Abstract
Depression is a risk factor for morbidity and mortality, and immune dysregulation may be partially responsible for this link. Proinflammatory cytokines such as interleukin 6 (IL-6) are reliable predictors of quality of life, morbidity, and many causes of mortality. The current study evaluated relationships between depressive symptoms, as assessed by the CES-D, and stress-induced inflammation. The participants, 138 healthy adults, were evaluated at rest, and after a standardized laboratory speech and mental arithmetic stressor. Compared with individuals with fewer depressive symptoms, those with more depressive symptoms produced more IL-6 in response to the stressor, as well as significantly higher levels of IL-6 both 45 minutes and 2 hours after the stressor. These findings add to our emerging understanding of the complex interactions among stress, depression, and immune dysregulation, and provide one potential pathway to explain relationships between depressive symptoms and disease.
1. Introduction
Depression is a risk factor for morbidity and mortality (Rovner et al., 1991; Wulsin, Vaillant, & Wells, 1999). Both major depression and subthreshold depressive symptoms have been linked to many diseases of older adulthood (Bush et al., 2001; Frasure-Smith et al., 2009; Pan et al., 2011; Satin, Linden, & Phillips, 2009; Wouts et al., 2008). Immune dysregulation may partially contribute to these links. Inflammation is a key immunological mechanism that promotes many age-related diseases including diabetes, certain cancers, cardiovascular disorders, frailty and mortality (Maggio, Guralnik, Longo, & Ferrucci, 2006). In this study, we examined how depressive symptoms interact with acute stress to induce proinflammatory cytokine production.
Acute stress produces transient increases in systemic inflammation. Stress-induced elevations in inflammation have been detected among medical students preparing for an academic exam, individuals giving an oral presentation, and couples engaging in a marital disagreement (Heinz et al., 2003; Kiecolt-Glaser et al., 2005; Maes et al., 1998). Standardized experimental performance tasks, such as the Trier Social Stress Test (TSST), promote reliable elevations in interleukin-6 (IL-6) (Carpenter et al., 2010; Pace et al., 2006; Steptoe, Hamer, & Chida, 2007). These stress-induced inflammatory responses may vary in magnitude between individuals.
Prior stress and depression may enhance stress-induced inflammatory rises by sensitizing the inflammatory response to stress (Glaser, Robles, Sheridan, Malarkey, & Kiecolt-Glaser, 2003). For example, rats that were previously exposed to inescapable tailshock produced larger inflammatory responses to bacterial endotoxin than controls that were not shocked (Johnson et al., 2002). Studies in humans provide preliminary evidence that depression may promote greater stress-induced increases in circulating IL-6. In a study comparing 14 males with a history of early life stress and major depression with 14 controls, major depression patients with early life stress produced a more pronounced plasma IL-6 response to the TSST than controls (Pace et al., 2006). In another study 16 women with a lifetime history of depression showed greater IL-6 increases after giving birth than 50 women without a history of depression (Maes, Ombelet, De Jongh, Kenis, & Bosmans, 2001). These findings provide initial evidence that major depression may enhance systemic inflammation. However, sample size was limited in both studies, and we do not know if these findings extend to those with depressive symptoms more generally.
Minor elevations in depressive symptoms are associated with higher baseline levels of proinflammatory cytokine production (Lutgendorf et al., 1999). Furthermore, in a community sample of older adults, those with more depressive symptoms had a greater IL-6 increase after an annual influenza vaccination compared to those with fewer symptoms, suggesting that even modest elevations in depressive symptoms may sensitize the inflammatory response (Glaser et al., 2003). The current study sought to extend work demonstrating that those with major depression have more pronounced IL-6 elevations in response to a stressor by examining whether stress-induced IL-6 responses are exaggerated among individuals with more depressive symptoms compared with fewer depressive symptoms.
