al., 1996). Additionally, greater production of the chloroplast lmw Hsp, both within (Park et al., 1996) and among species (Downs et al., 1997), is positively correlated with whole-plant thermotolerance. Also in support of this, several chloroplast fractionation studies indicate that the lmw Hsp is a stromal protein that associates with the thylakoid membranes in response to heat stress (Restivo et al., 1986; Glaczinski and Kloppstech, 1988; Adamska and Kloppstech, 1991; Debel et al., 1997; also see Vierling, 1991; Clark and Critchley, 1994). However, a definite role of this or other Hsps in photosynthetic thermotolerance has not been demonstrated.
In contrast to most hmw Hsps, which are constitutively expressed and are essential for protein folding and import into organelles (i.e. they are molecular chaperones) (Gatenby and Viitanen, 1994; Hartl, 1996), lmw Hsps (approximately 17–30 kD) are generally produced only in response to environmental stress and little is known about their function (Vierling, 1991; Howarth and Ougham, 1993; Parsell and Lindquist, 1994; Boelens and de Jong, 1995; Waters et al., 1996). Purified lmw Hsps from both plants and animals have been shown to prevent aggregation or facilitate reactivation of other proteins in vitro (Jakob et al., 1993; Merck et al., 1993; Lee et al., 1995). Also, natural in vivo production of lmw Hsps is often correlated with cell or organismal thermotolerance (Vierling, 1991; Howarth and Ougham, 1993; O'Connell, 1994; Parsell and Lindquist, 1994; Boelens and de Jong, 1995; Waters et al., 1996), and thermotolerance of mutant mammalian, protistan, and fungal cells that over- or underexpress lmw Hsps increases or decreases, respectively (Loomis and Wheeler, 1982; Landry et al., 1989; Plesofsky-Vig and Brambl, 1995). These studies indicate that lmw Hsps are an important adaptation to heat stress, perhaps by functioning as molecular chaperones.
lmw Hsps are often the most abundant group of Hsps in plants, whereas in other organisms hmw Hsps are the most abundant (Vierling, 1991; Howarth and Ougham, 1993; O'Connell, 1994; Parsell and Lindquist, 1994; Boelens and de Jong, 1995; Waters et al., 1996). Plants typically produce more than 10 lmw Hsps in response to heat stress, with each Hsp belonging to one of six distinct gene classes. In other organisms, only one or two lmw Hsps of a single class are produced. Two of these gene classes encode proteins that localize to the cytosol, one class each encodes proteins that localize to the ER, mitochondria, and chloroplasts, and localization of the sixth class is unknown (Vierling, 1991; Waters, 1995; Waters et al., 1996).
The chloroplast lmw Hsp, first described in 1986 (Vierling et al., 1986), has two conserved regions in common with other lmw Hsps, but has a third domain that is unique. This Met-rich domain is predicted to form an amphipathic α-helix, similar to the 54-kD signal-recognition particle (Vierling, 1991). This Hsp is nuclear encoded, is produced only in response to environmental stress, and is usually the most heat responsive of the chloroplast Hsps. The function of the chloroplast lmw Hsp remains unknown; however, as noted above, this protein may be involved in PSII thermotolerance. In this study we directly test whether the chloroplast lmw Hsp confers thermotolerance to PSII function or to other aspects of thylakoid electron transfer.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tomato (Lycopersicon esculentum Mill. cv UC82B) plants were grown from seed in 8-L pots containing commercial potting soil, and were watered daily and fertilized weekly with one-half-strength Hoagland solution. Plants were raised in growth chambers under 25/18°C day/night temperatures and at 900 μmol m−2 s−1 PPFD. Plants were heat stressed by gradually increasing the chamber temperature over 2 h to 42°C, holding this temperature for 6 h, and then gradually decreasing the temperature over 2 h back to control conditions. Control plants were maintained throughout at 25°C. During heat stress the plants were kept well watered and the growth chambers were humidified by misting to prevent water stress.
