Abstract
Individuals with body dysmorphic disorder (BDD) often experience anxiety, as well as perceptual distortions of appearance. Anxiety has previously been found to impact visual processing. This study therefore tested the relationship between anxiety and visual processing of faces in BDD. Medication-free participants with BDD (N=17) and healthy controls (N=16) viewed photographs of their face and a familiar face during functional magnetic resonance imaging. Blood-oxygen-level dependent signal changes in regions involved in anxiety (amygdala) and detailed visual processing (ventral visual stream – VVS) were regressed on anxiety scores. Significant linear relationships between activity in the amygdala and VVS were found in both healthy controls and individuals with BDD. There was a trend of a quadratic relationship between anxiety and activity in the right VVS and a linear relationship between anxiety and activity in the left VVS for the BDD sample, and this was stronger for own-face stimuli versus familiar-face. Results suggest that anxiety symptoms in BDD may be associated with activity in systems responsible for detailed visual processing. This may have clinical implications related to heightened perceptual distortions associated with anxiety.
Keywords: BDD, fMRI, amygdala, ventral visual stream, limbic
1. Introduction
Individuals with body dysmorphic disorder (BDD) are preoccupied with perceived appearance defects. They subsequently believe that they look disfigured and ugly, and suffer distress and functional impairment. BDD affects approximately 1–2% of the population (Otto et al., 2001; Rief et al., 2006; Koran et al., 2008), and is associated with high lifetime rates of psychiatric hospitalization (48%) and suicide attempts (22–27.5%) (Phillips, 2007).
Despite its prevalence and severity, little is known of the pathophysiology or neurobiology of BDD. Clinical observation suggests that patients focus primarily on details of their appearance at the expense of global or configural aspects, which may account for their perceptual distortions. Patients most often perceive “defects” of their face and head areas, such as skin, hair, and nose (Phillips, 2005), although perceived defects of other body parts are sometimes present. Neuropsychological data suggest that individuals with BDD demonstrate abnormal patterns of information processing consisting of selective recall of details rather than global features (Deckersbach et al., 2000).
A previous functional magnetic resonance imaging (fMRI) study reported abnormal neural correlates of own-face processing in BDD relative to healthy controls. Results demonstrated correlations in the BDD group between BDD symptoms and activity in visual processing and frontostriatal systems (Feusner et al., 2010). Participants in this study had varying degrees of anxiety, and in some cases comorbid generalized anxiety disorder (GAD), major depressive disorder (MDD) and/or dysthymia. The complex relationship between different symptom dimensions and brain pathophysiology is not entirely clear. Because lifetime prevalence of other Axis I comorbid disorders are high in BDD: 36–76% for major depressive disorder, 34–47% for social phobia, 21–39% for OCD, 16–26% for other anxiety disorders (including 18.8% for GAD (Zimmerman and Mattia, 1998)), and 10–32% for eating disorders (Gunstad and Phillips, 2003; Phillips et al., 2005; Ruffolo et al., 2006), it is important to understand the relationship between co-occurring symptom dimensions and brain pathophysiology. Thus, the present study analyzed data from this previous study, focusing on the impact of a frequently comorbid psychiatric symptom: anxiety.
We focus on the effects of anxiety in the present study because previous studies suggest that anxiety may influence visual processing. Degree of trait anxiety correlates with enhanced contrast detection (Laretzaki et al., 2008), and viewing fearful faces appears to enhance contrast sensitivity both independently of, and synergistically with, attention (Phelps et al., 2006). Several functional imaging studies have demonstrated that, in healthy controls (Bradley et al., 2003; Junghofer et al., 2005; Sabatinelli et al., 2005) and social phobia (Goldin et al., 2009) (Straube et al., 2005), viewing of pictures with emotional content is associated with enhanced activation in the amygdala, as well as occipital and inferior temporal regions.
Connections between the amygdala and the ventral visual stream (VVS) may carry top-down signals regarding emotional valence of stimuli to the visual cortex, resulting in enhanced visual processing of emotionally salient stimuli. Evidence of this comes from neuroimaging studies in which amygdala activation was found to correlate with activation in the visual cortex (Morris et al., 1998; Pessoa et al., 2002). In patients with amygdala damage this correlation is attenuated (Vuilleumier et al., 2004).
Given these previous findings of anxiety effects on visual processing, and given the prevalence of the symptom of anxiety and evidence for abnormal visual processing in BDD, the primary objective of the current study was to use fMRI to determine the relationship between anxiety and neural systems associated with visual processing in individuals with BDD. We selected the VVS as our region of interest because of the importance of this region visual processing, as well as the aforementioned correlations between activity in the amygdala and visual cortex in healthy controls. Although the fusiform face area has been implicated as an important region for face processing in prior studies (Kanwisher et al., 1997), we focused on the VVS as a whole because the relationship between anxiety/limbic system activity and activity in the visual system appears to encompass a broader visual processing network (Morris et al., 1998; Bradley et al., 2003; Vuilleumier et al., 2004; Junghofer et al., 2005; Sabatinelli et al., 2005).
