Abstract
Rationale
Manipulation of the stress neuropeptide corticotropin-releasing factor (CRF), specifically central antagonism of the type-1 receptors (CRF-R1), effectively reduces alcoholic-like ethanol drinking in rodents. Escalated consumption is largely controlled by neurocircuitry that is important for reward and affect such as the ventral tegmental area (VTA) and the dorsal raphé nucleus (DRN).
Objective
The current studies investigated the role of CRF-R1 within the VTA and DRN, and their relation to escalated ethanol drinking in two species. An additional goal was to explore whether high alcohol-drinking individuals would be more affected by CRF-R1 antagonism than low alcohol-drinking individuals.
Methods
With a two-bottle choice drinking procedure, adult male C57BL/6J mice and Long-Evans rats were given 24 hr access to 20% ethanol and water on an intermittent schedule. Rats and mice were implanted with cannulae targeting the VTA or DRN. Doses of the CRF-R1 antagonist CP-154,526 were microinfused to modulate drinking of ethanol and water over the course of 24 hr.
Results
In both mice and rats, intra-VTA CP-154,526 selectively decreased ethanol intake while identical doses (0.3 and 0.6 μg) infused intra-DRN reduced both ethanol and water drinking. Long-Evans rats displayed a range of individual differences for ethanol preference, and CP-154,526 suppressed ethanol drinking in the high-preferring animals regardless of brain site manipulation.
Conclusions
The current findings confirm previous studies that blockade of CRF-R1 efficaciously reduces escalated drinking while also suggesting that the effects of intermittent access on alcohol consumption may require CRF interaction with dopamine in the VTA.
Keywords: ALCOHOL, CRF, VTA, DORSAL RAPHE, DRINKING
INTRODUCTION
The progression from social drinking to alcohol dependence can be characterized by the emergence of excessive, binge-like drinking. These episodes of binge drinking tend to occur intermittently between periods of abstinence (Sinclair and Senter 1968; Becker 1998). Over time, a maladaptive pattern of heavy drinking and withdrawal induces neuroadaptations indicative of dependence (Koob and Le Moal 1997). Studies in rats (Pinel and Huang 1976; Wayner and Greenberg 1972; Tomie et al. 2006; Simms et al. 2008; Cippitelli et al. 2012) and more recently mice have demonstrated that intermittent access to ethanol engenders higher voluntary and preferential ethanol consumption than continuous access to ethanol (Hwa et al. 2011; Melendez 2011).
One hypothesized mechanism for the transition to dependence is the dysregulation of brain stress systems, most notably the neuropeptide corticotropin-releasing factor (CRF) and its receptors (Koob 2003). CRF is considered to play a key role in the anxiogenic effects of acute withdrawal, protracted abstinence, and reinstatement induced by mild footshock stress (Merlo-Pich et al. 1995; Valdez et al. 2002; Finn et al. 2007; Valdez and Koob 2004). Most pharmacological and genetic evidence suggests that CRF exerts its effects on ethanol consumption through activation of CRF type-1 receptors (CRF-R1) (Chu et al. 2007; Treutlein et al. 2006; Hansson et al. 2006), with opposing action on CRF-R2 (Valdez et al. 2004; Funk and Koob 2007; Sharpe and Phillips 2009). CRF-R1 antagonists have been identified as potential candidates for therapeutic agents in the treatment of alcohol use disorders (Lowery and Thiele 2010) since these compounds reduce ethanol drinking in dependent animals but not non-dependent animals (Sabino et al. 2006; Funk et al. 2007; Richardson et al. 2008). Recently, systemic application of CRF-R1 antagonist antalarmin was shown to decrease ethanol drinking in Wistar rats (Cippitelli et al. 2012). The current experiments investigate the effect of a prototypic CRF-R1 antagonist, microinjected into extrahypothalamic brain sites, on escalated alcohol consumption that resulted from intermittent access.
