Abstract
Background
Appalachia is a geographic region with high cervical cancer incidence and mortality rates, yet little is known about human papillomavirus (HPV) vaccination in this region. We determined HPV vaccine coverage among adolescent females from Appalachia, made comparisons to non-Appalachian females, and examined how coverage differs across subregions within Appalachia.
Methods
We analyzed 2008–2010 data from the National Immunization Survey-Teen (NIS-Teen) for adolescent females ages 13–17 (n=1,951 Appalachian females and n=25,468 non-Appalachian females). We examined HPV vaccine initiation (receipt of at least one dose), completion (receipt of at least three doses), and follow-through (completion among initiators). Analyses used weighted logistic regression.
Results
HPV vaccine initiation (Appalachian=40.8% vs. non-Appalachian=43.6%; OR=0.92, 95% CI: 0.79–1.07) and completion (Appalachian=27.7% vs. non-Appalachian=25.3%; OR=1.12, 95% CI: 0.95–1.32) were similar between Appalachian and non-Appalachian females. HPV vaccine follow-through was higher among Appalachian females than non-Appalachian females (67.8% vs. 58.1%; OR=1.36, 95% CI: 1.07–1.72). Vaccination outcomes tended to be higher in the Northern (completion and follow-through) and South Central (follow-through) subregions of Appalachia compared to non-Appalachian U.S. Conversely, vaccination outcomes tended to be lower in the Central (initiation and completion) and Southern (initiation and completion) subregions.
Conclusions
In general, HPV vaccination in Appalachia is mostly similar to the rest of the U.S. However, vaccination is lagging in regions of Appalachia where cervical cancer incidence and mortality rates are highest.
Impact
Current cervical cancer disparities could potentially worsen if HPV vaccine coverage is not improved in regions of Appalachia with low HPV vaccine coverage.
Keywords: Human papillomavirus, HPV vaccine, Appalachia, Cancer, Adolescent
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection (STI) in the United States (U.S.) (1). About 43% of U.S. females ages 14–59 have a current genital HPV infection (2). Most infections will clear within one year without intervention (3–5), but females with persistent infections can develop severe disease if left untreated. Oncogenic HPV types (mainly types 16 and 18) cause almost all cervical and anal cancers and lower percentages of vulvar, vaginal, and oropharyngeal cancers (6). Nononcogenic HPV types (mainly types 6 and 11) are associated with the development of anogenital warts (7), a diagnosis reported by about 7% of adult women in the U.S. (8).
Two HPV vaccines are currently available in the U.S. for females: a quadrivalent vaccine against types 6, 11, 16, and 18 (available since 2006) and a bivalent vaccine against types 16 and 18 (available since 2009). Guidelines currently recommend that all 11–12 year old females receive three doses of either HPV vaccine, with catch-up vaccination for 13–26 year old females (9). However, as of 2011, just over half (53%) of 13–17 year old females in the U.S. had received at least one dose of HPV vaccine, and only 35% had received all three doses (10). Widespread HPV vaccination may reduce cervical cancer incidence by as much as 77% (11), so vaccination may be particularly important for populations at high risk for cervical cancer.
One such population is females living in the Appalachian region of the U.S. Appalachia is a 13 state region (420 counties) extending from New York to Mississippi, with a total population of about 25 million people (about 8% of the U.S. population)(12). Appalachian residents tend to have lower socioeconomic status compared to the rest of the country, and there is less racial diversity among residents of the Appalachian region (12, 13). Appalachia can be divided into five subregions (Northern, North Central, Central, South Central, and Southern; Figure 1) (14). Each subregion represents a contiguous portion of Appalachia with similar characteristics (e.g., topography, demographics, and economics). The Northern and Southern subregions are the most populated subregions, with over 7.5 million residents each (other subregions have 1.9–4.7 million residents)(12, 13). Southern Appalachia is more racially diverse compared to the other subregions, while residents of the Central subregion tend to have lower socioeconomic status (12, 13).
Figure 1.
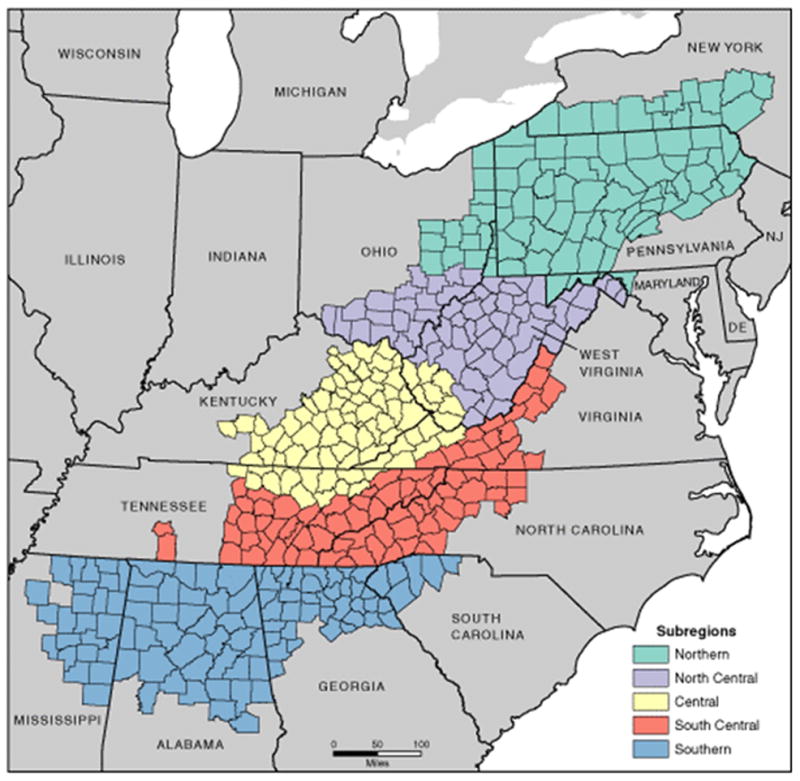
Subregions of Appalachia. Map by Appalachian Regional Commission, November 2009 (14).
