Abstract
Background
Toll-like receptors (TLRs) have been implicated in myocardial ischemia/reperfusion (I/R) injury. The TLR9 ligand, CpG-ODN has been reported to improve cell survival. We examined effect of CpG-ODN on myocardial I/R injury.
Methods
Male C57BL/6 mice were treated with either CpG-ODN, control-ODN, or inhibitory CpG-ODN (iCpG-ODN) one hr prior to myocardial ischemia (60 min) followed by reperfusion. Untreated mice served as I/R control (n=10/each group). Infarct size was determined by TTC straining. Cardiac function was examined by echocardiography before and after myocardial I/R up to 14 days.
Results
CpG-ODN administration significantly decreased infarct size by 31.4% and improved cardiac function after myocardial I/R up to 14 days. Neither control-ODN nor iCpG-ODN altered I/R-induced myocardial infarction and cardiac dysfunction. CpG-ODN attenuated I/R-induced myocardial apoptosis and prevented I/R-induced decrease in Bcl2 and increase in Bax levels in the myocardium. CpG-ODN increased Akt and GSK-3β phosphorylation in the myocardium. In vitro data suggested that CpG-ODN treatment induced TLR9 tyrosine phosphorylation and promoted an association between TLR9 and the p85 subunit of PI3K. Importantly, PI3K/Akt inhibition and Akt kinase deficiency abolished CpG-ODN-induced cardioprotection.
Conclusion
The CpG-ODN, the TLR9 ligand, induces protection against myocardial I/R injury. The mechanisms involve activation of the PI3K/At signaling pathway.
Keywords: Myocardial I/R, TLR9, PI3K/Akt signaling, Apoptosis
Introduction
The innate immune and inflammatory responses are involved in myocardial ischemia/reperfusion (I/R) injury [5, 12, 19, 26]. Toll-like receptors (TLRs) play a critical role in the induction of innate immune and inflammatory responses [1]. Recent studies have demonstrated that TLRs could be the target as a new treatment approach for myocardial I/R injury. We and other investigators have previously reported that TLR4 deficiency or modulation of TLR4 mediated signaling protected the myocardium from ischemic injury [4, 5, 12, 19, 26, 30]. TLR2 is also implicated in myocardial I/R injury [2, 9, 24, 28]. In addition, administration of ligands for TLR2 [6, 9, 13, 24], TLR4 [10], and TLR9 [32] induces a protection against ischemic injury in heart [6, 10, 24] and brain [13, 32] through a preconditioning mechanism.
TLR9 is located intracellularly in endosomes and endoplasmic reticulum and recognizes unmethylated CpG-DNA from bacteria and endogenous DNA [18]. CpG-motifs activate the TLR9-mediated MyD88-dependent NF-κB signaling pathway and mitogen activated protein kinases (MAPKs) [18]. Synthetic CpG-oligodeoxynucleotide (ODN) has been demonstrated to activate TLR9 [17, 18], improve cell survival and prevent cell apoptosis [29]. Recently, several studies reported a benefit of CpG-ODN in sepsis and cardiac function after I/R. Rice et al [27] and Weighardt et al [34] have shown that treatment of experimental animals with CpG-ODN improved polymicrobial sepsis survival rate. Mathur et al showed that pretreatment of mice with CpG-C isoform 24 hrs prior to endotoxin challenge improved ejection fraction six hrs after LPS stimulation [21]. Pretreatment of mice with CpG-C isoform 24 hrs prior to myocardial I/R improved ejection fraction after myocardial I/R [21]. The protection induced by 24 hrs pretreatment is through preconditioning dependent mechanisms. However, the role of B-isoform of CpG-ODN in myocardial I/R injury has not been investigated. In addition, the mechanisms by which CpG-ODN exerts the protective benefits have not been elucidated.
Phosphoinositide 3-kinases (PI3Ks), and their downstream target serine/threonine kinase Akt [also known as protein kinase B (PKB)], are a conserved family of signal transduction enzymes which are involved in regulating cellular activation, inflammatory responses, and apoptosis [3]. The PI3K/Akt signaling pathway may serve as an endogenous negative feedback regulator and/or compensatory mechanism that limits pro-inflammatory and apoptotic events in response to injurious stimuli. Activation of PI3K/Akt-dependent signaling has been shown to prevent cardiac myocyte apoptosis and protect the myocardium from I/R injury [7, 22]. Recent studies have identified cross talk between TLR signaling and the PI3K/Akt pathway [25].
In the present study, we examined the effect of CpG-ODN on myocardial I/R injury. We have observed that administration of CpG-ODN one hr prior to myocardial I/R significantly reduced myocardial infarct size and improved cardiac function after myocardial I/R injury. We demonstrated that CpG-ODN-induced cardioprotection was mediated by a PI3K/Akt signaling dependent mechanism.
Materials and methods
Animals
Male C57BL/6 mice (25–30 g) were obtained from Jackson Laboratory (Indianapolis, IN). Transgenic mice with cardiac specific expression of kinase defective Akt (kdAkt) with an FVB background were provided by Dr. McMullen and have been described previously [31]. The mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University. The experiments outlined in this article conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, Revised 1996). All aspects of the animal care and experimental protocols were approved by the East Tennessee State University Committee on Animal Care.