Antisera Production
Polyclonal rabbit antiserum specific to the Met-rich domain of the chloroplast lmw Hsp (Abmet) was produced using a synthetic oligopeptide antigen (as described by Downs et al., 1997). Antiserum with broad specificity (among species and lmw Hsp classes) to the highly conserved α-crystallin region of plant lmw Hsps (Abα) was generated in a similar way, using an amino acid sequence that encompasses domain II and part of the segment between domains I and II of the carboxyl-terminal region of lmw Hsps (i.e. the α-crystallin region) (Vierling, 1991; Caspers et al., 1995; Waters, 1995; Waters et al., 1996). The sequence of the oligopeptide antigen, designed using amino acid sequences obtained from GenBank (see Waters, 1995), is as follows: NH2-RVERSSGKFVRRFRLPENAKVDQVKASMENGVLTVTVPK-COOH. The oligopeptide and antiserum were produced by Bio-Synthesis (Lewisville, TX).
Electron-Transport Assays
Intact chloroplasts were isolated as described by Downs et al. (1997), but without protease treatment, inspected microscopically for purity and intactness (approximately 80–90%), and lysed in 50 mm Hepes, pH 7.6, 50 mm sorbitol, 5 mm MgCl2, 5 mm NaCl, 19 mm NH4Cl, to which was added: 5 mm (final concentration) NaN3 and 50 μm MV, for whole-chain assays; 10 μm dichlorophenyl dimethylurea, 100 μm dichlorophenol indophenol, and 5 mm ascorbate for PSI assays; or 50 μm parabenzoquinone and 25 μm dibromomethyl isopropyl benzoquinone for PSII assays (Allen and Holmes, 1986). Chloroplasts were incubated at either 25 or 47°C for 2 min, and then Abmet or Abα was added (1:300) to some chloroplast samples; either nothing, whole-molecule goat IgG (1:300), or BSA (250 μg/mL) was added to other samples as negative controls. Similar results were obtained whether IgG, Abmet, and Abα were added at dilutions of 1:60, 1:150, 1:300, or 1:1,500; however, at 1:10,000, the efficiency of Abmet and Abα began to decrease.
Proteins were added 1 min after steady-state rates of electron transport were observed (approximately 90 s after samples were illuminated and 2 min after incubation began); similar results were obtained when proteins were added before illumination. The rate of electron transport for whole-chain (water-to-MV), PSII (water-to-parabenzoquinone), and PSI (ascorbate/dichlorophenol indophenol-to-MV) assays was determined by monitoring liquid-phase O2 uptake (whole-chain and PSI) or evolution (PSII) (Walker, 1990). The chlorophyll concentration of the samples was 25 μg/mL. The light intensity was 1000 μmol m−2 s−1 PPFD. Results were analyzed by three-way (control/heat stress × incubation temperature × protein addition) ANOVA. Differences among protein additions within each control/heat-stress × incubation-temperature combination were analyzed by Sheffe's multiple-comparison test, following significant ANOVA results for effects of protein additions.
Purification of lmw Hsp
Abmet was purified from whole serum by first removing all IgGs using protein A-Sepharose, and then separating Abmet from other IgGs using immobilized purified antigen. Affinity-purified Abmet was then covalently conjugated to 3M Emphaze Biosupport medium AB1 (Pierce), following the suppliers' protocols. Chloroplasts were isolated from heat-stressed tomato plants, lysed, and resuspended in 50 mm Hepes, pH 7.8, 1 mm PMSF, 1 mm benzamide, 100 mm EDTA, 10 μm leupeptin, and 10 μm antipain. Chloroplast samples were filtered (0.45 μm) and passed by gravity flow through a 3-mL column (60 × 5 mm) containing 1.5 mL of beads with Abmet. The column was washed with PBS and bound proteins were eluted using 0.1 m Gly, pH 2.5. The elution was titrated to pH 7.0 using KOH and then centrifuged in a 30-kD pore-size microconcentrator (Millipore) to separate Hsp from any eluted IgG. Hsp purity was determined by SDS-PAGE (12.5% gel; 5 μg of total protein per lane), followed by staining with silver or Coomassie blue (as described by Heckathorn et al., 1996; Downs et al., 1997). The identity of the lmw Hsp was confirmed by electroblotting proteins in unstained replicate lanes to PVDF membranes, and then probing with Abmet, followed by secondary antibody conjugated to alkaline phosphatase. Secondary antibody was detected with nitroblue tetra- zolium/5-bromo-4-chloro-3-indolyl phosphate.