We hypothesized that anxiety scores would correlate positively with activity in the VVS in individuals with BDD, as a result of a greater propensity for emotional arousal and therefore subsequent enhanced analytic and detailed visual processing, and that these relationships would be similar across BDD participants regardless of comorbid diagnoses of anxiety or depressive disorders. Although individuals with comorbid diagnoses will tend have higher levels of anxiety (hence meeting threshold criteria for diagnosis), the effect of anxiety depending on comorbidity is more likely to be a quantitative rather than qualitative one. We also predicted that these relationships would be stronger for own-face relative to familiar-face stimuli because of greater emotional salience in BDD. Finally, we hypothesized that correlations between amygdala and VVS activity would be stronger for own-face relative to familiar-face viewing as a result of greater emotional salience. We predicted these correlations would be similar between BDD and healthy control groups, although the resultant effect in the BDD group would likely be heightened because they experience greater emotional arousal for their own face.
2. Methods
2.1. Participants
The UCLA Institutional Review Board approved the study protocol. We obtained informed consent from 17 right-handed participants with BDD and 16 healthy control participants of equivalent age and gender, all recruited from the community. All participated in a previously-reported study comparing BDD participants to healthy controls (Feusner et al., 2010) and some participated in a study of object processing (Feusner et al., 2011). All BDD participants met DSM-IV BDD criteria, as determined by the last author (JDF), who has clinical expertise with this population. Diagnoses were made using the Body Dysmorphic Disorder Module (Phillips et al., 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM. In addition, we screened participants with the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al., 1998). All BDD participants were required to have a score of ≥20 on the BDD version of the Yale-Brown Obsessive-Compulsive Scale (BDD-YBOCS) (Phillips et al., 1997). We allowed participants with delusional beliefs about their appearance, as delusional beliefs are common in BDD. Moreover, delusional variants appear to exist on a continuum with nondelusional variants, as they are similar in most demographics, clinical features, and course of illness (Phillips et al., 2006; Mancuso et al., 2010). In line with this research, we believe that visual processing of faces would be similar across this continuum.
Exclusion criteria for both BDD participants and healthy controls included: active substance abuse, neurological disorder, pregnancy, or current medical disorders that might affect cerebral metabolism. We excluded BDD participants with concurrent Axis I disorders besides dysthymia, MDD, or GAD. As depression and anxiety are frequently comorbid in this population, we believed it would not be a representative sample to exclude these. Although other disorders are also common in BDD, such as OCD, eating disorders, and social phobia, we only allowed comorbid disorders that did not have overlapping symptom presentations (e.g. obsessionality, anxiety in social situations, or detail-focused processing). This allows us to ensure that group differences between BDD and healthy controls are more likely due to BDD rather than related comorbidity. However, we required that BDD be the primary diagnosis as defined by the MINI (“Which problem troubles you the most or dominates the others or came first in the natural history?”). Although this approach limits the generalizability of the findings across all comorbidities, disallowed comorbidities resulted in the exclusion of 13 (37%) of the 35 total participants evaluated. We excluded participants whom the investigator judged were suicidal. We excluded healthy control participants with any Axis I disorder. We administered the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), the Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1969) to all participants, and the BDD-YBOCS to the BDD group.
All participants were free from psychoactive medications for at least eight weeks prior to entering the study, and none were receiving cognitive-behavioral therapy. Participants had normal or corrected vision, as verified by Snellen eye chart.
2.2. Procedures
2.2.1 Stimuli
Stimuli consisted of digital photographs of participants’ frontal view, neutral expression faces. We used Adobe Photoshop® CS3 software to create standard black backgrounds for the face and neck and to convert to grayscale. A neutral expression, grayscale photograph of a famous male actor provided one of the control conditions, matched for size and luminosity. We chose the actor based on 100% familiarity and a medium degree of attractiveness (4.25±1.75 on a scale of 0 to 10), as tested prior to the experiment in 10 healthy volunteers. All participants in the study recognized the actor. A low-level baseline control consisted of grey ovals approximately the same size as the faces and of the same luminosity. Participants wore fMRI-compatible goggles to view the stimuli. If participants wore eyeglasses, appropriate corrective lenses for the goggles were inserted. We used MacStim 3.0 (White Ant Occasional Publishing, West Melbourne, Australia) to present stimuli and record responses.
2.2.2. Task
The task consisted of viewing own-face, familiar-face, and oval images while in the MRI scanner. Participants were equipped with a button-box in their right hand and were instructed to push the button whenever the face or oval image disappeared from the screen. This ensured that they attended to the image for its full duration.
Own-face and familiar-face images appeared for 3 seconds, with a 1 second interstimulus interval following the face stimuli, followed by oval images. Twelve of each of the own-face, familiar-face, and oval images were presented in an event-related design. The order of the own-face and familiar-face stimuli was randomized and jittered with respect to the oval control, to minimize anticipation; the oval randomly occurred for either 3, 6, or 9 seconds, while the faces all appeared for 3 seconds. The oval stimuli were jittered to allow varying degrees of deconvolution to occur in visual and emotional processing systems between presentations of face stimuli, as these oval stimuli served as a baseline given that they contained only low-level visual and no emotional features. We used Optseq (http://surfer.nmr.mgh.harvard.edu/optseq/), a genetic algorithm, to create jittered presentation timing with the highest efficiency. There were three different sets of stimuli order, which we counterbalanced between participants. Total time for each run was seven minutes. There were two runs per experiment per participant, the second presented in a different order.