Induction of myocardial I/R injury
Myocardial I/R injury was induced as described previously[9, 10, 12, 19]. Briefly, the mice were anaesthetized by 5.0% isoflurane, intubated and ventilated with room air using a rodent ventilator. Anesthesia was maintained by inhalation of 1.5% to 2% isoflurane driven by 100% oxygen flow. Body temperature was regulated at 37°C by surface water heating. Following the skin incision, the hearts were exposed through a left thoracotomy in the fourth intercostal space. The left anterior descending (LAD) coronary artery was ligated with an 8-0 silk ligature over a 1 mm polyethylene tube (PE-10). After completion of 60 min of occlusion, the coronary artery was reperfused by pulling on the exteriorized suture to release the knot. The skin was closed, anesthesia discontinued, and the animal allowed to recover in pre-warmed cages. After reperfusion for indicated time, the mice were sacrificed by CO2 inhalation and the hearts were harvested.
Experimental protocol
To examine the role of TLR9 activation in myocardial I/R injury, we employed the TLR9 ligand, CpG-ODN. Mice were treated with CpG-ODN (n=10, 10 μg/25 g body weight) or control-ODN (n=10, 10 μg/25 g body weight) by intraperitoneal injection one hr before the hearts were subjected to I/R. To evaluate the role of TLR9 inactivation in myocardial I/R injury, we chose inhibitory CpG-ODN (iCpG-ODN) which binds C-terminal fragment of TLR9 resulting in preventing TLR9 activation[36]. iCpG-ODN (100 μg/25 g body weight) was administered to mice (n=10) by i.p. injection one hr prior to myocardial I/R. Mice that did not receive any treatment served as untreated controls. The dose of CpG-ODN used in the present study was selected based on our pilot study using 10, 50, 75 and 100 μg/25 g body weight of CpG-ODN. We observed that administration of CpG-ODN at 10 μg/25 g body weight also significantly decreased myocardial infarct size. The CpG-ODN (CpG-ODN 1826), Control ODN (control-ODN 1826), and iCpG-ODN (iCpG-ODN 2088) were purchased from InvivoGen (San Diego, CA).
We also examined the effect of CpG-ODN on cardiac function following myocardial I/R injury. Mice (n=5 each group) were treated with and without CpG-ODN, control-ODN or iCpG-ODN one hr before the hearts were subjected to 60 min of ischemia followed by reperfusion up to 14 days. Cardiac function was examined by echocardiography before I/R, 3 and 14 days after reperfusion as described previously [9, 11].
To determine the role of the PI3K/Akt signaling pathway in TLR9 ligand-induced cardioprotection, mice (n=8) were injected with the PI3K inhibitor LY924002 (I mg/25 g body weight, Sigma-Aldrich, St Louis, MO) 15 min prior to CpG-ODN administration (10 μg/25 g body weight). In separate experiment, kdAkt transgenic mice (n=6 each group) were treated with or without CpG-ODN one hr before the hearts were subjected to I/R [9, 10].
Assessment of myocardial infarct size
Infarct size was evaluated by TTC (Sigma-Aldrich) staining as described previously [9, 10, 12, 19]. Briefly, the hearts were perfused with saline on a Langendorff system to wash blood from the coronary vasculature before staining with 1% Evans Blue. Each heart was then sliced horizontally to yield five slices. The slices were incubated in 1% TTC for 15 min at 37°C, fixed by immersion in 10% neutral buffered formalin. The area of infarction on both sides of each slice was determined by an image analyzer, corrected for the weight of each slice, and summed for each heart. Ratios of risk area vs. left ventricle area (RA/LV) and infarct area vs. risk area (IA/RA) were calculated and expressed as a percentage.
Echocardiography
Transthoracic two-dimensional M-mode echocardiogram and pulsed wave Doppler spectral tracings were obtained using a Toshiba Aplio 80 Imaging System (Tochigi, Japan) equipped with a 12-MHz linear transducer as described previously [9, 11]. The mice were anesthetized by isoflurane and the body temperature was maintained at 37°C using a heating pad. M-mode tracings were used to measure left ventricular (LV) wall thickness, LV end-systolic diameter (LVESD), and LV end-diastolic diameter (LVEDD). Ejection fraction (EF) and percent fractional shortening (%FS) were calculated as described previously [9, 11].
In situ apoptosis assay
Myocardial apoptosis was examined as described previously [9, 10, 12, 19] using the in situ cell death detection kit, fluorescein (Roche, USA) according to the instructions of the manufacturer. Briefly, hearts were harvested 4 hrs after reperfusion and slices cut horizontally. One slice was immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin and cut at 5 μm thick. The sections were incubated with the commercially prepared labeling mixture supplied by the manufacturer at 37°C for one hr. Nuclei of living and apoptotic cells were counterstained with Hoechst 33342 (Invitrogen). Three slides from each block were evaluated for percentage of apoptotic cells and four fields on each slide were examined at the border areas using a defined rectangular field area with 20X magnification. Numbers of apoptotic cardiac myocytes are presented as the percentage of total cells counted.
Immunoblot
Immunoblots were performed as described previously [9, 10, 12, 19]. Briefly, hearts were harvested 4 hrs after reperfusion. The cellular proteins were prepared for Western blot analysis. The primary antibodies used in the present study were anti-phospho-Akt, anti-phospho-GSK-3β (anti-Ser9), anti-ERK, anti-Akt, anti-GSK-3β and anti-ERK (1:500, Cell Signaling Technology Beverly, MA), anti-BcL2, anti-Bax, anti-phosphotyrosine (anti-pTyr20), anti-TLR9, and anti-p85 subunit of PI3K (1:1000, Santa Cruz), anti-GAPDH (1:8000, Biodesign). The secondary antibodies were anti-rabbit and anti-mouse (Sigma-Aldrich). The signals were detected with the ECL system (Amersham Pharmacia). The signals were quantified using the G:Box gel imaging system by Syngene (Syngene, USA, Fredrick, MD).