Appalachia is a traditionally underserved geographic region, and many parts still lack adequate healthcare facilities (15, 16). These conditions having contributed to residents of Appalachia having an increased burden of cancer (17, 18), including cervical cancer. Areas within Appalachia have among the highest cervical cancer incidence and mortality rates in the U.S., and many Appalachian counties have cervical cancer mortality rates that exceed the national rate by 40% or more (19–24). For states containing Appalachian counties, within-state comparisons indicate that cervical cancer incidence and mortality rates are often higher in the Appalachian portions of these states (22, 24, 25).
Despite the existing cervical cancer disparities, few studies have addressed HPV vaccination in Appalachia. Most healthcare facilities in Appalachia offer HPV vaccine (26, 27), and a majority of adults in the Appalachian region are accepting of HPV vaccine for adolescent females (28–30). Data on HPV vaccine uptake in Appalachia are, however, sparse. The only published data indicate modest HPV vaccine uptake (less than 50% received the first dose) among adult women ages 18–26 from Kentucky given vouchers for free vaccine (31, 32). Less than 5% of these adult women received the recommended three doses of HPV vaccine (31).
Importantly, HPV vaccine uptake among adolescent females, the primary target population for HPV vaccination, from Appalachia has not yet been examined. We address this important research gap by analyzing national data to examine HPV vaccination among adolescent females from Appalachia and make comparisons to non-Appalachian adolescent females. Given the large size of the Appalachian region, we also examine potential differences in HPV vaccination across Appalachian subregions. Results from this descriptive study not only clarify HPV vaccine coverage in Appalachia, but also suggest how current cervical cancer disparities in Appalachia may be affected by this coverage. We conducted this study in conjunction with the Center for Population Health and Health Disparities (P50) housed at The Ohio State University Comprehensive Cancer Center. This Center used the framework for population health and health disparities (33) to understand and address the high cervical cancer burden in Appalachia.
Materials and Methods
Study Design
This study used data from the National Immunization Survey-Teen (NIS-Teen), which has been described in great detail elsewhere (34) and briefly here. The NIS-Teen is an annual survey conducted by the Centers for Disease Control and Prevention (CDC) in all 50 states, the District of Columbia, and selected local areas to monitor adolescent vaccination among 13–17 year-olds. It acts as an add-on to the National Immunization Survey (NIS), which examines vaccination among children 19–35 months old. The NIS-Teen uses a complex stratified sampling strategy to produce a national probability sample of adolescents ages 13–17. It is a two-phase survey that includes: 1) a random-digit-dialed telephone survey with parents/guardians (usually mothers but fathers or other guardians participate when mothers are not available; all referred to as “parents”) of adolescents ages 13–17; and 2) a mailed survey to adolescents’ healthcare providers identified by parents and for whom consent to contact is granted. Vaccination data are collected during both parent surveys (through immunization cards or recall) and from provider records, though provider records are used in generating vaccination estimates (35–37). If a household contains more than one adolescent ages 13–17, one is randomly selected to be the index adolescent for the NIS-Teen.
We examined NIS-Teen data from 2008–2010 (all years with public use datasets at the time of this report) for adolescents with provider-verified vaccination records. This included 17,835 adolescents from 2008 (household response rate=58.7% (35)), 20,066 adolescents from 2009 (excluding U.S. Virgin Islands; household response rate=58.0% (36)), and 19,257 adolescents from 2010 (excluding U.S. Virgin Islands; household response rate=58.0% (37)). We report data on 27,419 adolescent females (2008: 8,595; 2009: 9,611; 2010: 9,213) with provider-verified vaccination records, excluding 29 females whose data did not allow for determination of Appalachian residence. We also excluded males (n=29,710) since the NIS-Teen did not collect data on HPV vaccination among males until 2010.
Data collection for the NIS-Teen was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB). Analysis of deidentified data from the survey is exempt from the federal regulations for the protection of human research participants. Analysis of restricted data through the NCHS Research Data Center is also approved by the NCHS ERB. The Institutional Review Board at The Ohio State University determined this study was exempt from review.
Measures
Similar to previous reports using NIS-Teen data (37), we examined three dichotomous outcomes regarding HPV vaccination: 1) HPV vaccine initiation: receipt of at least one dose of HPV vaccine; 2) HPV vaccine completion: receipt of at least three doses of HPV vaccine; and 3) HPV vaccine follow-through: vaccine completion among only those females who initiated the vaccine regimen. Females who had not initiated the vaccine regimen were excluded when examining HPV vaccine follow-through. All HPV vaccination outcomes were based on provider-verified vaccination records.
We determined whether adolescent females lived in the Appalachian region (yes or no) for our main independent variable. Appalachian residence was determined using each adolescent female’s county of residence and the Appalachian Regional Commission’s classification scheme (38). We used the most recent classification scheme, which was established in 2008. For adolescent females living within Appalachia, we also determined which Appalachian subregion they resided in (Northern, North Central, Central, South Central, or Southern (14)) based on their current county of residence. These subregions allowed us to examine potential differences in HPV vaccination outcomes across Appalachia. County of residence is not available in the public use NIS-Teen datasets, so these restricted data were accessed through the Research Data Center in order to establish Appalachian residence.