2.2.3. Functional MRI
We used a 3-Tesla Allegra (Siemens) MRI scanner to evaluate BOLD contrast, using T2*-weighted echo planar imaging gradient-echo pulse sequence (TR = 2.0 seconds, TE = 35 milliseconds, Flip-Angle = 90°, Matrix = 64 × 64, field-of-view = 24 × 24 cm, in-plane voxel size 3.125 mm × 3.125 mm, slice thickness 3mm, 1mm intervening spaces, and 28 total slices). We also obtained matched-bandwidth high-resolution T1-weighted images for each participant to provide detailed brain anatomy.
We conducted image pre-processing using FMRIB Software Library (FSL), www.fmrib.ox.ac.uk/fsl. This included motion correction, skull stripping, spatial smoothing of 5mm full-width/half-maximum Gaussian kernel, mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering. We did not utilize slice-timing correction in order to avoid interpolation of data. We coregistered functional images of each participant to corresponding structural images in native space, and registered structural images to structural standard images, defined by the Montreal Neurological Institute averaged 152 standard brain.
2.3. Statistical analysis
To analyze functional neuroimaging datawe used FEAT software (FMRI Expert Analysis Tool) Version 5.4, part of FSL. At the individual subject level, we modeled the hemodynamic response function using a convolution of the experimental paradigms of each stimulus type vs. control task with the canonical hemodynamic response function and its temporal derivative (Aguirre et al., 1998). To analyze the relationship between percentage signal change in regions of interest and HARS scores, we used the general linear model in SPSS®.
2.3.1. Region-of-interest (ROI) analyses
To test our hypotheses about relationships between anxiety and brain activation in VVS and relationships between amygdala and VVS, we performed anatomical ROI analyses. Masks for these regions were obtained from the probabilistic Harvard-Oxford Cortical and Subcortical Structural Atlases supplied with FSL. The amygdala masks consisted of 50% probabilistic masks for the amygdala. The VVS masks consisted of combined 5% probabilistic masks from bilateral occipital fusiform gyrus plus bilateral temporal occipital fusiform cortex plus bilateral posterior temporal fusiform cortex (Figure 1). Parameter estimates were extracted from each ROI for each participant using FSL command line tools (Poldrack, 2007). We created scatter plots of mean BOLD percentage signal change as a function of HARS scores. To test linear and quadratic effects of HARS scores and amygdala activity, as well as to test the impact of comorbid GAD, MDD or GAD+MDD/dysthymia (entered as comorbidity or no comorbidity) and BDD Y-BOCS scores on effects, we conducted stepwise regression analyses predicting VVS activity on the left and right side. To test the impact of stimulus type (own face vs. oval compared to familiar face vs. oval), we conducted mixed model analyses of the stepwise regressions looking at HARS scores and amygdala activity predicting VVS activity. These analyses utilized log likelihoods for each model (stepwise regressions, with and without stimulus type) to generate a chi square difference to determine best fit. For this set of analyses, we corrected for multiple comparisons of the 4 main regressions: anxiety scores/amygdala activity predicting VVS activity on each hemisphere and for each stimulus type. Thus, we used Bonferroni corrected α=0.0125. The healthy controls had very low anxiety scores with limited range so we did not include them in the anxiety analyses but did include them in the analyses of BOLD percentage signal change in the amygdala vs. VVS.
Figure 1.
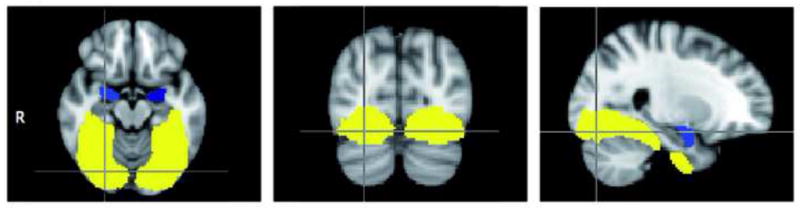
Regions of interest for study analyses. Ventral visual stream = bilateral occipital fusiform gyrus + bilateral temporal occipital fusiform cortex + bilateral posterior temporal fusiform cortex (yellow); Amygdala (blue).
3. Results
3.1. Demographics and psychometrics
Table 1 summarizes the demographic and psychometric data. One BDD participant had comorbid MDD, two had GAD, four had both MDD and GAD, and one had both dysthymic disorder and GAD. All 17 BDD participants had preoccupations with perceived facial defects. HARS and HDRS scores were highly correlated (r=0.94, P<0.0001). BDD-YBOCS and HARS scores were also correlated (r=0.36, P=0.037).
Table 1.