In vitro experiments
The H9C2 rat cardiomyoblasts were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) under 5% CO2 at 37°C. When cells reached 70–80% confluence, they were treated with CpG-ODN (50 nM), control-ODN (50 nM), in the presence and absence of LY924002 (10 uM) for 30 min, respectively. Each group contained four replicates. The cells were harvested and cellular proteins were prepared for immunoprecipitation and Western blot analysis as described previously [9, 19].
Immunoprecipitation
Approximately 800 μg of cellular proteins were immunoprecipitated with 2 μg of antibody against TLR9 (Santa Cruz, Biotechnology, Inc., CA) for 2 hrs at 4°C on a rotator followed by addition of 20 μL protein A/G-agarose beads (Santa Cruz) as described previously[9, 19]. The immunoprecipitations were washed three times in lysis washing buffer, suspended in 25 μl of loading buffer, and boiled for 5 min before the immunoprecipitates were subjected to immunoblots with primary antibodies (anti-pTyr20, 1:500, and anti-p85, 1:500, Santa Cruz), respectively, followed by secondary antibody (anti-rabbit and anti-mouse, Sigma-Aldrich).
Statistical analysis
Data are expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance (ANOVA), and Tukey’s procedure for multiple-range tests was performed. P< 0.05 was considered to be significant.
Results
CpG-ODN administration reduced myocardial infarction and improved cardiac function following I/R
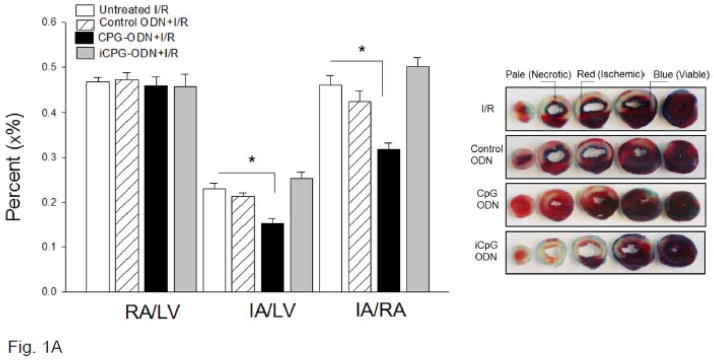
To investigate the effect of TLR9 ligand, CpG-ODN, on myocardial infarction following I/R, we administered CpG-ODN to mice one hr prior to myocardial ischemia (60 min) followed by reperfusion (4 hrs). Figure 1A shows that CpG-ODN treatment significantly reduced infarct size by 31.3% compared with untreated I/R hearts (15.3 ±1.15% vs. 22.2 ± 1.38%). There was no significant difference in the ratio of risk area/left ventricle area (RA/LV) between CpG-ODN-treated mice and untreated I/R mice, suggesting that the coronary artery was ligated in the same place between the groups (Figure 1A). Administration of either control-ODN or iCpG-ODN to mice did not alter I/R-induced myocardial infarction.
Figure 1.
(A) TLR9 ligand, CpG-ODN, decreased myocardial infarct size following I/R injury. Mice were treated with CpG-ODN (n=10) or control CpG-ODN (n=10) or inhibitory CpG-ODN (iCpG-ODN, n=10) by i.p. injection one hr prior to myocardial Ischemia (60 min) followed by reperfusion (4 hrs). The infarct area (white) and the area at risk (red + white) from each section were measured using an image analyzer. Ratios of risk area vs. left ventricle area (RA/LV) and infarct area vs. risk area (IA/RA) were calculated and are presented in the graph. * P<0.05 compared with indicated group.
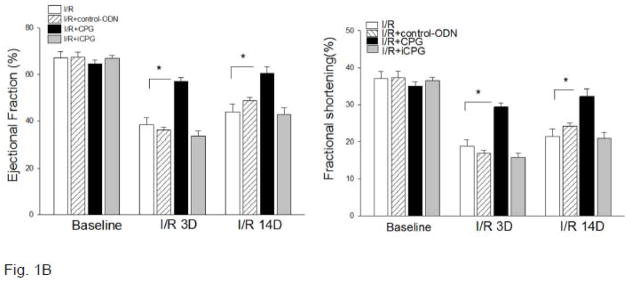
(B) CpG-ODN administration improved cardiac function following myocardial I/R. Mice were treated with CpG-ODN (n=5/group) one hr before the hearts were subjected to myocardial ischemia (60 min) followed by reperfusion. Cardiac function was examined by echocardiography before I/R (Baseline), 3 days and 14 days after I/R. * P<0.05 compared with indicated groups.
We also examined the effect of CpG-ODN on cardiac function following myocardial I/R. Figure 1B shows that ejection fraction (EF%) and fractional shortening (%FS) were significantly decreased after myocardial ischemia followed by reperfusion up to 14 days. In contrast, CpG-ODN administration attenuated I/R-induced cardiac dysfunction. EF and %FS values in CpG-ODN-treated mice were significantly increased by 48.3% and by 56.9% at 3 days, and by 37.0% and by 50.7% at 14 days after reperfusion, respectively, compared with untreated I/R mice. Treatment of mice with either control-ODN or iCpG-ODN did not affect I/R-induced cardiac dysfunction.
