Abstract
Rationale
The density of native (pre-existing) collaterals varies widely and is a significant determinant of variation in severity of stroke, myocardial infarction and peripheral artery disease. However, little is known about mechanisms responsible for formation of the collateral circulation in healthy tissues.
Objective
We previously found that variation in VEGF expression causes differences in collateral density of newborn and adult mice. Herein, we sought to determine mechanisms of collaterogenesis in the embryo and the role of VEGF in this process.
Methods and Results
Pial collaterals begin forming between embryonic day (E) 13.5 and 14.5 as sprout-like extensions from arterioles of existing cerebral artery trees. Global VEGF-A overexpressing mice (Vegf hi/+) formed more—and Vegf lo/+ formed fewer—collaterals during embryogenesis, in association with differences in vascular patterning. Conditional global reduction of Vegf or Flk1 only during collaterogenesis significantly reduced collateral formation, but now without affecting vascular patterning, and the effects remained in adulthood. Endothelial-specific Vegf reduction had no effect on collaterogenesis. Endothelial-specific reduction of a disintegrin-and-metalloprotease-domain-10 (Adam10) and inhibition of γ-secretase increased collateral formation, consistent with their roles in VEGF-induced Notch1 activation and suppression of “pro-sprouting” signals. Endothelial-specific knockdown of Adam17 reduced collateral formation, consistent with its roles in endothelial cell migration and embryonic vascular stabilization, but not in activation of ligand-bound Notch1. These effects also remained in adulthood.
Conclusions
Formation of pial collaterals occurs during a narrow developmental window via a sprouting angiogenesis-like mechanism, requires paracrine VEGF-stimulation of Flk1-Notch signaling, and adult collateral number is dependent on embryonic collaterogenesis.
Keywords: collateral, angiogenesis, VEGF, ADAM, embryo
INTRODUCTION
Mounting evidence supports the concept that the extent (number and diameter) of native (pre-existing) collaterals in the microcirculation is a primary determinant of the severity of ischemic tissue damage after vascular obstruction in humans1-4 and mice.5,6 Collaterals, which are arteriole-to-arteriole anastomoses that interconnect adjacent arterial trees, are present in most tissues. They provide an alternative route around a vascular obstruction by allowing retrograde perfusion of the obstructed tree. In the cerebral cortex, collaterals are confined to the pia mater (“leptomeningeal” collaterals) where they cross-connect a small number of the distal-most arterioles of the middle (MCA), anterior (ACA) and posterior (PCA) cerebral artery trees.3, 5-7 These “pial” collaterals are thus major determinants of the volume of infarcted brain and ischemic penumbra after acute thromboembolic stroke1-4. Direct methods in mouse and indirect approaches in humans have indicated that native collateral extent in brain, heart and skeletal muscle varies widely among healthy individuals.1, 2, 4-7 Moreover, collateral extent in mice declines with aging.8 Whereas the mechanisms underlying anatomical lumen enlargement of collaterals after vascular obstruction (remodeling, arteriogenesis) have received considerable attention,9,10 very little is known about the mechanisms directing formation of the native collateral circulation in healthy tissues.
We previously found, using global (ie, non-cell- and time-specific) VEGF-A hyper (Vegf hi/+) and hypomorphic (Vegf lo/+) mice, that collateral number in the newborn (P1) and adult brain and skeletal muscle correlates with VEGF production and severity of ischemic injury after obstruction of the MCA or femoral artery respectively.7 These findings indicate that VEGF is involved in collateral formation in the embryo (collaterogenesis). However, since embryonic vascular development and conditional and cell-specific genetic models were not examined, and birth (“P1”) can occur between 18 and 21 days post coitum, precisely when and how VEGF affects collaterogenesis remained unknown. Furthermore, it is unclear whether the observed differences in collateral density at P1 reflect a specific role of VEGF in collateral formation or a secondary effect due to VEGF’s known role in growth and branching morphogenesis of the general arterial-venous circulation. Previously, Chalothorn and coworkers examined embryonic collaterogenesis in two mouse strains, C57BL/6 and BALB/c, with large differences in collateral extent in the adult.11 Different numbers of collaterals formed in utero, resulting in the same relative differences in the adult. Analysis of expression in the pia and underlying cortical tissue found that Vegf-a expression was higher in the C57BL/6 strain (which form abundant collaterals) than in BALB/c mice. These findings suggest that VEGF plays a specific role in collaterogenesis, and that differences in its expression may underlie variation among individuals in native collateral extent. However, the associative nature of these findings, inherent differences in genomic expression patterns between the two mouse strains, as well as the different cell populations present in the sampled tissue, limit these conclusions.
In the present study, using global, conditional and cell-specific manipulation of expression, we demonstrate that VEGF-Notch signaling regulates pial collateral formation during a narrow developmental window by a unique arteriole-specific sprouting angiogenesis-like process. We further show that the Notch sheddases, a disintigrin and metalloprotease (ADAM) 10 and 17, have opposing roles in collateral formation. Lastly, we show that disturbance in collaterogenesis in the embryo results in life-long collateral deficiency. These findings are not only of fundamental importance to understanding collateral formation but they may also provide information needed to develop therapies to induce formation of new collaterals in adults with few of them or in individuals who have or are at risk for developing ischemic disease.
METHODS
An expanded Methods section is available in the online Data Supplement at http://circres.ahajournals.org.
Animals
Mice that underexpress or overexpress VEGF-A (Vegf lo/+, Vegf hi/+, respectively) were maintained on a CD1 background. Global inducible knockdown of VEGF-A (Vegf iΔ) or Flk1 (Flk1iΔ) were created by crossing inducible B6.CAG-Cre positive mice to Vegf fl/fl or Flk1fl/fl mice. Conditional endothelial-specific (EC) VEGF knockdown was accomplished with Tie2-ERT2 or Cdh5(PAC)-ERT2 driver lines. Conditional EC knockdown of Adam10 (Adam10iΔEC) or Adam17 (Adam17iΔEC) was achieved by crossing and backcrossing floxed females to Cdh5-ERT2 males. Tamoxifen-exposed pups were delivered by cesarean section, raised by foster dams, and aged to adulthood. A constitutive VE-Cadherin-Cre driver line was used to create a permanent EC knockdown of Adam17 (Adam17ΔEC) and Adam10 (Adam10ΔEC). All experiments compared Cre-positive mice to Cre-negative littermates. Online Table I lists Cre-positive-to-Cre-negative littermate ratios.