Demographics and psychometric scoresa
| Total BDD group (N=17) | Comorbid BDD group (N=8) | Non-comorbid BDD group (N=9) | P valueb | Control group (N=16) | P Valuec | |
|---|---|---|---|---|---|---|
| Age | 29.18±7.4 | 28.67±5.55 | 29.1±8.97 | .90 | 27.38±5.3 | .426 |
| Gender (F/M) | 9/8 | 5/3 | 4/5 | .64 | 8/8 | 1 |
| Handedness | 17R | 8R | 9R | >.99 | 16R | 1 |
| Years of education | 15.35±2.7 | 14.67±3.12 | 16.3±2.06 | .21 | 16.94±2.3 | .08 |
| BDD-YBOCS score | 28.82±5.1 | 31.12±4.49 | 26.78±4.87 | .075 | N/A | N/A |
| HDRS-17 score | 10.77±7.32 | 15.12±6.33 | 7.11±6.66 | .023 | 1.44±1.5 | <0.001 |
| HARS score | 12.83±7.76 | 17.75±5.90 | 8.67±7.31 | .013 | 1.56±1.4 | <0.001 |
Abbreviations: BDD: body dysmorphic disorder; BDD-YBOCS: BDD version of the Yale-Brown Obsessive-Compulsive Scale; HDRS-17: 17-item Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale
Data are given as mean±SD unless otherwise indicated.
two-sample, two-tailed t-test for all comparisons between comorbid and non-comorbid groups, except gender and handedness (Fisher’s Exact Test)
two-sample, two-tailed t-test for all comparisons between total BDD group and Control group, except gender and handedness (Fisher’s Exact Test)
3.2. Behavioral data
The mean response rate for the button-push was high, at 98.5% (SD=0.01) for BDD participants and 97.1% (SD=0.03) for controls (t=1.48, df=31, P=0.15).
3.3. ROI results
3.3.1 HARS and VVS in BDD sample
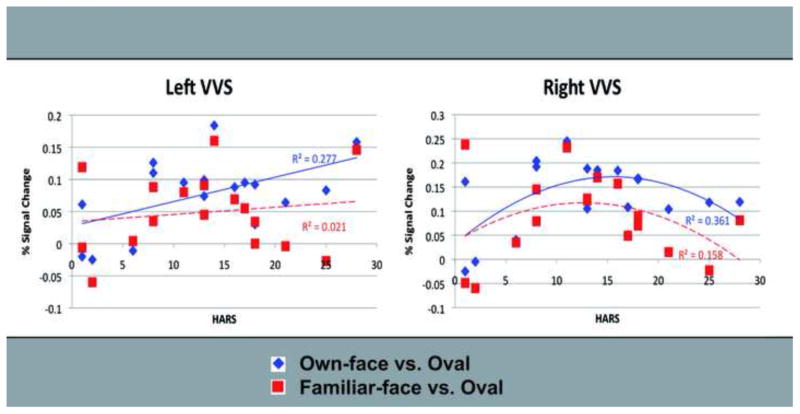
For own-face stimuli vs. oval, regression analyses revealed significant relationships between anxiety and brain activity. Specifically the model with the quadratic term produced a better fit than the linear-only model for the relationships between HARS scores and activity in the right VVS (β=−1.728, R2=0.361, P=0.020 for the quadratic term, ΔR2=0.281, P=0.026 for the change in model with the addition of the quadratic term). The relationship between HARS scores and activity in the left VVS was better fit by a linear equation (β=1.257, R2=0.277, P=0.030 for the linear term, ΔR2=0.056, n.s., for the change in model with the addition of the quadratic term) (Figure 2). Using Bonferroni corrected alpha of 0.0125, these relationships were not significant, although the trend suggests that anxiety may have a nonlinear relationship to brain activation in right VVS and a linear relationship to activity in the left VVS. Figure 3 shows the (unthresholded) Z-scores for voxels within the VVS ROI that were positively associated with HARS score during own-face and familiar-face processing.
Figure 2.
Linear and nonlinear regressions between anxiety and activity in VVS for the BDD group. VVS = ventral visual stream. BDD = body dysmorphic disorder. HARS = Hamilton Anxiety Rating Scale.
Figure 3.
Z-score map (unthresholded) within the VVS region of interest demonstrating the relationship between anxiety (Hamilton Anxiety Rating Scale scores) and activity during: a) own face vs. oval processing, and b) familiar face vs. oval processing. Voxels shown reflect the areas with a positive correlation between anxiety and activation for each contrast.
For familiar-face vs. oval, there were no significant linear (β=.146, R2=.021, P=.576) or quadratic (β=−.325, R2=.031, P=.710) effects in the left VVS, nor were there significant linear (β=−.058, R2=.003, P=.824) or quadratic (β=−1.281, R2=.158, P=.132) effects in the right VVS (Figures 2 and 3).
Comparison of mixed models for HARS scores predicting VVS activity including stimulus type (own-face compared to familiar-face) showed a significant effect of stimulus type on the overall model for the left VVS (χ2(2, N=17) = 13.834, P<.001)and right VVS (χ2(2, N=17) = 15.346, P<.001). As outlined above, the relationship between anxiety and VVS activity was stronger in the own-face contrast than the familiar-face contrast.