CpG-ODN administration attenuated I/R-increased myocardial apoptosis
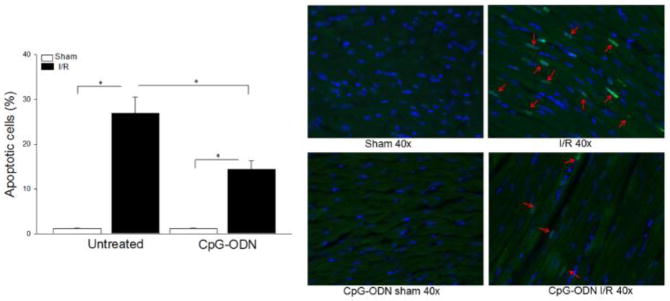
It is well known that myocardial apoptosis contributes to myocardial ischemic injury. We examined the effect of CpG-ODN treatment on I/R-induced myocardial apoptosis. Figure 2 shows that I/R markedly induced myocardial apoptosis. In contrast, CpG-ODN administration significantly attenuated I/R-induced myocardial apoptosis by 46.6% compared with untreated I/R hearts. Control-ODN administration did not affect I/R-induced apoptosis in the myocardium (data not shown).
Figure 2.
CpG-ODN administration attenuated I/R-increased myocardial apoptosis. Mice were treated with or without CpG-ODN (n=5/group) and were subjected to myocardial I/R. The hearts were harvested and sectioned for analysis of apoptosis by the TUNEL assay on the border zones. Green color indicates positive myocardial apoptosis which is marked by red arrows. Blue color indicates the nucleus of each cell. * P<0.05 compared with indicated groups. S= Sham; I/R = ischemia/reperfusion.
CpG-ODN administration attenuated I/R-decreased BCL-2 and increased Bax levels in the myocardium
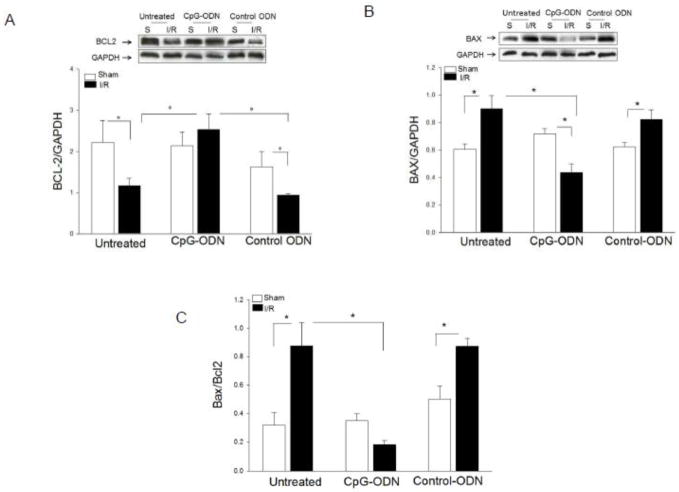
We investigated whether CpG-ODN-attenuated myocardial apoptosis will be involved in regulation of Bcl2/Bax mediators. Figure 3 shows that I/R significantly decreased Bcl-2 levels by 47.3% and increased Bax levels by 48.9% in the myocardium compared with sham control. The ratio of Bax/Bcl2 in untreated I/R mice was increased by 175% compared with sham control. In contrast, CpG-ODN treatment prevented I/R-decreased Bcl2 levels and attenuated I/R-increased Bax levels in the myocardium observed in untreated I/R hearts. I/R-increased ratio of Bax/Bcl2 was attenuated by CpG-ODN administration. In control-ODN treated I/R mice, the levels of Bcl2 and Bax were similar to that of untreated I/R mice.
Figure 3.
CpG-ODN treatment prevented I/R-decreased Bcl2 levels and attenuated I/R-increased Bax levels in the myocardium. Mice were treated with or without CpG-ODN or control-ODN one hr prior to myocardial I/R (n=6/group). Sham operated mice served as sham control (n=4). Cytoplasmic proteins were isolated from heart tissues for Western blot analysis of Bcl2 (A), Bax (B) and the ratio of Bax/Bcl2 (C) levels. * P<0.05 compared with indicated groups. S= Sham; I/R = ischemia/reperfusion.
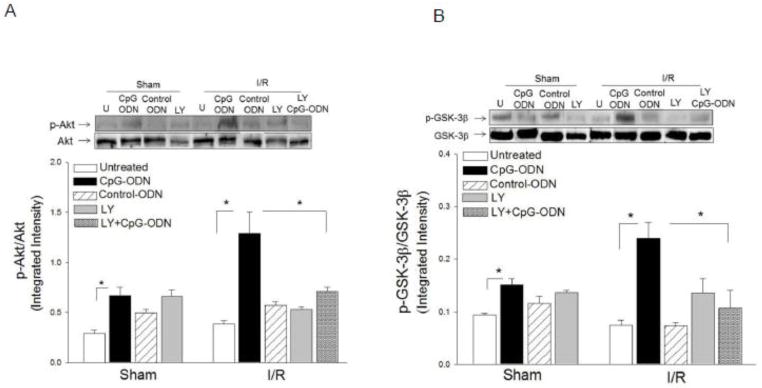
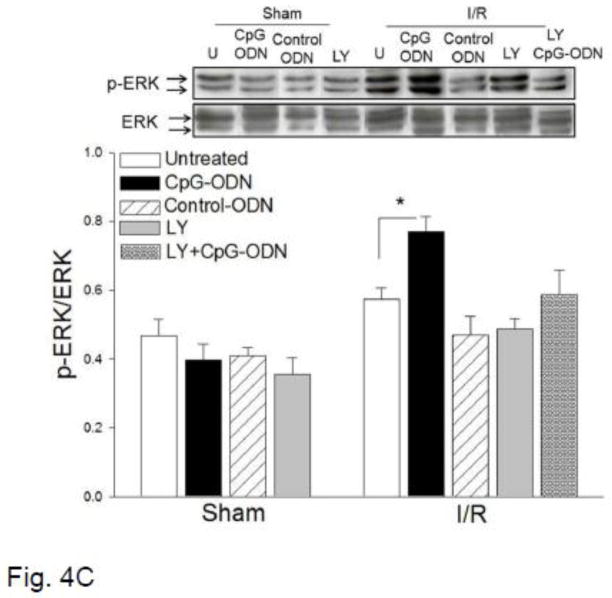
CpG-ODN increased the levels of phosphorylated Akt, GSK-3-β and ERK in the myocardium following myocardial I/R
Activation of the PI3K/Akt signaling pathway has been shown to protect the myocardium from ischemic injury [10, 19, 22]. We examined whether CpG-ODN administration will induce the activation of PI3K/Akt signaling in the myocardium following I/R. Figure 4 shows that CpG-ODN treatment significantly increased the levels of phospho-Akt by 2.3 fold and phospho-GSK-3β by 2.2 fold, respectively, compared with the untreated I/R group. CpG-ODN also increased the levels of ERK phosphorylation by 35% compared with untreated I/R hearts. Control-ODN administration did not significantly alter the levels of phospho-Akt, phospho-GSK-3β and phospho-ERK in the myocardium of untreated sham and I/R mice. Treatment of mice with a specific PI3K inhibitor, LY924002, abolished CpG-ODN-increased levels of p-Akt and p-GSK-3β in the myocardium.