Treatment protocols
Cre activity was induced by injecting 2 mg/30g of 4-hydroxytamoxifen IP to dams on E12.5, E13.5 and E14.5 of gestation. Embryos were harvested on E16.5 unless otherwise stated. Gamma-secretase activity was inhibited by injecting 3 mg DAPT IP to dams on E13.5 and E14.5.
Immunohistochemistry
For vessel detection, staged embryo brains were fixed, rinsed, blocked and exposed to combined anti-eNOS and anti-Flk1 antibodies, followed by HRP-conjugated or fluorescein-labeled secondary antibody. Proliferation was detected with anti-phospho-histone H3.
Vascular morphometry
Collateral and capillary morphometrics were obtained digitally from 150x images of the vascular plexus in the collateral zone between the opposing crowns of the MCA and ACA trees. MCA, ACA, and PCA territories (areas) were obtained by drawing scalars between the ends of the distal-most arteriole branches of each tree and the tree’s trunk.5
Quantitative RT-PCR
RNA from whole tissue or sorted ECs was isolated using magnetic bead separation. The protocol separated a cell population enriched 15.5-fold higher for the EC marker VE-Cadherin (p>0.01) than the rest of the cell population. We designate this population the “EC fraction”. The EC and remaining fractions were prepared for qRT-PCR (Online Table II lists primer sequences).
Statistics
All values are expressed as mean±SE. Data were tested with independent t-tests, one-way ANOVA, or General Linear Model (significance at p<0.05).
RESULTS
Most pial collaterals form between E13.5 and E14.5 as an arteriole plexus
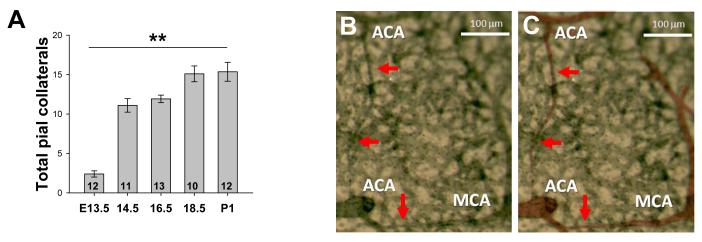
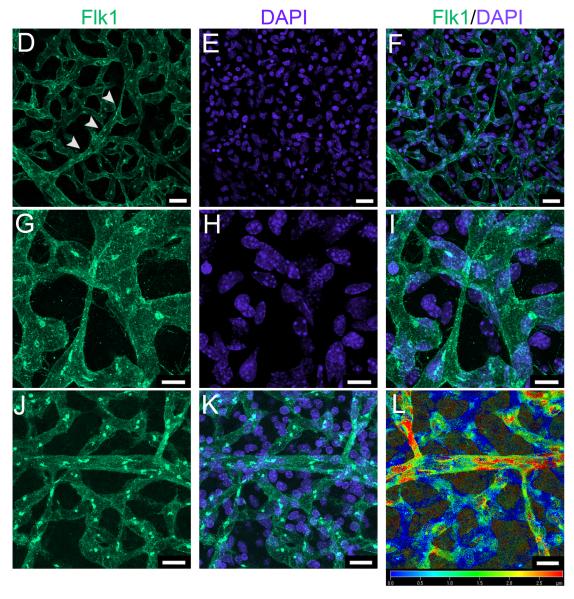
Collaterals interconnecting distal-most branches of the opposing ACA and MCA trees were rare at E13.5 but most of the postnatal population was present by E14.5 (Figure 1A). The number of collaterals was comparable in both hemispheres, in agreement with our previous studies5-7. Morphological features of these nascent vessels indicate that they form by a sprouting angiogenesis-like mechanism, rather than by intussusception or expansion of the diameter of certain capillaries present in the collateral zone. Whole mount immunohistochemistry and examination at low power showed that nascent collaterals course superficial to the pial capillary plexus and taper to the width of a single EC at the site of fusion (Figure 1B,C). Confocal microscopy of isolated pial membranes revealed arteriolar branches “led” by a single cell exhibiting a tip-cell phenotype (i.e. extending numerous filapodia in the direction of travel) (Figure 1D-I). Analysis of z-axis images confirmed that migrating branches and formed collaterals are always superficial to the pre-existing capillary plexus. Further, the collateral number increased—but the number of tapered collaterals decreased—from E14.5 to E18.5. N o intussusceptive-like pillar formation in nascent collaterals was observed using confocal or scanning electron microscopy (Online Figure I). Thus, we conclude that collaterals form by a novel type of EC sprouting from and fusion to pre-existing arterioles.
Figure 1. Most pial collaterals form between E13.5 and E14.5 from sprout-like extensions from established arterioles.
A, Time-course of collateral formation (total, all collaterals between ACA and MCA of both hemispheres of CD1 mice; **p<0.01; number of embryos from > 2 litters at base of bars). B, Collaterals form superficial to the pre-existing pial capillary plexus, are not yet tortuous, and taper as they extend from the parent vessel. C, Arterioles in B pseudocolored for clarity. D-F, Branches eminate from distal-most arterioles (arrowheads), course superficial to the plexus, and appear to be led by a tip cell. G-I, High magnification image of above tip cell. J-L. Depth coding on an image stack shows that collaterals form superficial to the plexus. Scalebars: A-C, J-L:20μm, D-F:10μm.