To investigate the effects of anxiety independent of BDD symptoms, we added BDD-YBOCS scores to the model to evaluate the contribution of symptom severity to the regression model. For all regressions, BDD symptoms did not significantly add to the model. Further, adding comorbidity to the model did not significantly increase prediction, thus it appears that comorbidity status does not impact the relation between anxiety and activity in the VVS.
3.3.2 Associations between amygdala and VVS activity in full sample (BDD + control)
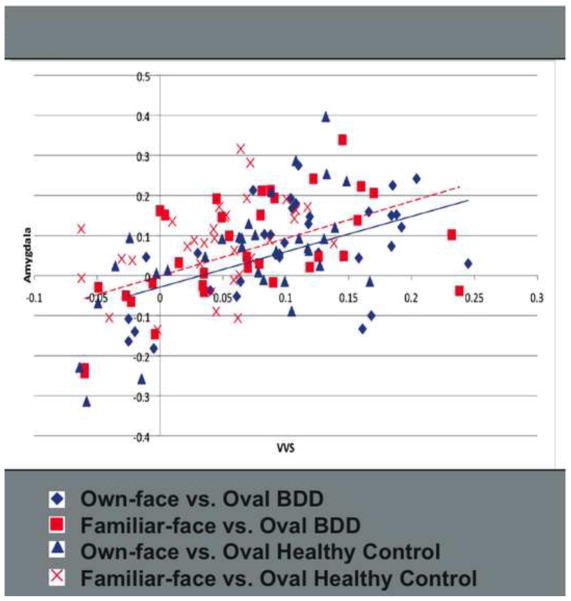
Regression analyses on the full sample revealed linear relationships between amygdala and VVS activity for own-face stimuli on the left (β=.668, P<.001, R2=.446), and right (β=.433, P=.012, R2=.205), and for familiar-face stimuli on the left (β=.534, P=.001, R2=.286) and right (β=.415, P=.016, R2=.172) (Figure 4). The addition of a quadratic term did not significantly improve the model fit. Effects were significant when adjusting alpha to .0125 for multiple comparisons, except for the relationship between right amygdala and right VVS for familiar-face stimuli. This effect, however, showed a strong trend in the same direction as the other effects.
Figure 4.
Linear regressions between activity in the amygdala and VVS for BDD and healthy control participants. VVS = ventral visual stream. BDD = body dysmorphic disorder.
We ran additional regressions including adding diagnostic group (BDD or healthy control) to the model. Diagnostic group did not significantly impact the relationship between amygdala and VVS activity. Furthermore, the diagnostic groups did not differ significantly in mean percentage signal change for the amygdala or VVS except for the signal change in response to own-face stimuli in the right VVS, which was significantly greater in the BDD (M=.133, SD=.074) group than healthy controls (M=.080, SD=.064, t(31)=2.151, P=.039).
We compared mixed models for regressions, testing the relationship between amygdala and VVS activity including stimulus type (own-face compared to familiar-face). There was a significant effect of stimulus type on the overall model for the relationship between activity in amygdala and VVS on both the left (χ2(2, N=33) = 25.798, P<.001)and right (χ2(2, N=33) = 16.880, P<.001).
3.3.3 Mediation of Anxiety by Amygdala in BDD sample
To examine whether the relationship between anxiety and VVS activity is mediated by amygdala activity, we followed the Baron and Kenny (1986) procedures to test mediation effects (Baron and Kenny, 1986). We already established in the previous regression that HARS scores predict VVS activity in the BDD sample, so we also tested whether HARS scores predicted amygdala activity and whether the addition of the amygdala activity to the regression model with HARS scores predicting VVS activity reduced the HARS coefficient value. Results suggest there was significant mediation of anxiety by amygdala activity on the right. HARS scores predicted amygdala activity (β=1.694, R2=.359, P=.001), and when right amygdala activity was added to the regression with HARS scores predicting right VVS activity, only the amygdala remained a significant predictor of VVS activity (β=.443, R2=.126, P=.025) and the coefficient for the HARS score was reduced (β=.696, P=.242). On the left side, however, requirements for mediation were not met, as HARS scores did not significantly predict left amygdala activity (β=.191, R2=.036, P=.280).
3.3.4 Post-hoc Gender Correlations in BDD sample
In order to investigate the potential effect of gender, we calculated correlations between anxiety and VVS activity, as well as between amygdala and VVS activity, for each gender. Due to small cell sizes for each gender, we were unable to directly compare the correlations, however. Correlations between anxiety and VVS activity in the own-face condition were r=0.42 for females and r=0.43 for males, and in the familiar-face condition were r=0.11 for females and r=0.003 for males. Correlations between amygdala and VVS activity in the own-face condition were r=0.55 for females and r=0.44 for males, and in the familiar-face condition were r=0.70 for females and r=0.37 for males.