Parents provided data on various demographic characteristics during the survey (Table 1). This included information on the daughter (age and race), mother (age, education level, and marital status), and household (income and location). If someone other than the mother completed the parent survey, they were asked to provide data on the mother’s age, education level, and marital status. Parents also provided data on health-related characteristics, including whether their daughters had visited a healthcare provider in the last year and their daughters’ current healthcare coverage. Parents indicated if they had ever heard of HPV and HPV vaccine and if they had ever received a HPV vaccine recommendation from a healthcare provider for their daughters.
Table 1.
Characteristics of parents and adolescent daughters, 2008–2010 National Immunization Survey-Teen
| Appalachian n=1951 | Non-Appalachian n=25468 | p | |
|---|---|---|---|
| n (weighted %) | n (weighted %) | ||
| Year | |||
| 2008 | 667 (32.9) | 7928 (33.9) | |
| 2009 | 630 (34.9) | 8981 (33.2) | |
| 2010 | 654 (32.2) | 8559 (32.9) | |
| Daughter characteristics | |||
| Age | |||
| 13 yr | 424 (20.7) | 4975 (19.0) | |
| 14 yr | 382 (18.7) | 5325 (20.1) | |
| 15 yr | 401 (20.7) | 5214 (21.5) | |
| 16 yr | 393 (19.8) | 5245 (20.4) | |
| 17 yr | 351 (20.1) | 4709 (19.1) | |
| Race/ethnicity | ** | ||
| White, non-Hispanic | 1664 (80.6) | 17419 (58.0) | |
| Black, non-Hispanic | 163 (12.1) | 2750 (15.4) | |
| Other, non-Hispanic | 70 (3.9) | 2030 (7.4) | |
| Hispanic | 54 (3.4) | 3269 (19.1) | |
| Visited healthcare provider in last year | |||
| No | 244 (14.6) | 3656 (16.1) | |
| Yes | 1699 (85.4) | 21640 (83.9) | |
| Healthcare coverage | |||
| Through parent employer or union | 1296 (63.3) | 17708 (63.9) | |
| Other insurance | 559 (31.3) | 6247 (28.8) | |
| No insurance | 93 (5.4) | 1377 (7.3) | |
| Parent characteristics | |||
| Mother’s agea | ** | ||
| <35 yr | 179 (9.9) | 1821 (8.5) | |
| 35–44 yr | 957 (50.7) | 10769 (45.7) | |
| 45+ yr | 815 (39.4) | 12878 (45.9) | |
| Mother’s educationa | ** | ||
| Less than high school | 199 (11.1) | 2343 (13.8) | |
| High school | 485 (33.2) | 5054 (26.5) | |
| Some college | 597 (27.0) | 7509 (25.9) | |
| College graduate | 670 (28.7) | 10562 (33.8) | |
| Mother’s marital statusa | |||
| Married | 1457 (74.3) | 19331 (73.0) | |
| Other | 494 (25.7) | 6137 (27.0) | |
| Heard of HPV | |||
| No | 304 (16.4) | 3203 (15.6) | |
| Yes | 1623 (83.6) | 21832 (84.4) | |
| Heard of HPV vaccine | ** | ||
| No | 101 (5.0) | 1435 (8.9) | |
| Yes | 1835 (95.0) | 23764 (91.1) | |
| Received provider recommendation to get daughter HPV vaccine | |||
| No | 856 (43.8) | 10584 (46.6) | |
| Yes | 1045 (56.2) | 14095 (53.4) | |
| Household characteristics | |||
| Poverty status | ** | ||
| Below poverty | 309 (18.9) | 3189 (18.3) | |
| Above poverty, ≤$75,000 | 908 (50.7) | 10170 (42.0) | |
| Above poverty, >$75,000 | 658 (30.4) | 11084 (39.8) | |
| Urbanicity | ** | ||
| Non-MSA | 775 (36.9) | 5765 (14.7) | |
| MSA, non-central city | 723 (38.8) | 9569 (46.3) | |
| MSA, central city | 453 (24.3) | 10134 (39.1) | |
| Appalachian subregion | |||
| Northern | 343 (32.3) | -- | |
| North Central | 402 (10.1) | -- | |
| Central | 200 (8.0) | -- | |
| South Central | 309 (19.0) | -- | |
| Southern | 696 (30.7) | -- |
Note. Totals may not sum to stated sample size due to missing data. Percents may not sum to 100% due to rounding.
HPV=human papillomavirus; MSA=metropolitan statistical area.
If someone other than the mother completed the parent survey, they were asked to provide data on the mother’s age, education level, and marital status
p<0.05,
p<0.001
Data Analysis
We used chi-square tests and logistic regression models to determine if demographic and health-related characteristics differed by: 1) Appalachian residence; and 2) Appalachian subregion. We calculated HPV vaccination estimates using data for all three years combined and for each year separately in order to examine potential temporal trends. We used logistic regression models to compare Appalachian and non-Appalachian females on HPV vaccination outcomes. For all data years combined, we constructed a multivariate model for each HPV vaccination outcome that controlled for year of data collection and all demographic and health-related characteristics. These multivariate models produced adjusted odds ratios (ORs) and 95% confidence intervals (CIs). We also used logistic regression models to determine how the different Appalachian subregions compared to the rest of the U.S. in terms of HPV vaccination outcomes. Non-Appalachian U.S. served as the referent group in making these comparisons, and these logistic regression models controlled for year of data collection. Analyses applied sampling weights and accounted for the complex design of the NIS-Teen (39). Frequencies are not weighted. Statistical tests using SAS Version 9.2 (Cary, NC) were two-tailed with a critical alpha of 0.05.