RESULTS AND DISCUSSION
To examine the importance of the lmw Hsp to photosynthetic electron transport, we used polyclonal antibodies specific to either the unique Met-rich domain of the chloroplast lmw Hsp (Abmet), or to another conserved domain common to most plant lmw Hsps (Abα), to disrupt the function of the lmw Hsp in chloroplasts isolated from tomato plants. We then monitored photosynthetic electron transport by measuring O2 exchange of chloroplasts from unstressed control plants and plants that had been heat stressed at 43°C (pre-heat-stressed plants). Electron transport in control and pre-heat-stressed plants was assayed at both 25 and 47°C.
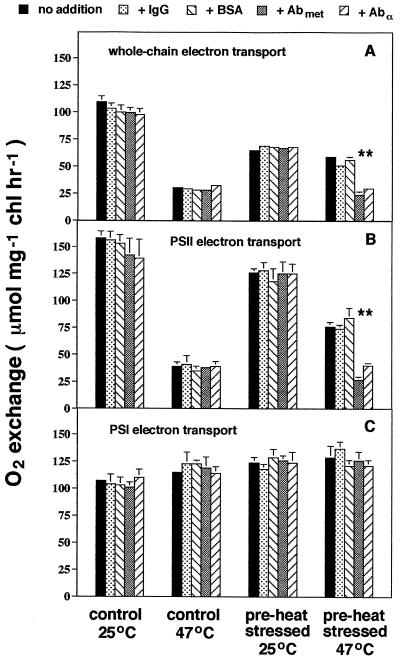
The rate of whole-chain electron transport was lower when assayed at 47°C than at 25°C (ANOVA; P < 0.0001), and pre-heat-stressed plants had lower rates of whole-chain electron transport compared with control plants (P < 0.0001) (Fig. 1A). Whole-chain electron transport was greater in pre-heat-stressed plants relative to controls at 47°C, which indicates that acclimation to high temperature occurred in pre-heat-stressed plants. This acclimation appeared to be entirely the result of the production of the chloroplast lmw Hsp, because the addition of Abmet and Abα, which we predicted would disrupt the function of the chloroplast lmw Hsp, decreased whole-chain electron transport in pre-heat-stressed plants incubated at 47°C by 54%, slightly less than that of control plants at the same temperature.
Figure 1.
The effect of Hsp-specific antibodies on photosynthetic electron transport. Chloroplasts were isolated from 10-week-old tomato plants grown at 25/18°C day/night temperatures or from plants heat stressed for 6 h at 43°C (control or pre-heat-stressed, respectively). Chloroplasts were lysed and then incubated at 25 or 47°C. Either nothing (no addition), IgG, BSA, Abmet, or Abα was added to chloroplast samples before determination of the rate of whole-chain (A), PSII (B), or PSI electron transport (C). Results are means ± 1 se; n = 3 to 4 (each from a separate set of chloroplasts). Differences (P < 0.05) among protein additions within each control/heat stress × incubation-temperature combination are indicated by asterisks (*). chl, Chlorophyll.
The addition of IgG or BSA had little effect on whole-chain electron transport of chloroplasts from pre-heat-stressed plants assayed at 25 or 47°C or in control plants at 25 and 47°C. Preimmune serum also had no effect on whole-chain electron transport, regardless of assay temperature or previous heat-stress status (not shown; limited availability of preimmune serum constrained the number of replicates to two and precluded conducting similar assays for PSII and PSI electron transport, but see below). No effect of Abmet or Abα was observed in control plants at either 25 or 47°C, as expected, because we observed no significant accumulation of the chloroplast lmw Hsp in leaves in the absence of heat stress (Downs et al., 1997). We also observed no effect of Abmet or Abα at 25°C in pre-heat-stressed plants. These results indicate that the chloroplast lmw Hsp protects photosynthetic electron transport at high temperature, but has no effect at control temperatures, and causes heat acclimation of whole-chain electron transport resulting from pre-heat stress.