2. Methods
2.1 Subjects
The study data were drawn from the baseline sample of a clinical trial addressing the potential benefits of fish oil. Participants were recruited through advertisements, brochures, and media announcements in the local community. Screening exclusions included a prior history of cancer (except basal or squamous cell), diabetes, chronic obstructive pulmonary disease, autoimmune disease, evidence of liver or kidney failure, symptomatic ischemic heart disease, GERD, ulcerative colitis, smoking status, excessively high triglycerides or LDL cholesterol, more than 3 hours a week of vigorous physical exercise, and a body mass index (BMI) above 40. Individuals could not participate if they were taking medications for depression, anxiety, cholesterol, or blood pressure. The Ohio State Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
2.2. Procedure
When participants arrived at the Clinical Research Center (a hospital research unit) at 7:45 a.m., a catheter was inserted in their arm. Once they had eaten a standardized breakfast (after fasting since midnight) and completed questionnaires (approximately 25 minutes after catheter insertion), they sat quietly in a chair for 20 minutes. At the end this relaxation period, blood was drawn to assess baseline IL-6 levels.
Next, subjects participated in the Trier Social Stress Test, a well validated stressor (Kirschbaum, Pirke, & Hellhammer, 1993). Participants were told they would deliver a speech in front of a committee of behavioral experts, and were briefly brought into the room to view the committee before they prepared the speech. Then, in another room, they spent 10 minutes preparing a speech about why they were the best candidate for a job. After that, a research assistant escorted them to a room where they saw a microphone, video camera, and an audience panel of 2 individuals wearing white laboratory coats. While seated, participants gave their 5-minute speech and then performed mental arithmetic serial subtraction tasks for 5 minutes in front of this panel. Then, 45 minutes post-stressor, another blood sample was drawn, followed by another blood draw 2 hours after the stressor.
2.3 Measures
The Center for Epidemiological Studies Depression Scale (CES-D) was used as a measure of depressive symptoms. It has been used extensively as a brief measure of depressive symptomatology (Basco, Krebaum, & Rush, 1997; Radloff, 1977). Studies have shown acceptable test-retest reliability and excellent construct validity (Basco et al., 1997).
We assessed major depression with the Structured Clinical Interview for DSM-IV, nonpatient version (SCID-NP) (First, Spitzer, & Williams, 1995) Inter-rater reliability for SCID-NP diagnoses were calculated using randomly selected audiotapes for 20% of the participants. This substantial interrater agreement was confirmed with McNemar's test for marginal proportions (p>0.99 for all diagnoses).
The Childhood Trauma Questionnaire provided data on early childhood abuse and neglect. Widely used, it has excellent normative data for its 5 scales: Physical, Sexual, and Emotional Abuse, and Physical and Emotional Neglect (Bernstein & Fink, 1998). We adopted the Walker cuts (Walker et al., 1999) to make categorical cut-offs (with sensitivity and specificity >.85 for each scale). Then, we created a categorical indicator variable representing any maltreatment (exceeding ≥ CTQ cut point threshold)(Walker et al., 1999).
We used the Beck Anxiety Inventory to assess both cognitive and physiological symptoms (Beck, Epstein, Brown, & Steer, 1988). This scale has been shown to have sound psychometric properties such as factorial validity, internal consistency, and test-retest stability.
Whole blood was drawn into a BD vacutainer serum tube (Becton-Dickinson, New Jersey) and allowed to clot at room temperature for 30 minutes. Serum tubes were then centrifuged for 10 minutes at 2700rpm. Serum was stored at -86OC until assayed in duplicate levels for IL-6 cytokine using MSD 96-well Multispot Custom Cytokine Kits (Meso Scale Discovery, Maryland) . Plates were read using an MSD Sector Imager 2400. A four parameter logistic fit standard curve was derived using the MSD Software and IL-6 concentrations were extrapolated from the standard curve. The IL-6 sensitivity was 0.26 pg/ml. The CV for intra-assay precision had a range of 5.64-6.70%. The CV for inter-assay precision had a range of 5.16-10.2%.
2.4 Analytic Method
All analyses were run using mixed models regression and restricted maximum likelihood estimation. IL-6 was log transformed before analysis. We adjusted for key potential confounds including age, BMI, and sex. We examined residuals from all analyses to confirm that they were distributed normally.
We hypothesized that individuals who had more depressive symptoms would have greater increased IL-6 in response to the stressor than those with fewer depressive symptoms. We ran a repeated measures analysis with depressive symptoms modeled as a continuous variable to test this hypothesis. There was considerable variability in correlations over the three segments. Hence, we employed an unstructured within-subjects covariance matrix for all repeated measures analyses. The Kenward-Roger option was used to correct the degrees of freedom in the model, which brought Type I errors rates back to the nominal level. All tests used a two-sided, α=0.05 significance level.