4. Discussion
This study demonstrates that symptoms of anxiety in individuals with BDD may be associated with activity in visual processing systems when viewing faces. These associations were largely nonlinear on the right side; intermediate (more than high or low) levels of anxiety symptoms were associated with the greatest activity. Moreover, these relationships were not impacted by BDD symptom severity, suggesting a distinct relationship between anxiety and activity in visual and emotional processing systems. These effects may be present in individuals without BDD, however, we were unable to test these relationships in our healthy control group due to restricted range of anxiety scores. Indeed, other studies have found relationships between anxiety and visual processing in healthy individuals in the past (e.g., Bradley et al., 2003; Junghofer et al., 2005; Sabatinelli et al., 2005). This study is focused on individuals with BDD because of the unique clinical implications this relationship has for this population.
4.1. Anxiety and regional brain activation
As hypothesized, anxiety scores were associated with activity in the VVS during own-face viewing, although their significance was at the trend level after correction for multiple comparisons. These associations were better explained by quadratic models on the right side. There was a stronger relationship between anxiety and VVS activity during own-face viewing than familiar-face viewing in BDD participants.
There were also significant associations, as hypothesized, between activity in the amygdala and VVS across diagnostic groups. Further, the relationship between amygdala activity and VVS activity was stronger for own-face stimuli than familiar-face stimuli. Adding diagnostic group to the model did not significantly improve the fit, nor did a quadratic term of amygdala activity.
Further analyses in the BDD sample revealed significant mediation of the relationship between anxiety and VVS activity by the amygdala on the right. This suggests that the amygdala plays a significant role by which anxiety impacts VVS activity (and/or vice versa). It was surprising that this was only found on the right, although there was a trend toward mediation on the left (anxiety did not significantly predict left amygdala activity, but the relationship was in the same direction). A previous study of brain morphology in individuals with BDD showed a significant correlation between right amygdala volume and BDD symptom severity, which could relate to the finding in this study of mediation being more robust on the right (Feusner et al., 2009).
In sum, there appears to be a relationship between anxiety and activity in the ventral visual system for own-face stimuli in the BDD group, as well as a relationship between activity in the amygdala and ventral visual system for own-face and familiar-face viewing in both the BDD group and healthy controls. This combination of findings suggests the presence of stimulus-specific relationships between brain activity in the VVS and anxiety in BDD, but that the intrinsic relationships between limbic and visual processing systems may be normal. It is also noteworthy that within the VVS, the strongest relationships between anxiety and brain activity appear to be within the fusiform face area bilaterally for both own and familiar faces (see Figure 3). This supports that idea that face processing may be related to anxiety in individuals with BDD.
This study revealed a trend in line with our hypotheses that anxiety in general was associated with activity in the VVS. These regions may represent nodes of an interacting system that is critical for the processing of visual emotional information. Studies in healthy controls have demonstrated structural and functional connections between the limbic system, in particular the amygdala, and occipital and temporal visual processing systems (Morris et al., 1998; Rudrauf et al., 2008). Emotional visual information appears to be conveyed from brainstem retinotectal regions (superior colliculus) to the thalamus, and then both to primary visual cortical regions and, via a parallel “short-cut,” directly to anterior emotion processing centers of the amygdala, temporal pole, and the orbitofrontal cortex (Liddell et al., 2005; Rudrauf et al., 2008). Information is also conveyed from V1 (intracalcarine cortex) and V2 (lingual gyrus and occipital pole) in the visual cortex to these temporal and prefrontal systems, which in turn provide top-down modulation of the VVS.
Considering such a model in interpreting this study’s results, one possibility is that anxiety may be associated with activation of the “short-cut” route from retinotectal regions to limbic systems, resulting in top-down enhancement of the VVS. Previous studies in social phobia have demonstrated that viewing of pictures with emotional content is associated with enhanced activation in occipital and inferior temporal regions (Straube et al., 2005; Goldin et al., 2009). If this is occurring in BDD it may contribute to an imbalance in ventral vs. dorsal visual streams, leading to heightened detail processing relative to holistic and configural processing (Iidaka et al., 2004). Such an imbalance is in line with prior evidence of enhanced detail processing in BDD from neuropsychological (Deckersbach et al., 2000) and neuroimaging (Feusner et al., 2007) studies. Subsequently, this may contribute to symptoms of perceptual distortions for their appearance if they are primarily honing in on small imperfections, with lesser contextualization of their whole face.
However, because we cannot determine the direction of this effect from this study due to limited temporal resolution, it could be that enhanced detail processing in the VVS leads to greater anxiety, or that the anxiety leads to enhanced detail processing of these stimuli. Confirmation about whether this interplay between limbic and VVS activity is occurring in BDD, and if so if it is initiated by a top-down or bottom-up process is unclear from this study due to the limited temporal resolution, and will need to be directly tested in future studies.
The observation that there was not a significant effect of own-face relative to familiar-face viewing on the relationship between amygdala and VVS activity was somewhat surprising given that own-face viewing likely has greater emotional salience relative to familiar face viewing. The absence of an effect of stimulus type on the relationship between amygdala and VVS activity may reflect a more general association between anxiety and limbic systems as opposed to anxiety related to symptom-provocation. Instead, it may be the case that individuals with BDD have greater processing in the VVS for symptom-provoking stimuli such as their own face.