Differences in VEGF expression alter collateral formation during embryogenesis
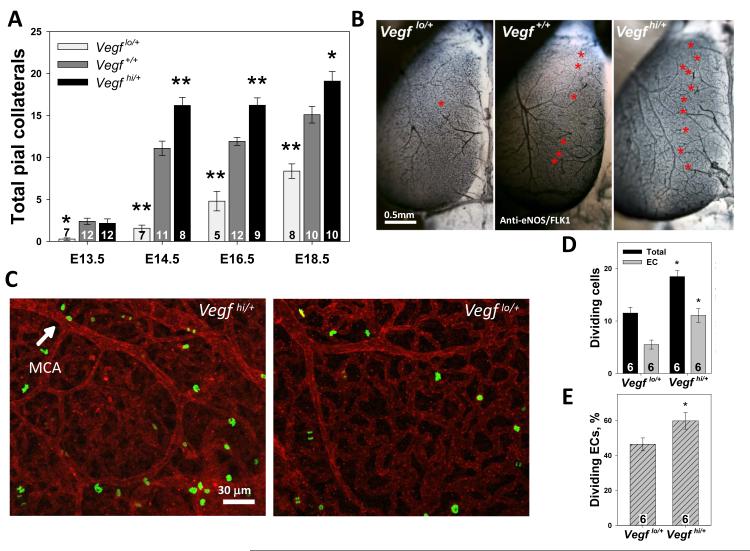
To determine the effect of changes in global VEGF-A (VEGF) expression on collaterogenesis, we quantified collaterals in mouse models functionally underexpressing (Vegflo/+) or overexpressing (Vegfhi/+) this gene. Homozygous mice of either genotype, which experience major disruptions in VEGF production, are embryonic-lethal.12, 13 Quantitative RT-PCR confirmed that Vegf was 43% reduced in isolated hypomorphic pial meninges and ~33% increased in the hypermorphs (p=0.01, ANOVA) as compared to controls. Almost no collaterals were observed at E13.5 in Vegflo/+embryos, while gene dose-dependent differences in number were evident thereafter (Figure 2A,B). Some but not all Vegflo/+ embryos were physically smaller than wildtype littermates or Vegfhi/+embryos at E14.5 and E16.5 (Online Figure IIA), as described previously.12 Not unexpectedly, collateral number correlated with brain size (and fetal maturation) within a given strain. However, collateral number was strongly dependent on VEGF expression and was independent of brain size (p>0.05) (Online Figure IIB). This indicates that VEGF-induced differences in collateral number arise from altered VEGF signaling rather than fetal size. Of note, neonates, juvenile, and adult Vegflo/+ and Vegfhi/+ mice were physically indistinguishable from WT littermates, and had similar lifespans and reproductive rates.
Figure 2. VEGF regulates pial collateral formation.
A, Vegf lo/+ embryos form fewer and Vegf hi/+ form more collaterals (*p<0.05, **p<0.01 vs WT). B, Asterisks denote individual collaterals. C, Representative immunolocalization of dividing cells (green) in the E14.5 vasculature (red). D, E, Total and endothelial cell (EC) proliferation in the MCA-ACA collateral zone is increased in E14.5 Vegf hi/+ brains.
We hypothesized that if collaterogenesis occurred by the addition of ECs to an existing plexus (i.e. sprout and branch formation from existing arterioles), then EC proliferation in the pial collateral zone should be increased in mouse strains having more collaterals. In contrast, if collaterals result from remodeling of the existing plexus, proliferation would be unchanged. If collaterals form via capillary pruning and arterialization of remaining arteriole-arteriole connections,14 proliferation would be lower. To discriminate among these possibilities, we examined cell proliferation in the MCA-ACA collateral zone during collaterogenesis (Figure 2,C). Total cell, EC-specific and percent EC proliferation were higher in Vegfhi/+ embryos compared to Vegflo/+ (Figure 2 D,E), consistent with VEGF’s role in inducing EC proliferation.ie,15, 16 These data suggest that collaterals form by the proliferation and addition of ECs to an arterial bed and not by rearrangement or loss of existing vessels.
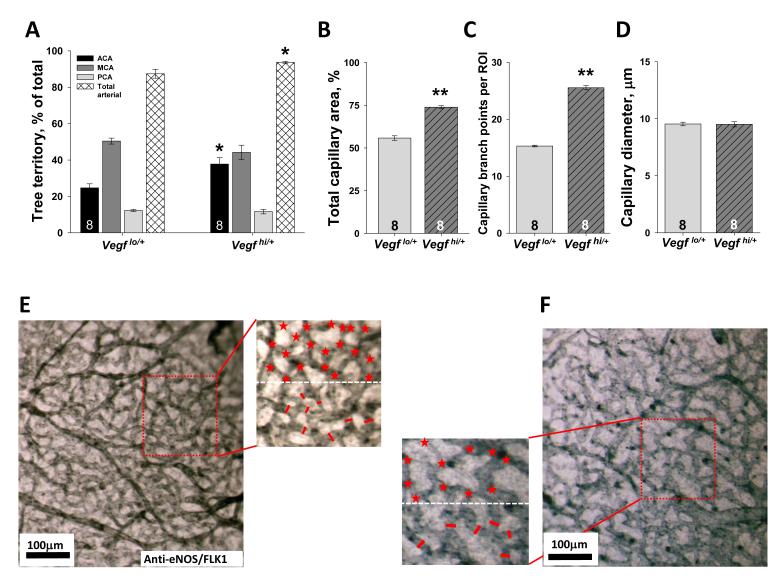
Differences in VEGF expression are known to affect the abundance and branching morphogenesis of the embryonic capillary plexus and arterial trees as they are remodeled out of this plexus. Because these differences could secondarily affect collateral formation, we examined tree size and morphometrics of the capillary plexus in the collateral zone at E14.5, when the majority of collaterals are forming. Global differences in VEGF expression caused differences in tree territory and area (overall vascular tissue/ECs) and branching of capillaries in the collateral zone (Figure 3). However, other data presented below demonstrate that these differences are not causal for differences in collateral formation.
Figure 3. VEGF regulates cerebral artery tree size and capillary density in E14.5 embryos.