CRF-R1 antagonists may modulate the monoamine pathways that are essential for the self-administration of alcohol, such as for example, the ventral tegmental area (VTA). Components of the mesolimbic dopamine (DA) system, originating in the VTA, are implicated in both responsiveness to stress (Deutch et al. 1991, 1987; Thierry et al. 1976) and in positively reinforcing effects of drugs of abuse, including ethanol (DiChiara and Imperato 1988; Gonzales et al. 2004; Koob et al. 1992; Wise 1996). CRF-DA synapses (Tagliaferro and Morales 2008) have been detected and are the site for stress-induced CRF release in the VTA (Wang et al. 2005, 2007). CRF-R1 activation through application of CRF transiently increases VTA DA neuronal firing (Korotkova et al. 2006; Wanat et al. 2006). However, CRF-R1 activation also facilitates slow D2- and GABAB-receptor mediated inhibitory synaptic transmission. This enhancement can be reduced by repeated exposure to stress or drugs of abuse (Beckstead et al. 2009). This evidence led us to consider that high drinking behavior, specifically at the level of the VTA, could be modulated by CRF-R1 antagonists. Alcohol-induced DA release within the NAcc is critically involved in the alcohol reinforcement process (DiChiara and Imperato 1988; Spanagel and Weiss 1999). However, the finding that 6-OHDA-induced lesion of the mesolimbic tract failed to alter voluntary self-administration in rats suggests a less central role of DA in maintaining alcohol consumption (Rassnick et al. 1993). Therefore, other neurotransmitters, such as serotonin, may also contribute to the development of intermittent ethanol drinking (LeMarquand et al. 1994).
Escalated alcohol drinking may also be based on impulse flow in other monoamine pathways, including those originating from the dorsal raphé nucleus (DRN) which is mostly serotonergic in nature. CRF-immunoreactive fibers densely innervate the cell bodies of the DRN in a topographically organized manner (Kirby et al. 2000; Sakanaka et al. 1986; Swanson et al. 1983), and particular serotonin (5-HT) cells constitute the major origins for 5-HT projections to the forebrain (Molliver 1987; Jacobs and Azmitia 1992). During acute stress, among other actions, CRF activates CRF-R1, inhibiting DRN 5-HT (Price et al. 1998; Price and Lucki 2001). This decreased 5-HT may promote the increased impulsivity and later on the compulsion that results in intense drug seeking behavior (Branchey et al. 1981; Tabakoff and Ritzmann 1975). Therefore, alcohol drinking may inhibit DRN 5-HT neuron firing as a consequence of activation by CRF-R1. In contrast, escalated alcohol drinking may increase VTA DA firing as a result of activation of CRF-R1, or also inhibit firing after chronic ethanol. We hypothesize that intermittent ethanol drinking may be differentially affected by CRF-R1 antagonism in the VTA compared to that in the DRN.
An additional goal of the current study was to investigate how selectively CRF-R1 antagonism can affect individuals in two commonly used species of animals using the intermittent access procedure (Simms et al. 2008; Melendez 2011; Hwa et al. 2011). The present research used Long-Evans rats and C57BL/6J (B6) mice to examine inherent differences in voluntary, intermittent drinking separated by brief periods of deprivation. Outbred rats may demonstrate a range of ethanol preferences in the intermittent access procedure including ethanol taste aversion (Keifer et al. 1994; Berman and Cannon 1974) while inbred B6 mice do not exhibit this taste aversion (McClearn and Rodgers, 1959). Another reason for species differences in intake may arise from functional differences in neuroanatomy. B6 mice show less of a DA excitatory response in VTA neurons caused by ethnaol in vitro (Brodie and Appel 2000), and they show fewer DAergic fibers innervating the prefrontal cortex (D’Este et al. 2007) compared to lower preferring animals. These neuroanatomical differences may suggest that B6 mice may drink more ethanol and be more impulsive than Long-Evans rats. By using two animal models that display different preferences for 20% ethanol, we intended to study how CRF acts in low drinking versus dependent-like drinking individuals, while being subjected to an intermittent schedule of access. We hypothesized that blockade of CRF-R1 will lead to greater suppression of ethanol drinking in those who drink in excess than in those who do not.