3. Results
Table 1 reports descriptive information for the participants. Table 2 summarizes the results of the repeated measures analysis that assessed whether changes in IL-6 over time differed depending on depressive symptoms.
Table 1. Sample Population Characteristics.
| Total (N=138) | |||
|---|---|---|---|
|
| |||
| Variable | n | M | SD |
| IL6 | 3.79 | 3.84 | |
| Log IL6 | 1.01 | 0.81 | |
| Age (yrs) | 51.04 | 7.75 | |
| BMI (kg/m2) | 30.59 | 4.50 | |
| CESD | 7.58 | 8.10 | |
| Current depression | |||
| No | 119 | ||
| Yes | 4 | ||
| Race | |||
| White | 109 | ||
| Black | 22 | ||
| Asian | 4 | ||
| Native American | 2 | ||
| Other | 1 | ||
| Sex | |||
| Female | 93 | ||
| Male | 45 | ||
| Menopause | |||
| No | 56 | ||
| Yes | 45 | ||
| NA | 37 | ||
| Marital Status | |||
| Single | 19 | ||
| Married | 92 | ||
| Common Law | 3 | ||
| Separated | 3 | ||
| Divorced | 16 | ||
| Widowed | 5 | ||
| Education | |||
| Some high school | 7 | ||
| Some college | 32 | ||
| College graduate | 52 | ||
| Graduate/Professional | 47 | ||
| Income | |||
| < 25 K | 11 | ||
| 25 K- 50 K | 34 | ||
| 50 K- 75 K | 35 | ||
| 75 K- 100 K | 26 | ||
| > 100 K | 24 | ||
| Prefer not to answer | 8 | ||
Table 2. Interleukin 6 across time based on CES-D scores.
| Effect | Time point | Log IL6 | |
|---|---|---|---|
|
|
|||
| Estimate | p | ||
| CESD total score | 0.008 | 0.240 | |
| Period | Baseline | ||
| Post stress 45 minutes | 0.424 | <0.001 | |
| Post stress 2 hours | 0.565 | <0.001 | |
| CESD × Period | Baseline | ||
| Post stress 45 minutes | 0.017 | 0.016 | |
| Post stress 2 hours | 0.014 | 0.035 | |
| Change per unit depression | Baseline | 0.008 | 0.240 |
| Post stress 45 minutes | 0.024 | 0.001 | |
| Post stress 2 hours | 0.022 | 0.002 | |
| Slope change per unit depression | Baseline to 45 minutes | 0.017 | 0.016 |
| across time points | Baseline to 2 hours | 0.014 | 0.035 |
| 45 minutes to 2 hours | −0.002 | 0.757 | |
Overall, IL-6 increased in response to the Trier Social Stress Test. There was a significant interaction between depressive symptoms and time. Compared to those with fewer depressive symptoms, individuals with more depressive symptoms had a greater IL-6 response to the Trier Social Stress Test. Specifically, individuals with more depressive symptoms displayed greater increases in IL-6 from baseline to 45 minutes post-stressor and baseline to 2 hours post-stressor (Figure 1). Depressive symptoms were unrelated to baseline levels of IL-6. However, those who had more depressive symptoms had significantly higher IL-6 levels both 45 minutes post-stressor and 2 hours post-stressor than those with fewer depressive symptoms. We ran an additional analysis controlling for baseline levels of IL-6, and all significance levels remained the same. In sum, those who had more depressive symptoms had greater IL-6 increases in response to the stressor and higher levels of IL-6 after the stressor than those with fewer depressive symptoms.
Figure 1.