A, VEGF regulates relative size of ACA tree territory (*p<0.05, **p<0.01 vs Vegflo/+). VEGF regulates capillary area (B) and branching (C) but not diameter (D). Data in B-D obtained from region of interests (ROI) in collateral zone, as shown in representative images, E, F, Magnified areas show quantification of capillary branch points and diameter (above and below white dotted line, respectively) per ROI.
To determine if Flk1-Notch signaling in endothelial cells was disturbed during collaterogenesis by modest changes in VEGF, we examined changes in Flk1-Notch pathway signaling in sorted endothelial cells (Figure 4). Vegf overexpression resulted in significant increases in signals downstream of VEGF (Flk1 and Nrp1), the Notch ligand dll4, the sheddases Adam10 and 17, and NICD-activated transcription of Hey1 and Hes1. Notch production was not affected. Similarly, expression of other endothelial proteins such as Pecam1, eNOS and Tie2 remained unchanged (Online Figure III). These results indicate that VEGF expression correlates with Notch activation, that compensatory mechanisms to normalize pathway signaling are not invoked and that Flk1-Notch activation participates in driving collateral formation. Ephrin B2 expression was increased in Vegfhi/+ ECs, suggesting an overall increased vascular arterialization as compared to Vegf lo/+.
Figure 4. Flk1-Notch pathway gene expression in endothelial cells isolated from mice differentially expressing VEGF.
Quantitative RT-PCR in E16.5 Vegfhi/+ (Vhi/+), control littermates (V+/+) and Vegflo/+ (Vlo/+) immunobead-sorted endothelial cells. At least 9 embryos from ≥2 litters were used (*p<0.05, **p<0.01).
Collaterogenesis depends on VEGF signaling during a narrow temporal window in mid-gestation
Given that the morphology of the general arterial-venous circulation correlates with modest differences in global VEGF levels (Figure 2), alterations in collateral formation could simply be secondary to stochastic events related to differences in early branch patterning. To determine if variation in VEGF signaling regulates collateral formation in an otherwise normal embryonic vascular bed, we used the tamoxifen-induced Cre-Lox system to achieve global reduction of Vegf and Flk1 expression specifically at the time that collaterals form but not earlier (Figure 5A). Quantitative RT-PCR homogenized lung lysate from each embryo showed that Vegf expression was reduced by 54% in Cre-positive embryos (VegfiΔ) as compared to Cre-negative littermates (Vegffl/fl(n=4, p<0.01), and Flk1 expression was reduced by 40% in Cre-positive embryos (Flk1iΔ) compared to Cre-negative littermates (Flk1fl/fl) (n=4, p<0.05). Incomplete knockdown was likely due the grouping of heterozygous and homozygous floxed embryos. To avoid potential complications of maternal origin, all dams were Cre-negative and were therefore unaffected by tamoxifen-induced gene deletion. Cre-negative littermates always served as controls.
Figure 5. Collaterogenesis, but not arterial tree formation or capillarity, is regulated by paracrine sources of VEGF during a narrow developmental window.
A, Experimental design. B, Tamoxifen-induced global knockdown of Vegf or its receptor, Flk1, impairs collateral formation in Cre-positive embryos (VegfiΔ) compared to Cre-negative littermates (Vegffl/fl) (*p<0.05). C, Arterial tree territories are similar between groups. D, Capillary plexus morphometrics are similar between groups. E, Two separate models of endothelial-specific tamoxifen-induced Vegf knockdown reveal no influence of autocrine VEGF signaling on collateral formation.
Similar to constitutive global manipulation of VEGF in Vegf hi/+ and Vegf lo/+ embryos, conditional global knockdown at the time of collaterogenesis reduced the number of collaterals formed (Figure 5B). Importantly, and in contrast to Vegf hi/+ and Vegf lo/+ embryos, Vegf iΔ and Vegf fl/fl littermates exhibited no differences in cerebral artery tree territories (Figure 5C), branch density or diameter of capillaries (Figure 5D). These findings demonstrate that collaterogenesis—and variation in it among individuals—depend on the spacio-temporal level of VEGF-dependent signaling and are not simply a function of programmed vascular branch patterning or differences in quantity of available vascular tissue.
To strengthen the link between collaterogenesis and temporally-specific VEGF signaling and to identify the responsible VEGF receptor type, we reduced Flk1 expression during the same time-frame as above (Figure 5A) in Cre-positive Flk1iΔ and Cre-negative Flk1fl/fl littermates. Collateral number was reduced in Flk1iΔbut not in mice heterozygous for Flt1 (VegfR1) (Online Figure IV), indicating that VEGF signaling via Flk1 drives collateral formation during a specific temporal window (Figure 5B).
Paracrine VEGF signaling mediates collaterogenesis
Autocrine and paracrine VEGF signaling pathways mediate different endothelial functions. In certain tissues, endothelial production of VEGF stimulates cell-autonomous signaling that is necessary for maintenance of normal vessel integrity.17 Paracrine sources of VEGF, which mediate other functions such as sprouting angiogenesis, cannot rescue dysfunction resulting from interrupting this loop. To determine whether autocrine or paracrine VEGF mediates collaterogenesis, we examined collaterogenesis in inducible endothelial-specific B6.Tie2-ERT2;Vegffl/fl and B6.Cdh5(PAC)-ERT2;Vegffl/fl mice. Tamoxifen injections and embryo harvest were performed as in Figure 5A. In contrast to global knockdown of VEGF, collateral formation was not impaired in either model of conditional endothelial-specific Vegf iΔEC mice (Figure 5E). Thus, paracrine VEGF signaling is required for collateral formation. These results are consistent with findings that autocrine VEGF signaling primarily functions in vessel maintenance rather than formation.17
Endothelial-specific ADAM10 and ADAM17 expression differentially affect collateral formation
Notch activity participates in VEGF-induced endothelial tip cell selection and sprout formation. Membrane-bound Notch is activated after cleavage steps release the intracellular domain (NICD), allowing it to transport to the nucleus and initiate gene transcription. While γ-secretase is believed to cleave NICD in the second cleavage step, numerous reports implicate ADAM10 and/or ADAM17 in the first.10 However, the role of each sheddase in this pathway is not completely clear and each has been known to cleave several molecules other than Notch.18 To investigate the role of Notch activation in collaterogenesis, we bred EC-specific Adam10 and Adam17 knockdown mice. Tamoxifen injections and harvest were performed as in Figure 5A. Quantitative RT-PCR confirmed a 15-fold reduction in Adam10 transcripts in sorted Cre-positive ECs (p<0.05), while Adam10 production remained unchanged in the remaining cell population (p=0.98), and a 6-fold reduction in EC-specific Adam17 production in Cre-positive ECs (p<0.01) without a change in the remaining cell types (p=0.75).