Results
Participant Characteristics
A total of 1,951 (7.5%) adolescent females were classified as living in the Appalachian region. A higher proportion of adolescent females from Appalachia were non-Hispanic white compared to non-Appalachian females (80.6% vs. 58.0%, p<0.001) (Table 1). Appalachian mothers were less likely to be age 45 or older (39.4% vs. 45.9%) or have a college degree (28.7% vs. 33.8%) than non-Appalachian mothers (both p<0.001). Appalachian parents were more likely to reside in a non-metropolitan statistical area (MSA) (36.9% vs. 14.7%) than non-Appalachian parents, and fewer reported household incomes greater than $75,000 (30.4% vs. 39.8%) (both p<0.001). HPV awareness was similar between Appalachian and non-Appalachian parents (83.6% vs. 84.4%, p>0.05), though Appalachian parents were more likely to report having heard of HPV vaccine (95.0% vs. 91.1%, p<0.001). The two groups did not differ in terms of having received a healthcare provider recommendation to get their daughters HPV vaccine (Appalachian=56.2% vs. non-Appalachian=53.4%, p>0.05).
Among Appalachian residents, 32.3% lived in the Northern subregion, 10.1% in the North Central subregion, 8.0% in the Central subregion, 19.0% in the South Central subregion, and 30.7% in the Southern subregion. The Southern subregion was more racially diverse, while more residents from the Central subregion had lower socioeconomic status and lived in a non-MSA (all p<0.05; Table 2). Mothers from the Central and Southern subregions tended to be younger, while fewer parents from these subregions had received a healthcare provider recommendation to get their daughters HPV vaccine (both p<0.05).
Table 2.
Characteristics of parents and adolescent daughters by Appalachian subregion, 2008–2010 National Immunization Survey-Teen
| Northern n=343 | North Central n=402 | Central n=200 | South Central n=309 | Southern n=696 | p | |
|---|---|---|---|---|---|---|
| n (weighted %) | n (weighted %) | n (weighted %) | n (weighted %) | n (weighted %) | ||
| Daughter characteristics | ||||||
| Age | ||||||
| 13 yr | 78 (21.8) | 84 (15.9) | 47 (22.2) | 60 (19.9) | 155 (21.3) | |
| 14 yr | 66 (16.5) | 82 (19.1) | 34 (20.0) | 58 (18.9) | 142 (20.4) | |
| 15 yr | 73 (20.8) | 67 (20.5) | 47 (22.1) | 57 (17.7) | 156 (22.0) | |
| 16 yr | 63 (19.4) | 95 (21.4) | 37 (17.8) | 66 (21.0) | 132 (19.5) | |
| 17 yr | 63 (21.4) | 74 (23.1) | 35 (17.8) | 68 (22.5) | 111 (16.9) | |
| Race/ethnicitya | ** | |||||
| White, non-Hispanic | 313 (87.9) | 367 (86.3) | 193 (95.6) | 272 (83.4) | 518 (65.5) | |
| Other | 30 (12.1) | 35 (13.7) | 7 (4.4) | 37 (16.6) | 178 (34.5) | |
| Visited healthcare provider in last year | ||||||
| No | 40 (12.0) | 43 (11.6) | 26 (13.8) | 43 (16.1) | 92 (17.8) | |
| Yes | 303 (88.0) | 357 (88.4) | 173 (86.2) | 266 (83.9) | 599 (82.2) | |
| Healthcare coverage | * | |||||
| Through parent employer or union | 238 (65.8) | 276 (68.2) | 110 (51.3) | 201 (59.6) | 470 (64.3) | |
| Other insurance | 93 (30.6) | 100 (25.7) | 82 (43.5) | 96 (35.4) | 188 (28.2) | |
| No insurance | 12 (3.6) | 25 (6.1) | 8 (5.2) | 11 (4.9) | 37 (7.5) | |
| Parent characteristics | ||||||
| Mother’s ageb | * | |||||
| <35 yr | 24 (7.0) | 38 (9.1) | 26 (13.7) | 14 (5.8) | 77 (14.7) | |
| 35–44 yr | 166 (49.0) | 198 (53.7) | 116 (59.8) | 152 (52.7) | 324 (47.8) | |
| 45+ yr | 153 (44.0) | 166 (37.2) | 58 (26.5) | 143 (41.5) | 295 (37.5) | |
| Mother’s educationb | * | |||||
| Less than high school | 22 (8.0) | 29 (12.6) | 38 (23.6) | 31 (9.1) | 78 (11.6) | |
| High school | 86 (36.2) | 123 (36.3) | 57 (32.7) | 78 (34.1) | 141 (28.6) | |
| Some college | 111 (26.6) | 114 (27.1) | 66 (27.8) | 84 (25.5) | 222 (28.2) | |
| College graduate | 124 (29.2) | 136 (24.0) | 39 (15.9) | 116 (31.3) | 255 (31.5) | |
| Mother’s marital statusb | ||||||
| Married | 257 (75.7) | 300 (77.4) | 152 (75.6) | 241 (76.7) | 506 (69.9) | |
| Other | 86 (24.3) | 102 (22.6) | 48 (24.4) | 68 (23.3) | 190 (30.1) | |
| Heard of HPV | * | |||||
| No | 45 (12.6) | 50 (12.6) | 38 (18.9) | 65 (23.6) | 106 (16.5) | |
| Yes | 294 (87.4) | 348 (87.4) | 160 (81.1) | 240 (76.4) | 580 (83.5) | |
| Heard of HPV vaccine | ||||||
| No | 11 (3.9) | 21 (5.2) | 13 (7.8) | 15 (6.9) | 41 (5.2) | |
| Yes | 330 (97.1) | 379 (94.8) | 186 (92.2) | 291 (93.1) | 648 (94.8) | |