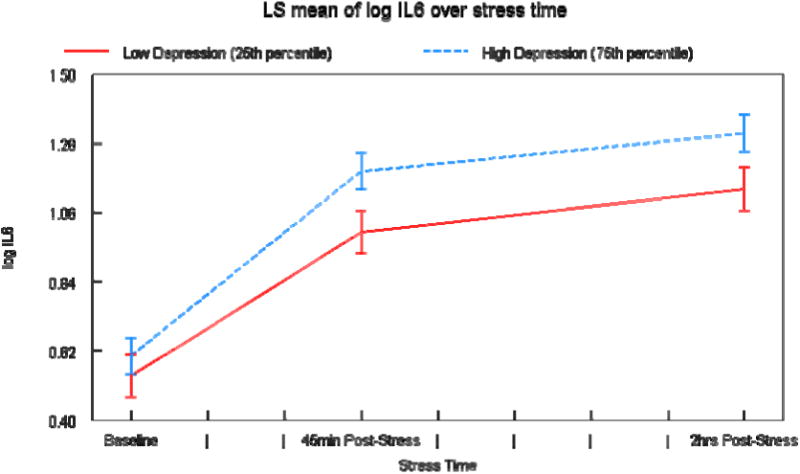
Mean (± SEM) IL-6 across experimental periods in people with more and fewer depressive symptoms based on the 75th and 25th percentile scores on the CES-D. The 25% represents a score of “2” on the CES-D and the 75% represents a score of “10.” These are both well below the clinical threshold for depression, which is “16.”
In ancillary analyses, we adjusted for a history of maltreatment, menopausal status, and anxiety levels. None of these variables were associated with changes in IL-6, and the significance levels of all results remained the same. When we dichotomized BMI at 30, it was not significantly associated with changes in IL-6. Furthermore, it did not interact with depressive symptoms.
4. Discussion
Individuals with more depressive symptoms had larger stress-induced increases in IL-6 to a standardized laboratory speech and mental arithmetic stressor. They also had significantly higher levels of IL-6 after the stressor than those with fewer depressive symptoms. With a considerably larger sample, this study extends work linking major depression to greater stress-induced increases in IL-6 by demonstrating that those who have more depressive symptoms show an exaggerated IL-6 response to a laboratory stressor.
Depression has been linked to premature mortality among those with cancer, heart disease, stroke, and diabetes (Bush et al., 2001; Frasure-Smith et al., 2009; Pan et al., 2011; Satin et al., 2009; Wouts et al., 2008). Elevated inflammation is a risk factor for these diseases (De Martinis, Franceschi, Monti, & Ginaldi, 2006; Maggio et al., 2006). Accordingly, these findings may provide one potential pathway to explain relationships between depressive symptoms and morbidity and premature morality.
Proinflammatory cytokines can act on the brain to induce sickness behaviors (Ahles et al., 2002; Bower, Ganz, Aziz, & Fahey, 2002; Luecken, Kraft, Appelhans, & Enders, 2009; Papanicolaou, Wilder, Manolagas, & Chrousos, 1998). Although there is ample evidence that depressive symptoms can elevate inflammation, there is also considerable evidence that inflammation contributes to depressive symptoms (Miller, Maletic, & Raison, 2009; Raison, Capuron, & Miller, 2006). The association between inflammation and depressive symptoms has been found in a variety of different populations (Alesci et al., 2005; Bouhuys, Flentge, Oldehinkel, & van den Berg, 2004; Miller, Stetler, Carney, Freedland, & Banks, 2002; Musselman et al., 2001). Minor elevations in depressive symptoms may be a catalyst for stress-induced inflammatory rises that subsequently promote more depressive symptoms through a feedback loop.
Mechanistically, both autonomic and neuroendocrine function may promote stress-induced inflammation. Norepinephrine enhances proinflammatory cytokines by inducing nuclear factor B (NF- B) transcription, an intracellular signaling molecule that regulates proinflammatory cytokine gene expression (Bierhaus et al., 2003; Straub & Härle, 2005). Furthermore, higher levels of parasympathetic activity can reduce inflammation via the cholinergic anti-inflammatory pathway that induces acetylcholine release (Tracey, 2009).
Our sample consisted of healthy adults. Accordingly, we do not know if these findings could be detected among those with chronic diseases. Furthermore, our sample was predominately college educated and white. Future studies that are more diverse are needed in order to ensure our findings generalize across different ethnic and socioeconomic groups. Finally, the type of stressor we used may have influenced the results. The stressor we imposed evokes negative self-evaluation (Kirschbaum et al., 1993), which may be particularly stressful for those with more depressive symptoms (Giesler, Josephs, & Swann Jr, 1996). In future work, it would be interesting to see if other types of acute stressors elicit similar inflammatory responses.