Knockdown of EC-specific Adam10 during embryonic collaterogenesis increased collateral number (Figure 6A). Knockdown of EC-specific Adam17 decreased collaterogenesis (Figure 6B). Similar to EC-specific Adam10 knockdown, pharmacological inhibition of γ-secretase activity with DAPT increased collaterogenesis (Figure 6C). These results indicate that Notch signaling through ADAM10 restrains collateral formation and support recent reports that Adam10 is required to cleave ligand-bound Notch prior to cleavage by γ-secretase.19 Further, these results support studies indicating that ADAM17 functions primarily in non-ligand-bound Notch signaling and may induce collaterogenesis through other mechanisms. To determine if constitutive alterations in endothelial Adam10 or 17 also permanently alters collaterogenesis, we bred a constitutive VE-Cadherin driven Cre mouse line to the floxed Adam lines. Sustained reduction of endothelial Adam10 (11-fold decrease, p<0.01) and 17 (8-fold reduction, p<0.01) increased and decreased, respectively, collateral number observed in adult mice (Figure 6D,E), with magnitudes similar to those seen in the embryo. Average collateral diameter was unaffected (Figure 6F).
Figure 6. Knockdown of endothelial Adam10 and Adam17 differentially affects collateral formation.
A,B, Same tamoxifen protocol as in Figure 4. Reduction in a disintegrin and metalloprotease (ADAM) 10 and 17 prior to collaterogenesis increases (A) and decreases (B) collateral formation. C, Inhibition of γ-secretase with N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester (DAPT) enhances collaterogenesis. Constitutive reduction of EC Adam10 (D) or Adam17 (E), beginning at conception, alters collateral number but not diameter (F) in adults.
Embryonic collaterogenesis specifies adult collateral number
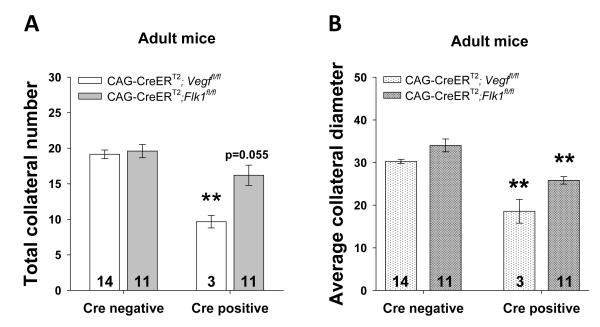
Since we established that VEGF-Notch signaling during a narrow developmental window affects collaterogenesis and that life-long knockdown of Adam10, Adam17 or VEGF7 cannot be compensated for, we hypothesized that specific patterns of VEGF-FLK1 signaling during embryonic collaterogenesis is necessary and sufficient to dictate adult collateral number. To investigate this, we induced Vegf and Flk1 deletion in embryos between E12.5 and E14.5 as described above and allowed the pups to grow. Pups were delivered and matured to 8 months of age without additional tamoxifen exposure. Cre-positive Vegfi adults had significantly fewer collaterals that were 62% smaller in diameter than the collaterals of Cre-negative Vegffl/fl littermates (Figure 7). Cre-positive Flk1iΔ adults tended to have fewer collaterals (p=0.055) with a smaller average collateral diameter. These data indicate that VEGF-determined adult collateral extent (number and diameter) is dependent on the number formed during a narrow window in embryogenesis, and cannot be recovered in adult life.
Figure 7. Temporal interruption of VEGF signaling during embryonic collaterogenesis permanently alters collateral number and diameter through adulthood.
Same tamoxifen protocol as in Figure 4 but pups were delivered and grown into adulthood. Reduced Vegf or Flk1 expression during embryonic collaterogenesis decreases collateral number (A) and diameter (B) in adults.
DISCUSSION
This is the first study to investigate when and how the native collateral circulation forms and the signaling pathways involved. Using global, cell-specific and conditional genetic approaches, we find that formation of pial collaterals occurs primarily during a narrow window of development between E13.5 and 14.5, via a unique angiogenesis-like mechanism that involves sprouting from pre-existing arterioles. This process is dependent on paracrine VEGF activation of an endothelial Flk1-ADAM10-Notch signaling pathway which when altered in the embryo results in permanent changes in collateral density in the adult.