We next measured PSII and PSI electron transport separately, while disrupting the function of the chloroplast lmw Hsp with antibodies, to determine more specifically which aspects of photosynthetic electron transport were protected by the lmw Hsp. Results for PSII electron transport were nearly identical to those obtained for whole-chain electron transport (Fig. 1B); PSII electron transport was lower at 47°C, relative to 25°C (ANOVA; P < 0.0001), and we observed an increase in electron transport in pre-heat-stressed plants at 47°C relative to controls at 47°C. This indicates that heat acclimation of PSII function occurred in pre-heat-stressed plants. Again, this acclimation was apparently related exclusively to the chloroplast lmw Hsp, because the addition of Abmet and Abα decreased PSII electron transport in pre-heat-stressed plants assayed at 47°C by 56%, to slightly less than that of control plants at 47°C. As before, the addition of IgG or BSA had little effect on PSII electron transport at 47°C in pre-heat-stressed plants. No effect of antibody/protein additions was observed in control plants at either 25 or 47°C or in pre-heat-stressed plants at 25°C. Similar results, including a negative effect of Abmet and Abα and no effect of preimmune serum, were obtained when PSII function was monitored by analysis of chlorophyll fluorescence from PSII (i.e. the ratio of variable to maximum fluorescence of dark-adapted chloroplast samples; data not shown).
In contrast to PSII function, PSI electron transport was slightly increased by heat stress (Fig. 1C), as is often the case (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux, 1993). Incubation of samples at 47°C increased PSI electron transport by 7% compared with 25°C (ANOVA; P < 0.0165), and pre-heat-stressed plants had slightly higher rates of electron transport than did controls (12%; P < 0.0001), but there was no significant interaction between incubation temperature and pre-heat-stress conditioning (P < 0.0893). PSI electron transport was also unaffected by antibody/protein additions (P < 0.9794). Collectively, results from this experiment indicate that the chloroplast lmw Hsp protects PSII and, consequently, whole-chain electron transport during heat stress.
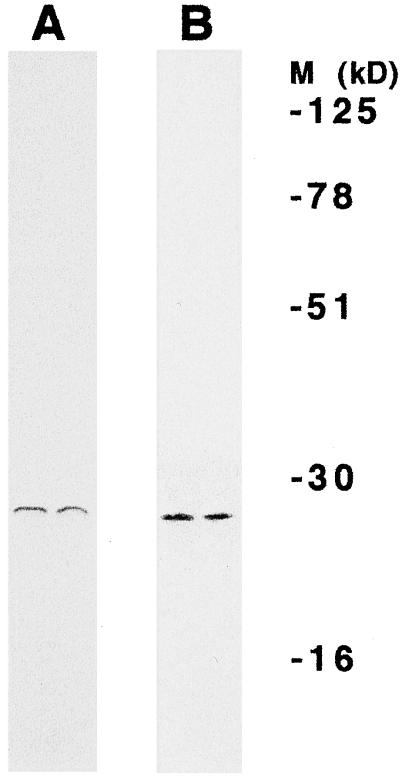
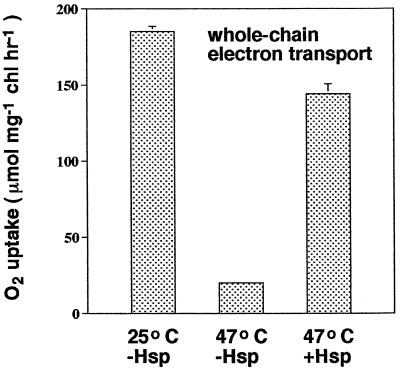
To determine if the chloroplast lmw Hsp could protect photosynthetic electron transport during heat stress when added to chloroplasts lacking this protein, we purified the chloroplast lmw Hsp from intact chloroplasts isolated from heat-stressed tomato plants to apparent homogeneity by antibody-affinity-column chromatography using Abmet, and then monitored the effects of purified lmw Hsp on whole-chain electron transport. Both silver (not shown) and Coomassie-blue staining of total protein eluted from the columns and subjected to SDS-PAGE indicated that only one protein was purified (Fig. 2A). Immunoblotting (western) on the other half of the same gels with Abmet indicated that this protein was the chloroplast lmw Hsp (Fig. 2B). When purified protein was added to thylakoid membrane preparations derived from chloroplasts isolated from unstressed control plants (i.e. plants that did not contain lmw Hsp) incubated at 47°C, the rate of whole-chain electron transport was 78% of that exhibited by chloroplast samples incubated at 25°C (Fig. 3). When purified lmw Hsp was not added to samples at 47°C, the rate of whole-chain electron transport was only 20% of the rate at 25°C. These results confirm that the chloroplast lmw Hsp confers thermotolerance to photosynthetic electron transport.