| Received provider recommendation to get daughter HPV vaccine | * | |||||
| No | 128 (36.4) | 165 (40.9) | 90 (49.7) | 135 (45.1) | 338 (50.2) | |
| Yes | 208 (63.6) | 228 (59.1) | 105 (50.3) | 164 (54.9) | 339 (49.7) | |
| Household characteristics | ||||||
| Poverty status | ** | |||||
| Below poverty | 40 (15.5) | 66 (21.4) | 51 (31.6) | 37 (16.3) | 115 (19.6) | |
| Above poverty, <$75,000 | 177 (56.3) | 210 (49.2) | 99 (50.8) | 139 (51.6) | 283 (44.8) | |
| Above poverty, >$75,000 | 107 (28.2) | 122 (29.4) | 46 (17.6) | 114 (32.1) | 269 (35.5) | |
| Urbanicity | ** | |||||
| Non-MSA | 101 (32.7) | 200 (54.6) | 151 (78.6) | 111 (39.3) | 211 (23.1) | |
| MSA, non-central city | 144 (38.9) | 112 (30.1) | 40 (17.0) | 94 (28.5) | 333 (53.7) | |
| MSA, central city | 98 (28.3) | 90 (15.3) | 9 (4.4) | 104 (32.2) | 152 (23.3) |
Note. Totals may not sum to stated sample size due to missing data. Percents may not sum to 100% due to rounding. Subregion could not be determined for one adolescent female living in Appalachia.
HPV=human papillomavirus; MSA=metropolitan statistical area.
Daughter’s race/ethnicity was dichotomized due to the small number of non-white participants
If someone other than the mother completed the parent survey, they were asked to provide data on the mother’s age, education level, and marital status
p<0.05,
p<0.001
HPV Vaccination
For 2008–2010 combined, fewer Appalachian adolescent females initiated the HPV vaccine regimen compared to non-Appalachian females (40.8% vs. 43.6%)(Table 3). HPV vaccine completion (27.7% vs. 25.3%) and follow-through (67.8% vs. 58.1%) were, however, higher among Appalachian females. In multivariate analyses, Appalachian and non-Appalachian females did not differ in terms of HPV vaccine initiation (OR=0.92, 95% CI: 0.79–1.07) or completion (OR=1.12, 95% CI: 0.95–1.32). Appalachian females did have higher HPV vaccine follow-through (OR=1.36, 95% CI: 1.07–1.72), even after controlling for demographic and health-related characteristics.
Table 3.
HPV vaccination among adolescent females, 2008–2010 National Immunization Survey-Teen
| HPV Vaccine Initiation | HPV Vaccine Completion | HPV Vaccine Follow-Througha | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weighted % | Bivariate OR (95% CI) | Multivariate ORb (95% CI) | Weighted % | Bivariate OR (95% CI) | Multivariate ORb (95% CI) | Weighted % | Bivariate OR (95% CI) | Multivariate ORb (95% CI) | |
| Appalachia | 40.8 | 0.89 (0.78–1.02) | 0.92 (0.79–1.07) | 27.7 | 1.13 (0.97–1.31) | 1.12 (0.95–1.32) | 67.8 | 1.53 (1.24–1.88)** | 1.36 (1.07–1.72)* |
| Non-Appalachia | 43.6 | ref. | ref. | 25.3 | ref. | ref. | 58.1 | ref. | ref. |
Note. Due to missing data, final models included n=25,106 for HPV vaccine initiation and completion and 11,140 for HPV vaccine follow-through.
HPV = human papillomavirus, OR = odds ratio, CI = confidence interval, ref. = referent group.
HPV vaccine completion among only those adolescent females who initiated the HPV vaccine regimen
Adjusted for year of data collection, daughter’s age, daughter’s race/ethnicity, whether daughters had visited a healthcare provider in the last year, daughter’s healthcare coverage, mother’s age, mother’s education, mother’s marital status, parent’s HPV awareness, parent’s HPV vaccine awareness, whether parent had ever received a healthcare provider recommendation to get daughter HPV vaccine, poverty status, and urbanicity
p<0.05,
p<0.001
Temporal Trends
HPV vaccination patterns were fairly consistent over time when comparing Appalachian and non-Appalachian females (Figure 2). HPV vaccine initiation has increased among both Appalachian (from 32.4% in 2008 to 45.4% in 2010) and non-Appalachian (from 37.6% in 2008 to 49.0% in 2010) females, and the two groups did not differ for any of the years examined (all p>0.05). Appalachian females had slightly higher HPV vaccine completion for each year, though none of the differences reached statistical significance (all p>0.05). Similar to initiation, HPV vaccine completion has increased among both Appalachian (from 19.2% in 2008 to 34.5% in 2010) and non-Appalachian (from 17.8% in 2008 to 31.8% in 2010) females. Appalachian females had consistently higher HPV vaccine follow-through, with differences reaching statistical significance for both 2008 (59.4% vs. 47.3%) and 2010 (76.1% vs. 64.9%)(both p<0.05).