In sum, those with more depressive symptoms exhibited enhanced inflammation to a stressor compared with those with fewer depressive symptoms. Accordingly, depressive symptoms may enhance the stress response system in ways that promote excessive inflammation. These findings add to our emerging understanding of the complex interactions among stress, depression, and immune dysregulation.
Highlights.
Compared with individuals with fewer depressive symptoms, those with more depressive symptoms produced more IL-6 in response to a laboratory stressor, as well as significantly higher levels of IL-6 both 45 minutes and 2 hours after the stressor.
Acknowledgments
Work on this project was supported in part by NIH grants R01AG029562, CA131029, CA126857, DE014320, UL1RR025755, CA016058, the S. Robert Davis endowment, the Kathryn & Gilbert Mitchell endowment, and American Cancer Society Postdoctoral Fellowship Grant PF-11-007-01-CPPB.
Footnotes
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. The Journal Of Clinical Endocrinology And Metabolism. 2005;90(5):2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clinical Science. 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. The Journal of Steroid Biochemistry and Molecular Biology. 2010;120(2-3):76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. The Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington D. C.: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. San Antonio, Texas: The Psychological Corporation; 1998. [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhuys AL, Flentge F, Oldehinkel AJ, van den Berg MD. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Research. 2004;127(3):237–245. doi: 10.1016/j.psychres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosomatic Medicine. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Brattsand R, Linden M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Alimentary Pharmacology & Therapeutics. 1996;10:81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- Bush DE, Ziegelstein RC, Tayback M, Richter D, Stevens S, Zahalsky H, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. The American journal of cardiology. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Experimental and molecular pathology. 2006;80(3):219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Williams J. Structured clinical interview for DSM-IV research version. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- Frasure-Smith N, Lespérance F, Habra M, Talajic M, Khairy P, Dorian P, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120(2):134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- Giesler RB, Josephs RA, Swann WB., Jr Self-verification in clinical depression: The desire for negative evaluation. Journal of Abnormal Psychology. 1996;105(3):358. doi: 10.1037//0021-843x.105.3.358. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Archives of General Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Heinz A, Hermann D, Smolka MN, Rieks M, Graf KJ, Pohlau D, et al. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: implications for coronary heart disease. Psychopharmacology. 2003;165(2):111–117. doi: 10.1007/s00213-002-1244-6. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain, Behavior, and Immunity. 2002;16(4):461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Kraft A, Appelhans BM, Enders C. Emotional and cardiovascular sensitization to daily stress following childhood parental loss. Developmental Psychology. 2009;45(1):296–302. doi: 10.1037/a0013888. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. The Journals of Gerontology: Series WA: Biological Sciences and Medical Sciences. 1999;54A(9):M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. Journal of affective disorders. 2001;63(1-3):85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Cohen S, Ritchey A. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. The American Journal Of Cardiology. 2002;90(12):1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American Journal of Psychiatry. 2001;158(8):1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Willett WC, et al. Increased mortality risk in women with depression and diabetes mellitus. Archives of General Psychiatry. 2011;68(1):42. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends In Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner BW, German PS, Brant LJ, Clark R, Burton L, Folstein MF. Depression and Mortality. JAMA: The Journal of the American r Medical Association. 1991;265(8):993. doi: 10.1001/jama.265.8.993. [DOI] [PubMed] [Google Scholar]
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115(22):5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behavior and Immunity. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Straub R, Härle P. Sympathetic neurotransmitters in joint inflammation. Rheumatic Disease Clinics of North America. 2005;31(1):43–59. doi: 10.1016/j.rdc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry. 1999;56(7):609. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Wouts L, Voshaar RCO, Bremmer MA, Buitelaar JK, Penninx BWJH, Beekman ATF. Cardiac disease, depressive symptoms, and incident stroke in an elderly population. Archives of General Psychiatry. 2008;65(5):596. doi: 10.1001/archpsyc.65.5.596. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosomatic Medicine. 1999;61(1):6. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]