Figure 2.
Homogeneity of the lmw Hsp purified from intact chloroplasts isolated from heat-stressed tomato plants. Coomassie-blue stain (A) and immunoblot (western) (B) of protein samples eluted from an Abmet column. Eluted proteins were fractionated by gel electrophoresis. One-half of the gel (two lanes) was stained with Coomassie blue to detect all proteins present. Proteins in unstained replicate lanes were electroblotted to membranes and probed with Abmet. The locations of molecular mass markers (M), which were included on both the Coomassie-blue-stained gel and the immunoblot, are indicated.
Figure 3.
The effect of purified chloroplast lmw Hsp on whole-chain electron transport. Chloroplasts were isolated from 6-week-old unstressed tomato plants grown at 25/18°C day/night temperatures (i.e. plants without detectable levels of lmw Hsp). Chloroplasts were lysed and then incubated at 25 or 47°C; samples at 47°C were incubated with or without the addition of purified chloroplast lmw Hsp (0.0017 μg Hsp/μL). Assuming that all of the added protein was lmw Hsp and assuming a chlorophyll:PSII stoichiometry of 400, we estimate that there were approximately 14 lmw Hsps available per PSII. Results are means ± 1 se; n = 3. chl, Chlorophyll.
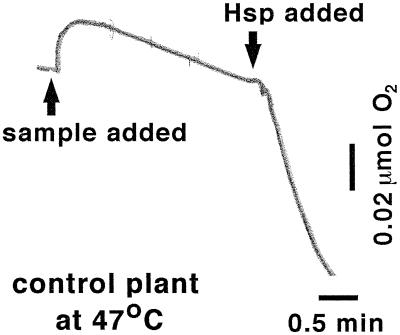
The rapidity with which the chloroplast lmw Hsp affects PSII function when disrupted with Abmet and Abα, or when purified protein is added to chloroplasts, is unusual among the stress-related functions of Hsps studied to date (Vierling, 1991; Howarth and Ougham, 1993; O'Connell, 1994; Parsell and Lindquist, 1994; Boelens and de Jong, 1995; Waters et al., 1996). For example, both lmw and hmw Hsps have demonstrated the capacity to reactivate previously aggregated or denatured proteins, but the time required to do so is at least 15 min (Showyra et al., 1990; Höll-Neugebauer et al., 1991; Jakob et al., 1993; Merck et al., 1993; Schröder et al., 1993; Lee et al., 1995). The addition of purified lmw Hsp to chloroplasts had an almost immediate (<1 min) effect on PSII function (Fig. 4). The disruptive effect of Abmet and Abα on PSII function (e.g. Fig. 1) was equally rapid (not shown). The speed with which the lmw Hsp protects PSII in vivo may not necessarily equal that observed in vitro.
Figure 4.
Time course of the effect of purified chloroplast lmw Hsp on whole-chain electron transport. Shown is a trace of an O2 electrode millivolt output through time of a representative chloroplast sample from unstressed control plants (i.e. lacking lmw Hsp). The sample was incubated with illumination (1000 μmol m−2 s−1 PPFD) at 47°C in a well-stirred, transparent, temperature-controlled cuvette. Chloroplast lmw Hsp was added approximately 2 min after steady-state rates of electron transport were observed. The rate of whole-chain electron transport was calculated from the rate of liquid-phase O2 uptake, which is proportional to the millivolt output generated by the O2-electrode sensor.