In the previous between-groups analysis of own-face and familiar-face processing (Feusner et al., 2010), which was performed on the same dataset as the current study, there was greater activity in the BDD group in frontostriatal systems for own-face relative to familiar-face stimuli. These results were essentially unchanged when controlling for HARS or HDRS scores, suggesting that those findings could not be ascribed to differences in anxiety. In that study the voxel-wise analysis did not reveal significant between-groups differences in activity in the VVS. However, the VVS was not used as an ROI, and therefore the analysis may have been underpowered for detecting smaller differences. In the current study, the greater VVS activity found in the BDD group additionally supports the model of an imbalance in detailed vs. holistic/configural visual processing (Feusner et al., 2010).
From clinical observation, individuals with BDD often report that they perceive themselves as more disfigured and ugly when they are anxious. As a clinical implication of this study, it may be important for individuals with BDD (and their treatment providers) to understand that anxiety itself may contribute further to perceptual distortions. Anxiety may therefore be an important target for therapeutic interventions in order to help reduce perceptual distortions.
4.2 Nonlinear relationships
A surprising finding is the quadratic relationship between anxiety and activity in right VVS for own-face processing. It may be that as anxiety symptoms exceed a certain level of severity, the brain may not mount as high a response to stressful stimuli, perhaps as a protective mechanism. For example, individuals with moderately high anxiety may show high amygdala and VVS response to viewing their own faces, which is emotionally distressing to individuals with BDD, while those with very high anxiety may engage modulatory mechanisms that down-regulate limbic systems. Due to the evidence from other studies of strong top-down modulation from limbic to ventral visual systems (Morris et al., 1998; Pessoa et al., 2002; Vuilleumier et al., 2004), the observed lower brain activity in the VVS with higher anxiety may be a downstream effect of modulatory mechanisms acting on the limbic system.
Other studies have not reported a quadratic relationship between brain activity and anxiety (Sabatinelli et al., 2005; Straube et al., 2005; Goldin et al., 2009). However, with some exceptions (Rao and Yeragani, 2001; Bob et al., 2006), many studies simply use correlation analyses or linear regression without exploring nonlinear terms in their models. As has been suggested elsewhere (Rabinovich et al., 2010), it will be important for future studies to investigate more complex models of the relationships between brain and behavior, rather than only testing linear trends and correlations.
4.3. Comorbidity
Relationships between anxiety and brain activation patterns were not impacted by comorbidity status. This suggests that anxiety may be a dimension in BDD, in that it demonstrates the same relationship with brain activation patterns in visual and emotional processing regions, regardless of DSM-IV criteria. The comorbid group, not surprisingly, tends to fall on the higher end of the entire sample’s regression line and so likely represents those with higher degrees of anxiety.
4.4. Limitations
Strong correlation between the HARS and HDRS limited our ability to separately investigate relationships between anxiety and depression on brain activation patterns. This correlation is not unique to BDD, as other studies have shown high correlations between these measures in samples of depressed and anxious patients, as well as healthy controls (e.g., Koeter & Brink, 1992; McDowell, 2010; Vaccarino et al., 2008). In addition, the fact that the healthy control group had very low HARS scores prevented meaningful analyses of brain activity related to anxiety in that cohort. The HARS allowed an understanding of brain activity associated with longer-term anxiety symptoms but it did not measure current state anxiety. We were not able to collect immediate anxiety ratings due to the design of the study being event-related and therefore involving a pseudo-randomized sequence of own-faces, familiar-faces and ovals (to minimize anticipation), and because of the fact that self-emotional labeling can itself influence brain activation patterns (Lane et al., 1997). Our use of the entire VVS as a region of interest, due to previously described relationships between limbic/emotional activation and the VVS, may limit our ability to make inferences specific to face processing.
The limited temporal resolution of fMRI and the correlational nature of the analyses limit our ability to determine cause and effect regarding the relationship between anxiety and activity in visual and emotional processing systems. Functional connectivity methods such as psychophysiological interaction analyses may improve our ability to understand the relationship between the amygdala and the ventral visual stream, but we were unable to utilize this method because of our event-related design and small sample, which both limit power. Although our use of correlations to characterize the relationship between activity in these regions can provide preliminary evidence for some functional connection between these regions, more advanced functional connectivity methods should be used in future research.
We were limited in our ability to examine effects of type of diagnostic comorbidity because of our small sample. Thus, there may be differential effects specific to type of comorbidity allowed in the study (e.g., only GAD versus GAD and MDD) that we were unable to detect. Because we excluded a number of common comorbid diagnoses, including OCD and social phobia, our sample of individuals with BDD may not be representative of all patients with BDD. Ideally, a larger number of participants would have allowed us to obtain a more inclusive sample, from which we would have had power to explore differences among types of comorbidities. However, in total, 37% of the evaluated participants were excluded because of disallowed comorbidities. Thus, our findings remain relevant to a majority of patients diagnosed with BDD, although future research would benefit from larger samples with the ability to examine multiple comorbid diagnoses. Furthermore, our small sample limited our power to detect effects given corrections for multiple comparisons. Larger samples may help us understand if these trends are in fact significant effects.