Surprisingly, little is known about collaterogenesis. Early microscopy studies examined the cerebral vascular architecture in perinatal rats20, 21 and mice22 and described “ring-like” anastomoses connecting some of the distal intra- and inter-tree branches of the cerebral arteries. We recently reported on pial collateral formation in the high collateral (C57Bl/6) and low collateral (BALB/c) abundance mouse strains.11 Potential mechanisms regarding how collaterals arise have been proposed11, 14 but not investigated. In the present study, we found that most collaterals (~70%) form between E13.5 and E14.5 in both CD1 and C57BL/6 wildtype strains, with the rest forming before E18.5. The density of pial collaterals present in both strains at E14.5 and E16.5 was 3-fold higher than what we reported at E15.5 in C57BL/6 in our previous study.11 In that report, formation of the collateral circulation appeared to begin at ~E15.5. The difference may result from use of different detection systems. In the present study, collaterals were identified using antibodies to the endothelial markers Flk1 and eNOS, while the previous study relied on β-galactosidase activity detection in EphrinB2lacZ haplotype reporter mice. Expression of this arterial marker was robust in branches of the cerebral arterial trees and in the few collaterals that were observed at E15.5 interconnecting the trees. It is likely that collateral-forming ECs—which our present study implies sprout from distal arterioles, proliferate to form EC cords, migrate, fuse to an adjacent arteriole, and lumenized—require exposure to hemodynamic forces for induction of robust ephrin-B2 expression. ECs in emergent vessels that experience pulsatile forward blood or a plasma flow profile at physiologically relevant shear forces23-25 gain expression of ephrin-B2.26 This expression is lost after grafting into non-arterial vasculature, indicating that shear forces drive ephrin-B2 expression.27 Because collaterals link two opposing pressurized trees, it is likely that shear stress is very low in them, as it is in adult collaterals.11 Thus, reliance on ephrin-B2 expression may not detect all collaterals present.
Next we found that collaterogenesis depends on VEGF signaling in a gene-dose-dependent manner. Collaterogenesis was inhibited with global knockout of VEGF or EC-specific reduction of Flk1, but not with EC-specific VEGF, when gene knockdown was induced just prior to the onset of collateral formation. Interestingly, reduced collateral number remained in adulthood. Cre-mediated recombination indelibly alters Cre-expressing cells and their descendants, and high doses of tamoxifen (3 injections of 8mg/mouse) can induce recombination for weeks after administration.28 However, Cre-mediated excision is not 100% penetrant, and unaffected cells could give rise to a significant population of daughter cells functionally expressing the gene of interest. Further investigation is needed to determine whether the depressed collateral number that persists in our knockdown models reflect sustained gene excision in the targeted cells 8 months after cessation of tamoxifen injection or the lack of compensatory post-natal collaterogenic mechanisms.
A recent study provides intriguing support for our findings. Murine overexpression of human VEGF165 using a neuron-specific enolase promotor increased total cerebral vascular volume, yet cerebral blood flow at rest or during maximal dilation was not increased.29 Construction of network models based on these results and micro-CT segmentation analysis led the authors to suggest that VEGF overexpression caused formation and insertion into the arterial trees of “ineffective micro-networks”—shown schematically as arterial-to-arterial anastomoses, ie, collaterals.30 Interestingly, this same transgenic mouse exhibited significantly smaller infarcts and increased perfusion of the infarct zone after MCA occlusion.31 Unfortunately, the pial circulation was not selectively imaged or segmented in these studies. These results are consistent with our previous reports in adult mice showing that variation in VEGF expression correlates with collateral density and inversely with infarct volume after MCA occlusion,7 and that collateral density correlates closely with infarct volume in 15 inbred strains with genetically-dependent differences in collateral density.6
Embryonic glial- and neuronal-derived VEGF stimulates Flk1-mediated signaling in ECs that, together with other factors and hemodynamic forces, directs EC specification and migration, plexus assembly and proliferation, growth and branching of the capillary plexus and its remodeling into vascular trees12, 13, 29-32. During the earliest stages of development, Flk1-positive (thus, VEGF-responsive) endothelial precursor cells migrate from the midline to the sites of early vasculogenesis. Cells retaining Flk1 expression proliferate, migrate, coalesce and form the early vascular plexuses. Other stimuli including fluid forces cause the plexus to remodel into a hierarchical arterial-venous bed, or the arterial tree to expand into nearby hypoxic tissue. We previously reported that the MCA is identifiable and is remodeling from the head plexus by E12.5.11 One can surmise that permanently reduced VEGF leads to fewer migrating and proliferating ECs, a sparser plexus EC population (which provides less “material” to remodel into an arterial bed), and impaired remodeling into mature vessels (i.e. recruitment of pericytes, impaired arterialization, etc.). All of these factors can lead to the persistence of the initial capillary plexus and a delay or stunting of arterial outgrowth. We suspect the opposite may occur during sustained increases in VEGF production. However, conditional global knockdown of VEGF specifically during the time of collaterogenesis reduced collateral formation but had no effect on the extent or branching of the capillary plexus or size of the cerebral artery trees (Figure 7) despite the reduction in collateral number. These findings support the concept that a direct action of VEGF-Flk1 signaling is primarily responsible for driving the collaterogenic process.
VEGF has a central role in angiogenic sprout formation.33, 34 When exposed to a local VEGF gradient, certain ECs acquire a migrating “tip cell” phenotype, while trailing ECs develop a supporting or “stalk cell” phenotype. The tip versus stalk phenotype, and maintenance of that phenotype, is regulated by bidirectional VEGF-Notch signaling between neighboring ECs. Activated Flk1 upregulates membrane-bound Delta-like ligand 4 (Dll4), which binds Notch on adjacent cells. Subsequent cleavage events free the Notch intracellular domain (NICD) allowing it to travel to the nucleus and regulate transcription of several genes. Primary NICD targets, Hes and Hey/Herp, encode basic helix-loop-helix proteins that are integral to capillary network formation and stalk cell maintenance.35, 36 Although active NICD is ultimately freed by γ-secretase activity, at least one prior cleavage event by an ADAM family member is required. Both Adam10 and Adam17 have been strongly implicated in angiogenic VEGF-Notch signaling, although their roles remain unclear.