Figure 4.
CpG-ODN treatment increased the levels of phosphorylated Akt, GSK-3β and ERK in the myocardium. Mice were treated with or without CpG-ODN one hr prior to myocardial I/R (n=6/group). Sham operated mice served as sham control (n=4). A PI3K inhibitor, LY294002 was also administered to the mice (n=6/group) treated with and without CpG-ODN. Cytoplasmic proteins were isolated from heart tissues for Western blot analysis of phosphorylated Akt (A), phosphorylated GSK-3β (B), and phosphorylated DRK (C). * P<0.05 compared with indicated groups. U= Untreated; I/R = ischemia/reperfusion; LY= LY294002.
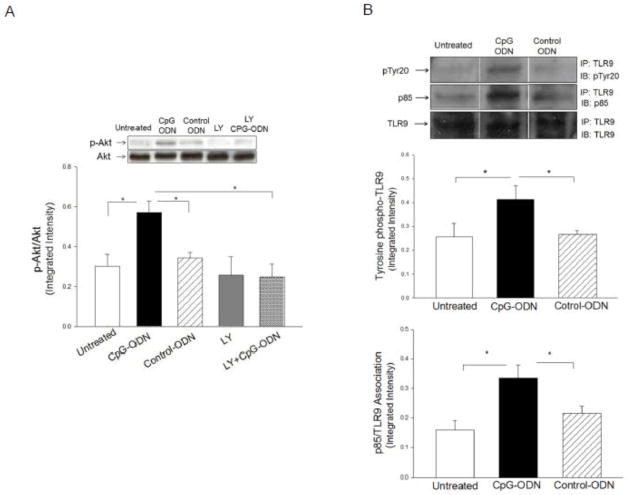
CpG-ODN treatment induced TLR9 phosphorylation and association with the p85 subunit of PI3K
To determine the mechanisms by which CpG-ODN administration increased myocardial phospho-Akt, we performed in vitro experiments using the cultured cardiomyoblast H9C2 cell line. H9C2 cells were treated with CpG-ODN for 15 min and the cells were then harvested. The cellular proteins were isolated for analysis of Akt phosphorylation. Figure 5A shows that CpG-ODN treatment increased the levels of phospho-Akt compared with untreated cells (0.57 ± 0.06 vs 0.30 ± 0.06). Control-ODN did not markedly induce Akt phosphorylation in H9C2 cells. PI3K inhibition by LY924002 abolished CpG-ODN-induced Akt phosphorylation, indicating that CpG-ODN-increased Akt phosphorylation is mediated by activation of PI3K.
Figure 5.
CpG-ODN administration increased TLR9 phosphotyrosine levels and association of the p85 subunit of PI3K with TLR9. H9C2 cells were treated with CpG-ODN or control ODN for 30 min and untreated cells served as control. Cellular proteins were isolated for Western blot analysis of the levels of phosphorylated Akt (A) and examined for immunoprecipitation with a specific anti-TLR9 antibody followed by immunoblots with antibodies to pTyr20 and p85 subunit of PI3K (B). Each group contained four replicates.
Next, we examined whether there is an association between TLR9 and PI3K following CpG-ODN treatment. H9C2 cells were treated with CpG-ODN for 15 min and cellular proteins were isolated for immunoprecipitation with anti-TLR9 specific antibody followed by immunoblots with indicated antibodies. As shown in Figure 5B, CpG-ODN administration increased the levels of TLR9 tyrosine phosphorylation by 38.5% and association with the p85 subunit of PI3K by 110%, respectively, compared with untreated cells. The data suggests that CpG-ODN treatment activated the PI3K/Akt signaling through promoting the association of TLR9 and PI3K.