METHODS
Animals
Adult, male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) and Long-Evans rats (Charles River, Wilmington, MA), aged 8 weeks old upon arrival, were given 1 week to habituate to vivarium conditions on a 12 hr reversed light/dark cycle (lights off at 8am) and maintained temperature (70 ± 5°F) and humidity (25%). Animals were then individually housed for the duration of the study. Mice were housed in polycarbonate cages (28×17×12cm) with pine shavings bedding with stainless steel wire mesh lids with 2 openings for bottle spouts. Rat homecages were custom-made Plexiglas chambers (25×25×30cm) lined with Cellu-Dri™ pellet bedding (Shepherd Specialty Papers, Kalamazoo, MI) with front panels that included 2 angled holes for drinking spouts. Food and water were available ad libitum. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Study Design
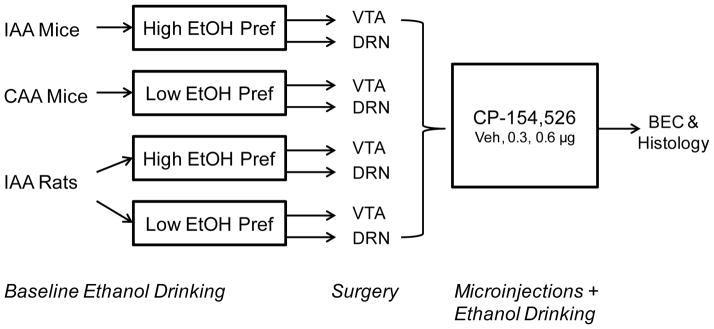
B6 mice and Long-Evans rats were given intermittent access to 20% ethanol and water for at least 4 weeks. Rats were then divided into high ethanol-preferring individuals versus low ethanol-preferring individuals based on their baseline ethanol preference. High preferring rats showed greater than 30% ethanol preference during both the 2 hr and 24 hr access periods. Subjects were randomly divided into groups for cannula implants targeting either the VTA or DRN. In a within-subjects design, animals underwent 3 microinjection tests (aCSF, 0.3 μg, and 0.6 μg CP-154,526) on 3 ethanol drinking test days at least 48 hours apart, consistent with Hwa et al. (2011) intermittent access protocol for mice and Simms et al. (2008) protocol for rats. Ethanol intake in grams of ethanol per kilogram of body weight (g/kg), volume of ethanol and water consumption (ml) and ethanol preference (%) were measured 2 hr and 24 hr after microinfusion. After microinjection testing, blood ethanol concentrations were measured on a final drinking day after 1 hr access to alcohol and water after vehicle microinjection using an ethanol assay kit (Diagnostic Chemical Limited, Oxford, CT). Cardiac blood was collected from rats, and submandibular blood was collected from mice. The study design is illustrated in Fig. 1.
Fig. 1. Study design to test intracerebral CRF-R1 antagonist manipulation of ethanol-drinking animals.
C57BL/6J mice were given intermittent (IAA) or continuous (CAA) 24 hr access to 20% ethanol and water. Long-Evans rats were also given intermittent access to alcohol (IAA) then characterized into high ethanol-preferring rats (High EtOH Pref) or low ethanol-preferring rats (Low EtOH Pref). After cannulation targeting either the ventral tegmental area (VTA) or the dorsal raphé nucleus (DRN), multiple doses of CP-154,526 were microinfused to suppress alcohol drinking behavior after 2 hours (2 hr) or 24 hours (24 hr) of fluid access. Blood ethanol concentrations (BEC) were taken after drug testing and histological analysis confirmed cannula placement at the end of experiments.
Ethanol Drinking
Mice and rats were given intermittent access to 2-bottle choice of 20% ethanol and water on Mondays, Wednesdays, and Fridays for 24 hr (Simms et al. 2008; Hwa et al. 2011). Between these ethanol and water sessions, animals were given 2 bottles of water in the homecage. In an additional group of B6 mice, continuous access was provided. Mice were given 2-bottle choice of 20% ethanol and water for 7 days per week. Animals were weighed before every ethanol drinking session. Fluid access began 3 hr into the dark cycle, as peak fluid consumption in outbred mice (Goldstein and Kakihana 1977) and one-bottle 20% ethanol consumption in B6 mice occurs at this time of day (Rhodes et al. 2005). To assess fluid consumption, differences in bottle weights were measured before and after 2-bottle choice to ethanol and water. On microinjection test days, 2 hr fluid intake was measured in addition to 24 hr intake. Although animals were given 24 hr total access, it was informative to monitor acute drinking during the first hours of 2-bottle choice after the previous day’s 24 hr alcohol deprivation. Also, 2 hr measurements allowed for drug comparisons influenced by shorter-acting microinjections.
Ethanol solutions (w/v) were prepared in tap water from 95% ethyl alcohol (Pharmaco-AAPER, Brookfield, CT). For mice, fluids were presented in 50 ml plastic centrifuge tubes (Nalgene) with no. 5 rubber stoppers and were securely held through the wire mesh cage lid. Fluids were presented to rats in 100 ml graduated plastic cylinders with no. 6 rubber stoppers (Fisher Scientific, Agawam, MA) containing stainless steel ball-bearing sippers. To control for extraneous spillage due to experimenter handling or evaporation, weekly “drip” averages (loss of fluid in a cage with no animal present) were subtracted from individual fluid intakes.