Adam17 (tumor necrosis factor alpha converting enzyme (TACE), CD156b), is a membrane-anchored sheddase responsible for many shedding events in multiple cell types.37, 38 Adam17 knockout mice phenocopy mice lacking TGFα, HB-EGF or EGRF, and succumb perinatally.39, 40 Recently, Adam17 has been shown to have a significant role in angiogenesis by modulating endothelial sprouting and invasion,41 embryonic vascular branching and stability—presumably via EC filapodial behavior,42 and membrane excision of Flk143, 44 and Notch.19 Consistent with this, global Adam17 deficiency disrupts hindbrain vascular branching and causes cerebral venous hemorrhage at E14.5.42 Other than impeding collateral formation in the embryo and collateral density in the adult, our constitutive or conditional endothelial-specific Adam17 knockdown mice did not evidence any obvious vascular abnormalities. Our results are consistent with the above emerging evidence that ADAM17 participates in sprouting angiogenesis and the formation of nascent collaterals, and with a recent report that ADAM17 does not participate in ligand-activated Notch activation.19
Adam10 is also critical in VEGF-Notch signaling. Knockout mice strongly phenocopy Notch knockout mice and perish at ~E9.5.45 Recent evidence shows that Adam10 is critical for proper signaling in Notch1 signal-receiving (i.e. Notch-containing) but not Notch1-signal sending (i.e. ligand-containing) cells.19 Recent findings describe interactions of ADAM10 with Flk1,46 Neuropilin-143 and VE-Cadherin47 and indicate a role for the sheddase in modulating endothelial cell migration. Blobel et al recently reported that EC knockout of Adam10 using Tie2–driven Cre causes increased numbers of branching tip cells and “vascular loops” in the P5 retinal plexus, but no obvious differences in early embryonic vascular patterning.48 This report is consistent with our observed increase in collaterogenesis (“vascular loops”?) without an obvious change in plexus morphology. It is possible that increased tip cell formation leads to increased “collateral sprouting”, and that the resultant collaterals become patent vessels. Furthermore, inhibition of Adam10 and γ-secretase both increased collateral number, suggesting that Adam10 and γ-secretase function in the same collaterogenic pathway. Our findings suggest a specific function for ADAM10 in collateral formation and intimate that increased sprouting results in enhanced collaterogenesis. Since we did not get 100% EC knockout in our mice and the half-life of DAPT may be relatively short, our protocols may have favored the creation of tip cells without inhibiting stalk cell formation (thus collateral extension toward an opposing arteriole) altogether. Interestingly, significant deficiencies in growth of the long bones of Adam10EC postnatal mice were reported.48 However, our Adam10EC mice were physically indistinguishable from Cre-negative mice, despite Cre-activity in the long bones (Online Figure V,D). Differential promoter activity during limb formation may explain these results.
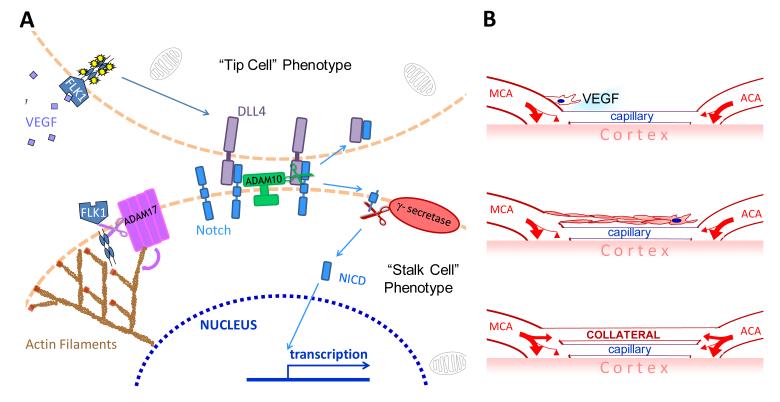
We propose a model for collateral formation based on mechanisms associated with sprouting/branching angiogenesis (Figure 8). Local isoform-generated gradients in VEGF, acting through Flk1, specify certain endothelial cells residing in distal-most arterioles of the embryonic arterial tree to express the tip-cell phenotype. Through bi-directional canonical VEGF-Notch signaling, these tip cells migrate and seek an opposing arteriole to fuse with. Trailing stalk cells proliferate to form a lumenizing EC cord. ADAM17’s pro-collaterogenic role is modeled from recent evidence that it facilitates angiogenesis and cell migration, which would be critically important to a migrating collateral “extension.”41, 43,41,43 In accordance with the process of sprouting angiogenesis, ADAM10 and γ-secretase are proposed to suppress formation of excessive tip cells in favor of trailing stalk cells. How these sprouts fuse with arterioles rather than venules is an intriguing question for future study. It is tempting to speculate that a specific subset of macrophage or microglial cells may serve as endothelial tip cell chaperones in this process.49
Figure 8. Model for collateral formation: collaterals form via a sprouting angiogenesis-like mechanism that occurs after the general arterial and venous trees have formed.
A, Paracrine VEGF-A activates FLK1 and initiates ligand (presumed DLL4) binding to Notch. This promotes a proliferating/migrating “tip cell” phenotype in an EC of a distal-most arteriole of the cerebral artery tree. A disintegrin and metalloprotease (ADAM) 10 and γ-secretase cleavage activity are required for Notch intracellular domain (NICD) signaling and promotion of a “stalk cell” phenotype in the trailing ECs. ADAM17 does not participate in ligand-dependent NICD formation but instead reduces collaterogenesis via cleaving the intracellular domain of FLK1 and altering actin activity. B, The collateral-forming arterial-fated EC sprout cell, followed by its lumenizing stalk cells, migrates over the pial capillary plexus and fuses with a distal-most arteriole of the opposing tree, establishing a nascent collateral. How the tip cell homes to and fuses with an arteriole of the opposing arterial tree and not to nearby capillaries or venules is not known.
Supplementary Material
Novelty and Significance.
What Is Known?
Collateral arteriole density varies widely in humans and mice, and is a significant determinant of variation in severity of stroke, myocardial infarction and peripheral artery disease.
Vascular endothelial growth factor (VEGF) dictates collateral formation in neonates and collateral function in adults.
Pial collaterals form in the prenatal period.
What New Information Does This Article Contribute?
Pial collateral arterioles form during a narrow window in late embryogenesis by a unique VEGF-dependent process of endothelial cell (EC) sprouting from a terminal arteriole and subsequent fusion to an opposing arteriole.
Collaterogenesis is independent of capillary patterning.