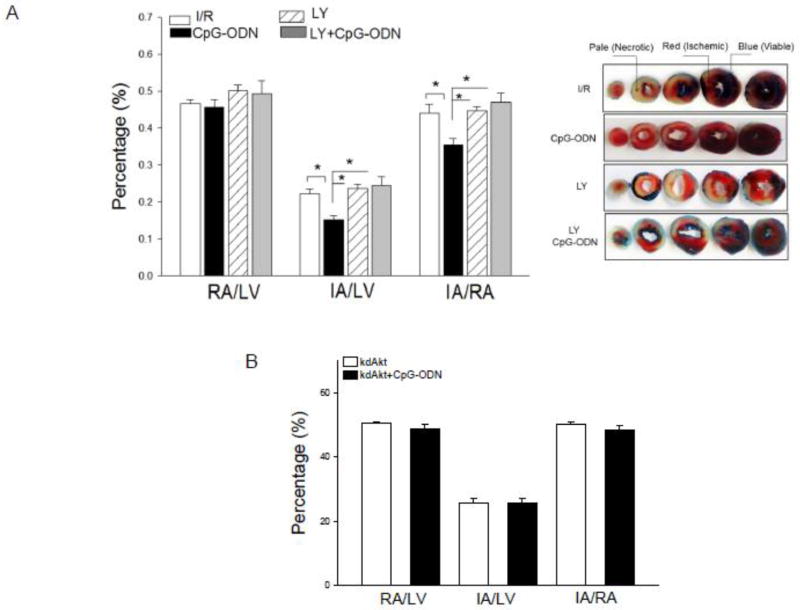
PI3K inhibition abolished CpG-ODN-induced cardioprotection
To determine the role of the PI3K/Akt signaling pathway in CpG-ODN-induced cardioprotection, we treated mice with LY924002, a PI3K inhibitor, 15 min prior to CpG-ODN administration. The hearts were subjected to ischemia (60 min) followed by reperfusion (4 hrs). Figure 6A shows that LY924002 administration abolished CpG-ODN-induced cardioprotection. Infarct size in CpG-ODN-treated mice in the presence of LY924002 was significantly increased compared with CpG-ODN treated mice that did not receive LY924002 (24.6 ± 2.23% vs.15.3 ±1.15%). There was no significant difference in the infarct size between the CpG-ODN + LY924002 group and the untreated I/R group.
Figure 6.
Pharmacologic inhibition of PI3K (A) and genetic deficiency of Akt (B) abrogated the cardio protection induced by CpG-ODN. (A) Mice were pretreated with the PI3K inhibitor LY924002 15 min prior to CpG-ODN administration. The hearts were subjected to myocardial ischemia (60 min) followed by reperfusion (4 hrs). (B) Mice with genetically defective Akt kinase (kdAkt) showed an abolishment of CpG-ODN-induced cardioprotection. Kinase defective Akt transgenic mice (kdAkt, n=8/group) were pretreated with or without CpG-ODN one hr before the hearts were subjected to ischemia (60 min) followed by reperfusion (4 hrs). Ratios of risk area vs. left ventricle area (RA/LV) and infarct area vs. risk area (IA/RA) were calculated and are presented in the graph. * P<0.05 compared with the indicated group. RA = risk area; LV = left ventricle; IA = infarct area. LY = LY924002.
Genetic deficiency of Akt abrogated CpG-ODN-induced cardioprotection
Akt is an important kinase downstream from PI3K [3]. To further determine the role of the PI3K/Akt signaling in CpG-ODN-induced cardioprotection, we examined the effect of Akt deficiency on CpG-ODN-induced cardioprotection using mice that express a kinase deficient form of Akt (kdAkt) in cardiac myocytes. CpG-ODN was administered to kdAkt transgenic mice one hr before the hearts were subjected to I/R. Untreated kdAtk Tg mice were subjected to I/R. Figure 6B shows that CpG-ODN administration to kdAkt mice did not induce cardioprotection against I/R injury. The infarct size/area at risk in CpG-ODN-treated kdAkt mice was similar to that of kdAkt I/R mice that did not receive CpG-ODN treatment. The data indicates that CpG-ODN-induced cardioprotection was abolished in kdAkt mice.
Discussion
A major finding in the present study is that administration of a TLR9 agonist, CpG-ODN, to mice decreased infarct size and improved cardiac function after myocardial I/R injury. Administration of inhibitory CpG-ODN, the TLR9 antagonist, did not affect I/R-induced myocardial infarction, suggesting that activation of TLR9 serves a protective role during myocardial I/R injury. More significantly, CpG-ODN treatment activated the PI3K/Akt signaling pathway through induction of TLR9 tyrosine phosphorylation and association with the p85 subunit of PI3K. Either PI3K inhibition with a specific PI3K inhibitor, LY924002, or genetic inactivation of Akt in cardiac myocytes abolished CpG-ODN-induced cardioprotection. The data suggests that the TLR9 agonist, CpG-ODN, induces cardioprotection involving activation of the PI3K/Akt signaling pathway.