Figure 2.
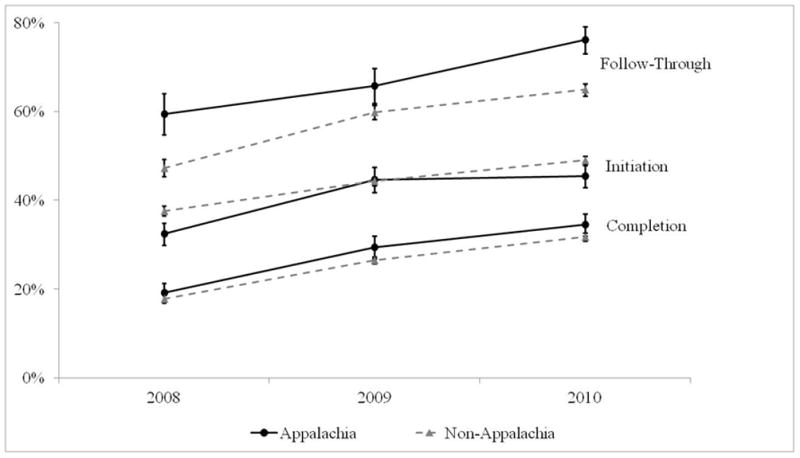
HPV vaccination by year, 2008–2010 National Immunization Survey-Teen. Error bars indicate standard errors.
Subregions
HPV vaccine coverage varied greatly by Appalachian subregion, with initiation among adolescent females ranging from 28.7% (Central) to 50.0% (Northern), completion ranging from 15.6% (Central) to 37.8% (Northern), and follow-through ranging from 54.5% (Central) to 76.4% (South Central)(Table 4). HPV vaccine initiation among females was lower in the Central (OR=0.51, 95% CI: 0.36–0.73) and Southern (OR=0.72, 95% CI: 0.59–0.87) subregions compared to non-Appalachian U.S. HPV vaccine completion was also lower among females from these two subregions (Central: OR=0.53, 95% CI: 0.35–0.82; Southern: OR=0.71, 95% CI: 0.57–0.89), while completion was higher among females from the Northern subregion (OR=1.80, 95% CI: 1.36–2.38). HPV vaccine follow-through was higher among adolescent females from the Northern (OR=2.29, 95% CI: 1.52–3.45) and South Central (OR=2.26, 95% CI: 1.36–3.74) subregions compared to those from non-Appalachian U.S.
Table 4.
HPV vaccination by Appalachian subregion, 2008–2010 National Immunization Survey-Teen
| HPV Vaccine Initiation | HPV Vaccine Completion | HPV Vaccine Follow-Througha | ||||
|---|---|---|---|---|---|---|
| Weighted % | ORb (95% CI) | Weighted % | ORb (95% CI) | Weighted % | ORb (95% CI) | |
| Non-Appalachia | 43.6 | ref. | 25.3 | ref. | 58.1 | ref. |
| Subregion | ||||||
| Northern | 50.0 | 1.29 (0.99–1.68) | 37.8 | 1.80 (1.36–2.38)** | 75.7 | 2.29 (1.52–3.45)** |
| North Central | 39.0 | 0.84 (0.59–1.20) | 24.6 | 0.99 (0.64–1.54) | 63.1 | 1.34 (0.75–2.41) |
| Central | 28.7 | 0.51 (0.36–0.73)** | 15.6 | 0.53 (0.35–0.82)* | 54.5 | 0.84 (0.47–1.52) |
| South Central | 39.6 | 0.85 (0.64–1.11) | 30.2 | 1.28 (0.96–1.72) | 76.4 | 2.26 (1.36–3.74)* |
| Southern | 35.6 | 0.72 (0.59–0.87)** | 19.5 | 0.71 (0.57–0.89)* | 54.9 | 0.84 (0.61–1.15) |
Note. Final models included n=27,418 for HPV vaccine initiation and completion and 12,132 for HPV vaccine follow-through. Subregion could not be determined for one adolescent female living in Appalachia.
HPV = human papillomavirus, OR = odds ratio, CI = confidence interval, ref. = referent group.
HPV vaccine completion among only those adolescent females who initiated the HPV vaccine regimen
Adjusted for year of data collection
p<0.05,
p<0.001
Discussion
Appalachia is a geographic region with high cervical cancer incidence and mortality rates (19–24), yet little is known about HPV vaccination in this region. In this descriptive study, we analyzed 2008–2010 data from the NIS-Teen to provide the most comprehensive examination to date on HPV vaccine coverage among adolescent females living in Appalachia. In general, HPV vaccine coverage was mostly similar among adolescent females from Appalachia compared to those from non-Appalachia U.S. Neither HPV vaccine initiation nor completion differed between the two groups. Appalachian females did, however, have higher HPV vaccine follow-through, with almost 70% of vaccine initiators completing the three-dose regimen. The difference in HPV vaccine follow-through persisted even after controlling for variables previously shown to be associated with this outcome (e.g., race (40, 41)).
The reason for the difference in follow-through is not clear but is encouraging given the high cervical cancer incidence and mortality rates in Appalachia (19–24). It is possible that these Appalachian parents had greater knowledge about HPV vaccine and better understood that the vaccine regimen consists of three doses, though research conducted soon after vaccine licensure suggested that many Appalachian residents lack knowledge about the vaccine (30). Fewer than three HPV vaccine doses may still offer health benefits (42), but it is important that all females who initiate the vaccine regimen receive all three recommended doses. Future research is needed to identify why HPV vaccine follow-through is higher among Appalachian females. Furthermore, although our results showed vaccination patterns between Appalachian and non-Appalachian females to be pretty consistent over time, it will be important to continue to monitor these vaccination patterns moving forward.