Finally, the familiar face used in the study was that of a male actor. The difference between own-face and familiar-face for female participants also involved a change in gender. Thus, effects could be confounded. Our sample was 47% male, however, so some of these effects could be minimized since these participants’ responses were analyzed across the group, and there were no differences between genders on anxiety scores. Although we could not statistically compare correlations between genders, it does not appear that the gender of the familiar face had a large impact on results.
4.5. Conclusions
This study demonstrates a relationship between anxiety and activity in visual processing systems in individuals with BDD when viewing faces. Moreover, associations between anxiety and visual processing are distinct from those associated with core BDD symptoms. Own-face as opposed to familiar-face viewing appears to have a more specific relationship with activity in visual systems. It is possible that anxiety may therefore be linked to enhanced detail processing and contribute to symptoms of perceptual distortions for their appearance, although future studies are needed to understand the mechanistic relationship between activity in these networks and specific visual processing abnormalities to confirm this.
Acknowledgments
We would like to thank Malin McKinley and Jennifer Townsend for their assistance with conducting this study, and Dr. Susan Bookheimer for her comments on the manuscript. This work was supported by a grant from the National Institute of Mental Health (K23 MH079212-02, Dr. Feusner), a grant from the Obsessive Compulsive Foundation, Inc. (Dr. Feusner), and a UCLA Faculty Research Grant (Dr. Feusner).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bob P, Kukleta M, Riecansky I, Susta M, Kukumberg P, Jagla F. Chaotic EEG patterns during recall of stressful memory related to panic attack. Physiological Research. 2006;55:S113–S119. doi: 10.33549/physiolres.930000.55.S1.113. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–681. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychological Medicine. 2011:1–13. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Neziroglu F, Wilhelm S, Mancusi L, Bohon C. What Causes BDD: Research Findings and a Proposed Model. Psychiatric Annals. 2010;40:349–355. doi: 10.3928/00485713-20100701-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64:1417–1425. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, McKinley M, Moller H, Bookheimer S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2009;172:161–167. doi: 10.1016/j.pscychresns.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry. 2003;44:270–276. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Iidaka T, Yamashita K, Kashikura K, Yonekura Y. Spatial frequency of visual image modulates neural responses in the temporo-occipital lobe. An investigation with event-related fMRI. Brain Research Cognitive Brain Research. 2004;18:196–204. doi: 10.1016/j.cogbrainres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Schupp HT, Stark R, Vaitl D. Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. Neuroimage. 2005;25:520–526. doi: 10.1016/j.neuroimage.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectrums. 2008;13:316–322. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Laretzaki G, Plainis S, Argyropoulos S, Pallikaris I, Bitsios P. Threat and anxiety affect visual contrast perception. Journal of Psychopharmacology. 2008 doi: 10.1177/0269881108098823. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Comprehensive Psychiatry. 2010;51:177–182. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121 (Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. American Journal of Psychiatry. 2001;158:2061–2063. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Science U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K, Atala K, Pope H. Diagnostic instruments for body dysmorphic disorder. New Research Program and Abstracts; American Psychiatric Association 148th Annual Meeting, Miami American Psychiatric Association..1995. [Google Scholar]
- Phillips KA. The Broken Mirror. Oxford University Press; New York: 2005. [Google Scholar]
- Phillips KA. Suicidality in body dysmorphic disorder. Primary Psychiatry. 2007;14:58–66. [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005;46:317–325. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. Journal of Psychiatric Research. 2006;40:95–104. doi: 10.1016/j.jpsychires.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. Tools of the trade, region of interest analysis for fMRI. SCAN. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich MI, Muezzinoglu MK, Strigo I, Bystritsky A. Dynamical Principles of Emotion-Cognition Interaction: Mathematical Images of Mental Disorders. PLoS One. 2010;5:e12547. doi: 10.1371/journal.pone.0012547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RKA, Yeragani VK. Decreased chaos and increased nonlinearity of heart rate time series in patients with panic disorder. Autonomic Neuroscience-Basic & Clinical. 2001;88:99–108. doi: 10.1016/S1566-0702(01)00219-3. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36:877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, David O, Lachaux JP, Kovach CK, Martinerie J, Renault B, Damasio A. Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. Journal of Neuroscience. 2008;28:2793–2803. doi: 10.1523/JNEUROSCI.3476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo J, Phillips K, Menard W, Fay C, Weisberg R. Comorbidity of body dysmorphic disorder and eating disorders: severity of psychopathology and body image disturbance. International Journal of Eating Disorders. 2006;39:11–19. doi: 10.1002/eat.20219. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. Body dysmorphic disorder in psychiatric outpatients: recognition, prevalence, comorbidity, demographic, and clinical correlates. Compr Psychiatry. 1998;39:265–270. doi: 10.1016/s0010-440x(98)90034-7. [DOI] [PubMed] [Google Scholar]