There are several isoforms of CpG-ODNs including A-, B-, and C-classes. These isoforms of CpG-ODN contain different CpG sequence motifs and palindromic sequences [33]. A isoform effectively stimulates the production of high amounts of IFN-alpha, while B-isoform primarily induces a strong activation and proliferation of B cells. C isoform of CpG-ODNs has both the immune effects of A- and B- isoforms but with lower effective [33]. A recent study by Mathur et al has investigated the effect of the three isoforms of CpG (A, B, and C) on endotoxin-induced cardiac dysfunction [21]. The authors have observed that CpG-C isoform was uniquely able to prevent LPS-induced cardiac dysfunction. In addition, pretreatment of mice with CpG-C isoform at 100 μg/mouse 24 hrs before the hearts were subjected to I/R prevented I/R-decreased ejection fraction [21]. In the present study, we employed CpG-ODN 1826 which is the CpG B isoform. Our data showed that administration of CpG-ODN B isoform at 10 μg/mouse one hr prior to myocardial I/R induced a protection against myocardial I/R injury and attenuation of I/R-induced cardiac dysfunction. We chose B isoform of CpG-ODN in the present study because it has been reported to protect cells against spontaneous apoptosis [29]. In addition, we have recently reported that this CpG-ODN prevented trauma-hemorrhage induced cardiac dysfunction [37]. Our results were different from Mathur’s study. First, we employed CpG-B isoform (1826) in our present study while Mathur et al used CpG-C isoform in their study [21]. Second, we treated mice with CpG-ODN at 10 μg/mouse one hr prior to myocardial I/R and observed that CpG-ODN-induced protection via a PI3K/Akt dependent mechanism. In Mathurs’ study, these authors treated mice with CpG-C class at 100 μg/mouse 24 hrs before myocardial I/R. The mechanisms are involved in preconditioning. Our data is consistent with our recent study showing that administration of CpG-ODN 1826 one hr prior to induction of trauma hemorrhage (T-H) in mice significantly prevented T-H-induced cardiac dysfunction [37]. In addition, we have found that CpG-ODN 1826 treatment prevented polymicrobial sepsis-induced cardiac dysfunction (unpublished data). Other studies have shown that CpG-ODN administration protects the organs in ischemic injury and septic model through preconditioning mechanisms [14, 27, 32]. For example, Stevens et al reported that systemic administration of CpG-ODN to mice 72 hrs prior to brain ischemia reduced ischemic damage up to 60% in a dose and time dependent manner through a preconditioning mechanism [32]. Hyakkoku et al reported that cerebral infarct volume in TRL9 knockout mice was similar to that of WT mice, suggesting that knockout of TLR9 did not protect the brain from ischemic injury [14]. However, Knuefermann et al reported that in vitro treatment of isolated wild type cardiac myocytes with CpG-ODN induced a loss of sarcomeric shortening but not in TLR9 knockout cardiac myocytes [16]. The differences observed in the effect of CpG-ODN treatment on cardiac myoctyte contractility may be due to use of different experimental models, i.e., in vitro isolated cardiac myocytes and in vivo cardiac function. The present study showed that treatment of mice with CpG-ODN significantly decreased myocardial infarct size and improved cardiac function after ischemia followed by reperfusion up to 14 days. Administration of the TLR9 antagonist, iCpG-ODN, did not alter I/R-induced myocardial infarction and cardiac dysfunction, indicating that modulation of TLR9 by its ligand can induce a protective effect on myocardial ischemic injury.
Cardiac myocyte apoptosis plays a role in myocardial I/R injury. Bcl-2 is an important anti-apoptotic and anti-necrotic molecule while Bax is proapoptotic mediator. During apoptotic process, Bax protein undergoes a conformational change and forms homodimers, and then translocates and integrates into the mitochondrial membrane thereby facilitating the release of cytochrome c. We have observed that CpG-ODN administration prevented I/R-decreased Bcl2 levels and attenuated I/R-increased Bax levels in the myocardium. The data suggests that increased Bcl-2 and decreased Bax levels in the myocardium treated with CpG-ODN could be one of the mechanisms by which the CpG-ODN protects against myocardial ischemic injury.
Recent studies have shown that CpG-ODN administration increased survival and prevented apoptosis in murine macrophages [20, 29] and neutrophils [15] through activation of the PI3K/Akt signaling pathway. In the present study, we have observed that administration of CpG-ODN increased the levels of phospho-Akt in the myocardium following myocardial I/R, indicating that CpG-ODN treatment activated the PI3K/Akt signaling pathway. It has been well documented that activation of the PI3K/Akt signaling pathway protects against I/R-induced cardiac myocyte apoptosis [7, 10, 19, 22]. Activation of PI3K/Akt inhibits Bax conformational change, thus preventing Bax translocation and integration into mitochondrial membrane. PI3K/Akt activation also phosphorylates Bim, leading to dissociation of Bim from BCL2. Numerous studies have demonstrated that activation of PI3K/Akt may serve as a negative feedback regulator that prevents excessive innate immune and/or inflammatory responses during myocardial I/R injury and polymicrobial sepsis[8, 10, 19, 35]. To determine the mechanisms by which CpG-ODN treatment activated the PI3K/Akt signaling, we examined whether there is an association between TLR9 and PI3K following CpG-ODN administration. We performed in vitro experiments using H9C2 cardiomyoblasts and observed that CpG-ODN administration increased the levels of phospho-Akt, while PI3K inhibition by LY924002 prevented CpG-ODN-increased Akt phosphorylation. CpG-ODN treatment rapidly induced TLR9 tyrosine phosphorylation and an association with the p85 subunit of PI3K. The data suggest that CpG-ODN treatment increased Akt phosphorylation through induction of TLR9 tyrosine phosphorylation which interacts with PI3K, resulting in activation of the PI3K/Akt signaling pathway. In vivo experiments showed that either PI3K inhibition by LY924002, a specific PI3K inhibitor, or using mice with genetically defective Akt kinase (kdAkt) abolished CpG-ODN-induced cardioprotection. This data confirmed that CpG-ODN-induced cardioprotection is mediated through a TLR9 mediated PI3K/Akt dependent mechanism.
We have observed that CpG-ODN administration significantly increased the levels of phospho-ERK in the myocardium following myocardial I/R, suggesting that attenuation of myocardial apoptosis by CpG-ODN involves activation of ERK signaling. ERK can be activated by Ref-mediated MEK signaling [23]. The role of Raf/MEK/ERK signaling pathway in anti-apoptosis has been well demonstrated[23]. Activation of Raf/MEK/ERK phosphorylates Bad, resulting in its inactivity. This process allows BCL2 to form homodimers and process an anti-apoptotic response. Activation of Raf/MEK/ERK also induces Bim phosphorylation, resulting in Bim disassociation from BCL2. BCL2 then binds to Bax and prevents Bax formation of homodimers and activation. In addition, the Raf/MEK/ERK and PI3K/Akt signaling pathways are synergistically regulated and that there is crosstalk between both signaling pathways [36].
In summary, administration of a TLR9 ligand, CpG-ODN, significantly decreased I/R-induced infarct size and improved cardiac function following myocardial I/R. CpG-ODN-induced cardioprotection is mediated through activation of the PI3K/Akt signaling pathway. The data suggest that the TLR9 ligand, CpG-ODN, may be a new approach for management and treatment of acute myocardial ischemic injury.