HPV vaccine coverage varied greatly across Appalachia. HPV vaccination outcomes tended to be higher in the Northern and South Central subregions and lower in the remaining subregions, particularly the Central and Southern subregions. In fact, the Central and Southern subregions had lower levels of both HPV vaccine initiation and completion compared to non-Appalachian U.S. Interestingly, the HPV vaccine disparities demonstrated by these analyses are very similar to existing cervical cancer disparities. Northern Appalachia (where HPV vaccine coverage was highest) has cervical cancer incidence rates similar to non-Appalachian U.S. and lower than other parts of Appalachia (17). Conversely, the more central and southern areas of Appalachia (where HPV vaccine coverage tended to be lower) have among the highest cervical cancer incidence and mortality rates in the U.S. (17, 21). Our results suggest that these existing cervical cancer disparities will persist, or may potentially worsen, with the current patterns of HPV vaccine coverage.
There are several factors that may be contributing to these HPV vaccine disparities in Central and Southern Appalachia. Access to care may be more of an issue for residents of Central and Southern Appalachia, as these areas have fewer healthcare providers and healthcare facilities compared to the Northern subregion (15, 16). For example, about 37% of counties in the Central subregion and 23% of counties in the Southern subregion rank below the 20th percentile for Health Care Resources Availability (HCRA), compared to only 8% of counties in the Northern subregion (15). Access to care is important since healthcare provider recommendation is one of the key determinants of HPV vaccine uptake among adolescent females (43, 44). Indeed, fewer parents from the Central and Southern subregions in our study had received a provider recommendation. The Central and Southern subregions may also differ in terms of political and religious views. These subregions consist of states in the “Bible Belt” of the U.S., many of which are among the most religious and politically conservative states in the country (45). Conservative political views and certain religious affiliations among parents (e.g., born-again or evangelical Christians) have been associated with lower HPV vaccine acceptability and uptake among adolescents (46–48). Lastly, these subregions tend to be more economically depressed, with many counties classified as “distressed” (i.e., the category for the most economically depressed counties)(49). Residents of the Central subregion, including those in our study, tend to have lower socioeconomic status compared to the other subregions (13), though findings concerning socioeconomic status and HPV vaccination have been inconsistent (50). There is an obvious need for public health programs to improve HPV vaccination among adolescent females living in the especially high-risk areas within Appalachia, and these factors (and potentially others) should be considered in designing and implementing such programs.
Study strengths include a large sample size, data on adolescent females throughout the U.S. including the entire Appalachian region, and HPV vaccination histories based on healthcare provider records. Our study also has a few limitations. The NIS-Teen identified potential participants through random-digit-dialing and is limited to households with landline telephones, though most U.S. households still have landlines (51). It is possible that provider vaccination records might be incomplete. The timing of HPV vaccine doses received was not included, so some adolescent females who initiated the HPV vaccine regimen may not have had adequate time to complete the three-dose regimen prior to data collection. Lastly, we did not have data on community-level or state-level factors that might influence HPV vaccination. Future research is needed to determine the potential effects of these factors on HPV vaccine coverage.
Appalachia as a whole is similar to the rest of the U.S. in terms of HPV vaccine initiation and completion, while HPV vaccine follow-through is higher among adolescent females from Appalachia. However, HPV vaccination is lagging in some regions within Appalachia where cervical cancer incidence and mortality rates are highest. These results suggest that current cervical cancer disparities among females in these regions of Appalachia will persist, or may potentially worsen, with the current patterns of HPV vaccine coverage. Public health efforts are needed to improve HPV vaccination among adolescent females living in the regions of Appalachia with low vaccine coverage.
Acknowledgments
We thank the Research Data Center for their help with this study. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Financial Support: Supported by the National Cancer Institute at the National Institutes of Health (P50CA105632 and P30CA016058).
Footnotes
Conflicts of Interest: PLR and EDP have received research grants from Merck Sharp & Dohme Corp., but neither has received honoraria or consulting fees from this company. These funds were not used to support this research study.
References
- 1.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among american youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132(2):277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 4.Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180(5):1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 5.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Suppl 3):S3/35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, National Health and Nutrition Examination Survey, 1999–2004. Sex Transm Dis. 2008;35(4):357–60. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–9. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [PubMed] [Google Scholar]
- 11.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 12.Pollard K, Jacobsen LA. The Appalachian region in 2010: A Census data overview. 2011 Available at: http://www.arc.gov/assets/research_reports/AppalachianRegion2010CensusReport1.pdf.
- 13.Pollard K, Jacobsen LA. The Appalachian region: A data overview from the 2006–2010 American Community Survey. 2012 Available at: http://www.arc.gov/assets/research_reports/PRB-DataOverview-2012.pdf.
- 14.Appalachian Regional Commission. Subregions in Appalachia. 2009 Available at: http://www.arc.gov/research/MapsofAppalachia.asp?MAP_ID=31.
- 15.PDA, Inc., and the Cecil B. Sheps Center for Health Services Research. Health care costs and access disparities in Appalachia. 2012 Available at: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=101.
- 16.Halverson JA. An analysis of disparities in health status and access to health care in the Appalachian region. 2004 Available at: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=82.