Temporal and constitutive EC-specific a disintegrin and metalloprotease (ADAM) 10 knockdown enhances collateral formation while ADAM17 knockdown inhibits it.
Interfering with VEGF-Flk1signaling during embryonic collaterogenesis permanently alters collateral number in adults.
Collateral arterioles connect two opposing arterial trees and are present in many tissues, including the pia mater. Pre-existing pial collateral number and diameter varies widely and significantly determines cerebral infarct volume, yet the mechanisms of collaterogenesis are poorly understood. We show, that collaterals form during a narrow developmental window in a VEGF-Flk1-ADAM dependent manner that is independent of native vascular patterning. While ADAM10 and 17 are both known to cleave Notch, they have opposing roles in collaterogenesis that cannot be compensated for in post-natal or adult life. We also found that interfering with embryonic VEGF-Flk1 signaling during the narrow window of collaterogenesis permanently alters collateral number in adults. These findings suggest that genetic and environmental factors during pregnancy can impact severity of stroke in adulthood.
ACKNOWLEDGEMENTS
The authors are grateful to Bernd Arnold of DKFZ-German Cancer Research Center for providing the Tie2-ERT2 mice, William McFadden for technical support, and Andrew Dudley of University of North Carolina at Chapel Hill for contributing expertise to immuno-bead sorting.
SOURCES OF FUNDING NIH-NHLBI HL090655 (JEF)
Non-standard Abbreviations
- ACA
anterior cerebral artery
- ADAM10
a disintegrin and metalloprotease 10
- ADAM17
a disintegrin and metalloprotease 17
- E
embryonic day
- EC
endothelial cell
- MCA
middle cerebral artery
- PCA
posterior cerebral artery
- VEGF
vascular endothelial growth factor A
- WT
wildtype
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 2.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- 4.Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, Hill MD, Demchuk AM, Goyal M. Regional Leptomeningeal Score on CT Angiography Predicts Clinical and Imaging Outcomes in Patients with Acute Anterior Circulation Occlusions. AJNR Am J Neuroradiol. 2011;32:1640–1645. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 11.Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–1892. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- 13.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez B. Embryonic development of collaterals. In: Schaper W, Schaper J, editors. Arteriogenesis. Kluwer Academic Publishers; 2004. pp. 11–19. [Google Scholar]
- 15.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 16.Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res. 2006;71:20–31. doi: 10.1016/j.mvr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pruessmeyer J, Ludwig A. The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Semin Cell Dev Biol. 2009;20:164–174. doi: 10.1016/j.semcdb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol. 2009;29:5679–5695. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar T, Miodonski A, Budi Santoso AW. Postnatal development of the vascular pattern in the rat telencephalic pia-arachnoid. A SEM study. Anat Embryol (Berl) 1986;174:215–223. doi: 10.1007/BF00824337. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhao Y, Zhou JW. The three-dimensional structure and the relationship between external and internal vascularizations in the brain of rat embryos. Chin Med J (Engl) 2004;117:280–285. [PubMed] [Google Scholar]
- 22.Wang DB, Blocher NC, Spence ME, Rovainen CM, Woolsey TA. Development and remodeling of cerebral blood vessels and their flow in postnatal mice observed with in vivo videomicroscopy. J Cereb Blood Flow Metab. 1992;12:935–946. doi: 10.1038/jcbfm.1992.130. [DOI] [PubMed] [Google Scholar]
- 23.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark EB, Hu N. Developmental hemodynamic changes in the chick embryo from stage 18 to 27. Circ Res. 1982;51:810–815. doi: 10.1161/01.res.51.6.810. [DOI] [PubMed] [Google Scholar]
- 25.Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res. 1989;65:1665–1670. doi: 10.1161/01.res.65.6.1665. [DOI] [PubMed] [Google Scholar]
- 26.Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, Shi Y, Duelsner A, Hoefer I, Gatzke N, Wang H, Lehmann K, Ulm L, Ritter Z, Hauff P, Hlushchuk R, Djonov V, van Veen T, le Noble F. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 27.Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenfuhr M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. [DOI] [PubMed] [Google Scholar]
- 28.Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, Powers AC. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS One. 7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel J, Gehrig M, Kuschinsky W, Marti HH. Massive inborn angiogenesis in the brain scarcely raises cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:849–859. doi: 10.1097/01.WCB.0000126564.89011.11. [DOI] [PubMed] [Google Scholar]
- 30.Heinzer S, Kuhn G, Krucker T, Meyer E, Ulmann-Schuler A, Stampanoni M, Gassmann M, Marti HH, Muller R, Vogel J. Novel three-dimensional analysis tool for vascular trees indicates complete micro-networks, not single capillaries, as the angiogenic endpoint in mice overexpressing human VEGF(165) in the brain. Neuroimage. 2008;39:1549–1558. doi: 10.1016/j.neuroimage.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- 32.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 34.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J Biol Chem. 2001;276:6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- 36.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 38.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Lee DC. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277:12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 39.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 41.Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol. 2009;28:470–479. doi: 10.1016/j.matbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Canault M, Certel K, Schatzberg D, Wagner DD, Hynes RO. The lack of ADAM17 activity during embryonic development causes hemorrhage and impairs vessel formation. PLoS One. 2010;5:e13433. doi: 10.1371/journal.pone.0013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 46.Donners MM, Wolfs IM, Olieslagers S, Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, van Buul JD, Caolo V, Molin DG, Post MJ, Waltenberger J. A disintegrin and metalloprotease 10 is a novel mediator of vascular endothelial growth factor-induced endothelial cell function in angiogenesis and is associated with atherosclerosis. Arterioscler Thromb Vasc Biol. 30:2188–2195. doi: 10.1161/ATVBAHA.110.213124. [DOI] [PubMed] [Google Scholar]
- 47.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102:1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glomski K, Monette S, Manova K, De Strooper B, Saftig P, Blobel CP. Deletion of Adam10 in endothelial cells leads to defects in organ-specific vascular structures. Blood. 118:1163–1174. doi: 10.1182/blood-2011-04-348557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.