Highlights.
CpG-ODN administration attenuated ischemia/reperfusion-induced myocardial infarction.
CpG-ODN improved cardiac function after myocardial reperfusion up to 14 days.
CpG-ODN administration increased Akt and GSK-3β phosphorylation in the myocardium.
PI3K inhibition or Akt deficiency abolished CpG-ODN-induced cardioprotection.
Acknowledgments
Funding:
This work was supported by NIH HL071837, NIH GM083016 and NIH GM53552.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128:170–179. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Dong JW, Vallejo JG, Tzeng HP, Thomas JA, Mann DL. Innate immunity mediates myocardial preconditioning through Toll-like receptor 2 and TIRAP-dependent signaling pathways. Am J Physiol Heart Circ Physiol. 2010;298:H1079–H1087. doi: 10.1152/ajpheart.00306.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt Promotes Survival of Cardiomyocytes In Vitro and Protects Against Ischemia-Reperfusion Injury in Mouse Heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends in Immunology. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 9.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, Isumo S, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 11.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against Myocardial Ischemia/Reperfusion Injury in TLR4 Deficient Mice is Mediated through a Phosphoinositide 3-Kinase Dependent Mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 13.Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. Journal of Neuroimmunology. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have meuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 15.Jozsef L, Khreiss T, Filep JG. CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. FASEB J. 2004;18:1776–1778. doi: 10.1096/fj.04-2048fje. [DOI] [PubMed] [Google Scholar]
- 16.Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rudiger M, Boehm O, Fink K, Dreiner U, Grohe C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, Meyer R. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res. 2008;78:26–35. doi: 10.1093/cvr/cvn011. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature Reviews Drug Discovery. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Advanced Drug Delivery Reviews. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. Modulating Toll-like receptor mediated signaling by (1-->3)-b-D-glucan rapidly induces cardioprotection. Cardiovascular Research. 2003;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Lim EJ, Park DW, Lee JG, Lee CH, Bae YS, Hwang YC, Jeong JW, Chin BR, Baek SH. Involvement of FoxO3a in toll-like receptor 9-mediated anti-apoptosis via FLIP. Exp Mol Med. 2010 doi: 10.3858/emm.2010.42.10.070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur S, Walley KR, Boyd JH. The Toll-like receptor 9 ligand CPG-C attenuates acute inflammatory cardiac dysfunction. Shock. 2011;36:478–483. doi: 10.1097/SHK.0b013e31822d6442. [DOI] [PubMed] [Google Scholar]
- 22.Matsui T, Ling L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral Gene Transfer of Activated Phosphatidylinositol 3′-Kinase and Akt Inhibits Apoptosis of Hypoxic Cardiomyocytes In Vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 23.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mersmann J, Berkels R, Zacharowski P, Tran N, Koch A, Lekushi K, Dimmeler S, Granja TF, Boehm O, Claycomb WC, Zacharowski K. Preconditioning by toll-like receptor 2 agonist Pam3CSK4 reduces CXCL1-dependent leukocyte recruitment in murine myocardial ischemia/reperfusion injury. Crit Care Med. 2010;38:903–909. doi: 10.1097/CCM.0b013e3181ce50e6. [DOI] [PubMed] [Google Scholar]
- 25.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 26.Oyama J, Jr, Blais C, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 27.Rice L, Orlow D, Ceonzo K, Stahl GL, Trianabos AO, Wada H, Aird WC, Buras JA. CpG oligodeoxynucleotide protection in polymicrobial sepsis is dependent on interleukin-17. J Infect Dis. 2005;191:1368–1376. doi: 10.1086/428452. [DOI] [PubMed] [Google Scholar]
- 28.Sakata Y, Dong J-W, Vallejo JG, Huang C-H, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 29.Sester DP, Brion K, Trieu A, Goodridge HS, Roberts TL, Dunn J, Hume DA, Stacey KJ, Sweet MJ. CpG DNA Activates Survival in Murine Macrophages through TLR9 and the Phosphatidylinositol 3-Kinase-Akt Pathway. The Journal of Immunology. 2006;177:4473–4480. doi: 10.4049/jimmunol.177.7.4473. [DOI] [PubMed] [Google Scholar]
- 30.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like Receptor 4 with Eritoran Attenuates Myocardial Ischemia-Reperfusion Injury. Circulation. 2006;114:I-270–I-274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 31.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/Protein Kinase B Promotes Organ Growth in Transgenic Mice. Mol and Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Advanced Drug Delivery Reviews. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Weighardt H, Feterowski C, Veit M, Rump M, Wagner H, Holzmann B. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J Immunol. 2000;165:4537–4543. doi: 10.4049/jimmunol.165.8.4537. [DOI] [PubMed] [Google Scholar]
- 35.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide-3-kinase signaling pathway alters host resistance to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Guo W, Zhang Q, Li H, Liu X, Yang Y, Zuo J, Liu W. Crosstalk between Raf/MEK/ERK and PI3K/AKT in Suppression of Bax Conformational Change by Grp75 under Glucose Deprivation Conditions. J Mol Biol. 2011;414:654–666. doi: 10.1016/j.jmb.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Gao M, Ha T, Kalbfleisch JH, Browder W, Williams DL, Li C, Kao RL. The TLR9 agonist, CpG-ODN 1826, ameliorates cardiac dysfunction after trauma-hemorrhage. Shock. 2012;38:146–152. doi: 10.1097/SHK.0b013e31825ce0de. [DOI] [PMC free article] [PubMed] [Google Scholar]