The method used in this study to rupture intact chloroplasts (mild hypotonic lysis) and the NaCl concentration of the medium in which broken chloroplasts were resuspended (5 mm) should have ruptured the chloroplast outer envelope without rupturing the thylakoids, and resulted in unstacked thylakoid membranes (Gegenheimer, 1990). Thus, accessibility to PSII may be greater in vitro than in vivo, where most PSII reaction centers are localized to relatively inaccessible grana regions. In vitro disruption of thylakoid organization may therefore increase the speed of Hsp function relative to in vivo speed, and may also increase antibody accessibility to Hsp.
Taken together, our results demonstrate that the chloroplast lmw Hsp is an important determinant of photosynthetic thermotolerance. The chloroplast lmw Hsp (a) increases PSII and, consequently, whole-chain electron transport during heat stress (by as much as 7-fold in our experiments; Fig. 3, 47°C for −Hsp versus 47°C for +Hsp); (b) completely accounts for all of the observed heat acclimation of PSII and whole-chain electron transport in pre-heat-stressed plants (Fig. 1); and (c) imparts protection to PSII very rapidly (Fig. 4). These observations are consistent with those of a number of studies demonstrating a correlation between the production of the chloroplast lmw Hsp and PSII or organismal thermotolerance (Stapel et al., 1993; Clarke and Critchley, 1994; Heckathorn et al., 1996; Park et al., 1996; Downs et al., 1997). To our knowledge, results from this study are the first direct evidence that Hsps (chloroplast or otherwise) play a role in photosynthetic thermotolerance. Because it is well established that PSII is a highly thermolabile component of photosynthetic electron transport in particular, and one of the most thermosensitive aspects of net photosynthesis and plant performance in general (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux, 1993), production of the chloroplast lmw Hsp represents an important adaptation of plants to acute heat stress.
ACKNOWLEDGMENTS
We thank E. Craig, J. Slovin, and anonymous reviewers for helpful comments on the manuscript and M. Shahan for assistance with affinity purification of Abmet.
Abbreviations:
- Ab
antibody
- ANOVA
analysis of variance
- hmw
high-molecular-weight
- Hsp
heat-shock protein
- lmw
low-molecular-weight
- MV
methyl viologen
Footnotes
This work was supported by grants from the National Science Foundation (IBN 9317900 to T.D.S. and IBN 9357302 and 9207203 to J.S.C.) and the Andrew W. Mellon Foundation (to J.S.C.). C.A.D. was an Advanced Opportunity Predoctoral Fellow.
LITERATURE CITED
- Adamska I, Kloppstech K. Evidence for the localization of the nuclear-coded 22-kDa heat-shock protein in a subfraction of thylakoid membranes. Eur J Biochem. 1991;198:375–381. doi: 10.1111/j.1432-1033.1991.tb16025.x. [DOI] [PubMed] [Google Scholar]
- Allen JF, Holmes NG. Electron transport and redox titration. In: Hipkins MF, Baker NR, editors. Photosynthesis Energy Transduction: A Practical Approach. Oxford, UK: IRL Press; 1986. pp. 116–126. [Google Scholar]
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Boelens WC, de Jong WW. α-Crystallins, versatile stress-proteins. Mol Biol Rep. 1995;21:75–80. doi: 10.1007/BF00986495. [DOI] [PubMed] [Google Scholar]
- Caspers GJ, Leunissen JAM, de Jong WW. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain.”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- Clarke AK, Critchley C. Characterization of chloroplast heat shock proteins in young leaves of C4 monocotyledons. Physiol Plant. 1994;92:118–130. [Google Scholar]
- Debel K, Sierralta WD, Braun HP, Schmitz UK, Kloppstech K. The 23-kDa light-stress-regulated heat-shock protein of Chenopodium rubrum L. is located in the mitochondria. Planta. 1997;201:326–333. doi: 10.1007/s004250050074. [DOI] [PubMed] [Google Scholar]
- Downs CA, Heckathorn SA, Bryan JK, Coleman JS (1997) The methionine-rich low-molecular-weight chloroplast heat-shock protein: evolutionary conservation and accumulation in relation to thermotolerance. Am J Bot (in press) [PubMed]