- 17.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001–2003. Cancer. 2008;112(1):181–92. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 18.Blackley D, Behringer B, Zheng S. Cancer mortality rates in Appalachia: Descriptive epidemiology and an approach to explaining differences in outcomes. J Community Health. 2012;37(4):804–813. doi: 10.1007/s10900-011-9514-z. [DOI] [PubMed] [Google Scholar]
- 19.Lengerich EJ, Tucker TC, Powell RK, Colsher P, Lehman E, Ward AJ, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: Disparities in Appalachia. J Rural Health. 2005;21(1):39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 20.Hall HI, Rogers JD, Weir HK, Miller DS, Uhler RJ. Breast and cervical carcinoma mortality among women in the Appalachian region of the U.S. 1976–1996. Cancer. 2000;89(7):1593–602. doi: 10.1002/1097-0142(20001001)89:7<1593::aid-cncr25>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(4):591–9. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopenhayn C, King JB, Christian A, Huang B, Christian WJ. Variability of cervical cancer rates across 5 Appalachian states, 1998–2003. Cancer. 2008;113(10 Suppl):2974–80. doi: 10.1002/cncr.23749. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Cancer mortality maps and graphs. 2009 Available at: http://ratecalc.cancer.gov/ratecalc//index.html.
- 24.Appalachia Community Cancer Network. Addressing the cancer burden in Appalachian communities, 2010. 2010. [Google Scholar]
- 25.Reiter PL, Fisher JL, Hudson AG, Tucker TC, Plascak JJ, Paskett ED. Assessing the burden of HPV-related cancers in Appalachia. Hum Vaccin Immunother. doi: 10.4161/hv.22389. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz ML, Reiter PL, Kluhsman BC, Kennedy S, Dwyer S, Schoenberg N, et al. Human papillomavirus (HPV) vaccine availability, recommendations, cost, and policies among health departments in seven Appalachian states. Vaccine. 2009;27(24):3195–200. doi: 10.1016/j.vaccine.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huey NL, Clark AD, Kluhsman BC, Lengerich EJ ACTION Health Cancer Task Force. HPV vaccine attitudes and practices among primary care providers in Appalachian Pennsylvania. Prev Chronic Dis. 2009;6(2):A49. [PMC free article] [PubMed] [Google Scholar]
- 28.Christian WJ, Christian A, Hopenhayn C. Acceptance of the HPV vaccine for adolescent girls: Analysis of state-added questions from the BRFSS. J Adolesc Health. 2009;44(5):437–45. doi: 10.1016/j.jadohealth.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Hopenhayn C, Christian A, Christian WJ, Schoenberg NE. Human papillomavirus vaccine: Knowledge and attitudes in two Appalachian Kentucky counties. Cancer Causes Control. 2007;18(6):627–34. doi: 10.1007/s10552-007-9007-7. [DOI] [PubMed] [Google Scholar]
- 30.Katz ML, Reiter PL, Heaner S, Ruffin MT, Post DM, Paskett ED. Acceptance of the HPV vaccine among women, parents, community leaders, and healthcare providers in Ohio Appalachia. Vaccine. 2009;27(30):3945–52. doi: 10.1016/j.vaccine.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosby RA, Casey BR, Vanderpool R, Collins T, Moore GR. Uptake of free HPV vaccination among young women: A comparison of rural versus urban rates. J Rural Health. 2011;27(4):380–4. doi: 10.1111/j.1748-0361.2010.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderpool RC, Casey BR, Crosby RA. HPV-related risk perceptions and HPV vaccine uptake among a sample of young rural women. J Community Health. 2011;36(6):903–9. doi: 10.1007/s10900-010-9345-3. [DOI] [PubMed] [Google Scholar]
- 33.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: The National Immunization Survey-Teen 2006. Public Health Rep. 2009;124(5):642–51. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) National, state, and local area vaccination coverage among adolescents aged 13–17 years--United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(36):997–1001. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–23. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years --- United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–23. [PubMed] [Google Scholar]
- 38.Appalachian Regional Commission. Counties in Appalachia. 2012 Available at: http://www.arc.gov/counties.
- 39.Centers for Disease Control and Prevention. National Immunization Survey-Teen. A user’s guide for the 2010 public-use data file. 2011 Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISteenPUF10_DUG.pdf.
- 40.Niccolai LM, Mehta NR, Hadler JL. Racial/Ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med. 2011;41(4):428–33. doi: 10.1016/j.amepre.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Chou B, Krill LS, Horton BB, Barat CE, Trimble CL. Disparities in human papillomavirus vaccine completion among vaccine initiators. Obstet Gynecol. 2011;118(1):14–20. doi: 10.1097/AOG.0b013e318220ebf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69(3):475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19(8):531–8. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallup, Inc. Gallup poll. 2012 Available at: http://www.gallup.com/home.aspx?ref=logo.
- 46.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: A representative statewide analysis. J Adolesc Health. 2007;40(2):108–15. doi: 10.1016/j.jadohealth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Reiter PL, McRee AL, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29(34):5595–602. doi: 10.1016/j.vaccine.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appalachian Regional Commission. County economic status in Appalachia, FY 2013. 2012 Available at: http://www.arc.gov/research/MapsofAppalachia.asp?MAP_ID=64.
- 50.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30(24):3546–56. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 51.Blumberg SJ, Luke JV. Wireless substitution: Early release of estimates from the National Health Interview Survey, January-June 2011. 2011 Available at: http://www.cdc.gov/nchs/data/nhis/earlyrelease/wireless201112.pdf.