- Gatenby AA, Viitanen PV. Structural and functional aspects of chaperonin-mediated protein folding. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:469–491. [Google Scholar]
- Gegenheimer P. Preparation of extracts from plants. Methods Enzymol. 1990;182:174–193. doi: 10.1016/0076-6879(90)82016-u. [DOI] [PubMed] [Google Scholar]
- Glaczinski H, Kloppstech K. Temperature-dependent binding to the thylakoid membranes of nuclear-coded chloroplast heat-shock proteins. Eur J Biochem. 1988;173:579–583. doi: 10.1111/j.1432-1033.1988.tb14038.x. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Havaux M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993;94:19–33. [Google Scholar]
- Havaux M, Tardy F, Ravenel J, Chanu D, Parot P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. Plant Cell Environ. 1996;19:1359–1368. [Google Scholar]
- Heckathorn SA, Poeller GJ, Coleman JS, Hallberg RL. Nitrogen availability alters patterns of accumulation of heat stress-induced proteins in plants. Oecologia. 1996;105:413–418. doi: 10.1007/BF00328745. [DOI] [PubMed] [Google Scholar]
- Höll-Neugebauer B, Rudolph R, Schmidt M, Bucher J. Reconstitution of a heat shock effect in vitro: influence of GroE on the thermal aggregation of α-glucosidase from yeast. Biochemistry. 1991;30:11609–11614. doi: 10.1021/bi00114a001. [DOI] [PubMed] [Google Scholar]
- Howarth CJ, Ougham HJ. Gene expression under temperature stress. New Phytol. 1993;125:1–26. doi: 10.1111/j.1469-8137.1993.tb03862.x. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- Loomis WF, Wheeler SA. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982;90:412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Merck KB, Groenen PJTA, Voorter CEM, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine α-crystallin and mouse small heat-shock protein. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- O'Connell MA. Heat shock proteins and thermotolerance. In: Basra AS, editor. Stress-Induced Gene Expression in Plants. Chur, Switzerland: Harwood; 1994. pp. 163–183. [Google Scholar]
- Park SY, Shivaji R, Krans JV, Luthe DS. Heat-shock response in heat-tolerant and nontolerant variants of Agrostis palustris Huds. Plant Physiol. 1996;111:515–524. doi: 10.1104/pp.111.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. Heat shock proteins and stress tolerance. In: Morimoto RI, Tissiéres A, Georgopoulos C, editors. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 457–494. [Google Scholar]
- Plesofsky-Vig N, Brambl R. Disruption of the gene for hsp30, an α-crystallin-related heat shock protein of Neurospora crassa, causes defects in thermotolerance. Proc Natl Acad Sci USA. 1995;92:5032–5036. doi: 10.1073/pnas.92.11.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo FM, Tassi F, Maestri E, Lorenzoni C, Puglisi PP, Marmiroli N. Identification of chloroplast associated heat-shock proteins in Nicotiana plumbaginifolia protoplasts. Curr Genet. 1986;11:145–149. [Google Scholar]
- Schröder H, Langer T, Hartl FU, Bukau B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993;12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- Showyra D, Georgopoulos C, Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990;62:939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Stapel D, Kruse E, Kloppstech K. The protective effect of heat shock proteins against photoinhibition under heat shock in barley (Hordeum vulgare) J Photochem Photobiol. 1993;B21:211–218. [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Vierling E, Mishkind ML, Schmidt GW, Key JL. Specific heat shock proteins are transported into chloroplasts. Proc Natl Acad Sci USA. 1986;83:361–365. doi: 10.1073/pnas.83.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D (1990) The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis. Hansatech Instruments, Kings Lynn, UK
- Waters ER. The molecular evolution of the small heat-shock proteins in plants. Genetics. 1995;141:785–795. doi: 10.1093/genetics/141.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. [Google Scholar]
- Weis E, Berry JA (1988) Plants and high temperature stress. In SP Long, FI Woodward, eds, Plants and Temperature. Company of Biologists Ltd, Cambridge, UK, pp 329–346