Stereotaxic Surgery
Animals underwent stereotaxic surgery for permanent indwelling cannulae in either the VTA or DRN. Mice received ketamine (100 mg/kg) and xylazine (10 mg/kg) combination and Rimadyl ® (5 mg/kg) as anesthetic (10 ml/kg, i.p.) and analgesic (10 ml/kg, s.c.), respectively, preceding surgical procedures in a stereotaxic frame (Kopf Instruments, Tujunga, CA). Rats received ketamine (100 mg/kg) and xylazine (6 mg/kg) combination and Rimadyl ® (5 mg/kg) as anesthetic (1 ml/kg, i.p.) and analgesic (1 ml/kg, s.c.), respectively, preceding surgery. Mice were implanted with a dual cannulae system to bilaterally target the mouse VTA (Plastics One, Roanoke, VA) with no angle from bregma (AP-3.2, ML±0.5, DV-4.5mm). Dummy cannulae and dust caps fit the length of the cannula while VTA dual injectors protruded 0.1 mm past the cannula. Other groups of mice were implanted with unilateral 6 mm, 26 ga. guide cannulae aimed at the DRN (AP-4.6, ML+0.5, DV-1.9mm from bregma) at a 26° angle (Paxinos and Franklin 2003). Fitted obdurators protruded 0.5 mm beyond the guide cannula, while injectors protruded 1 mm beyond the guide cannula. Rats were bilaterally implanted with 11 mm, 26 ga. guide cannulae targeting the VTA (AP-5.2, ML+1.8, DV-7.5mm from bregma) at a 10° angle, or unilaterally targeting the DRN (AP-7.5, ML+3.2, DV-5.8mm from bregma) at a 28° angle (Paxinos and Watson 1997). Dental cement (Jet Acrylic, Lang Dental, Wheeling, IL) and 2 0.5 mm tapered screws (Small Parts, Lexington, KY) anchored cannulae permanently to the skull surface. Obdurators fit 0.5 mm beyond the guide cannulae immediately after surgery. Animals recovered 3–7 days before resuming intermittent access to 20% ethanol drinking. Mice and rats were given at least 3 2-bottle choice sessions to return to pre-surgical drinking levels, which was defined as less than 15% variability in individual drinking across 3 sessions. Obdurators were handled before fluid presentation in these sessions to habituate animals to testing conditions.
Microinjection Procedures
Injectors were connected via flared polyethylene tubing to a glass syringe controlled by an automatic CMA/100 microinjection pump (CMA Microdialysis, Sweden). For mice, a 33 ga. injector microinfused 0.2 μl volume of aCSF, 0.3 μg or 0.6 μg CP-154,526 at a flow rate of 0.1 μl/min into the target brain sites. For rats, a 33 ga., 12 mm injector(s) infused a 0.5 μl volume of aCSF, 0.3 μg or 0.6 μg CP-154,526 at a 0.1667 μl/min flow rate into the target brain sites. Animals moved about freely during the infusion process. Injectors remained in place for 1 min to allow for diffusion and to minimize vertical action up the cannula. Nine min later (total of 10 min after drug administration), alcohol and water bottles were given to animals to assess CRF-R1 effects on fluid consumption.
Histology
After testing, mice and rats were deeply anesthetized with an i.p. injection of ketamine/xylazine combination (100 mg/kg ketamine, 10 mg/kg xylazine) or sodium pentobarbital (100 mg/kg), respectively. Animals were intracardiacally perfused with 0.9% saline and 4% paraformaldehyde, followed by brain removal. Brains were sliced in 55 μm coronal sections using a Cryostat (Leica CM1900, Bannockburn, IL). Nissl stained sections were used to check placement of guide cannulae. Animals with incorrect placements into target sites (VTA required two correct placements; DRN required one correct placement) were excluded from analysis (n=2 mice, n=9 rats).
Drugs
CP-154,526 [butyl-(2,5-dimethyl-7-[2,4,6-trimethylphenyl]-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-ethylamine] (Tocris, Ellisville, MO) was freshly suspended immediately before microinjections in a vehicle of artificial cerebral spinal fluid (aCSF). aCSF was composed of 147 mM NaCl2, 0.9 mM MgCl2, 4.0 mM KCl and filtered through a 25 mm syringe filter before use. CP-154,526 displays high affinity for the CRF-R1 (Ki < 10 nM), high selectivity for CRF-R1 (>10000 for CRF-R2), and blocks CRF-stimulated adenylate cyclase activity in rodent pituitary and cortical membranes (Lundkvist et al. 1996; Schulz et al. 1996). Drug doses were based on previous microinjection studies in our laboratory using CP-154,526 suspended in aCSF (Boyson et al. 2011; Quadros et al. in prep).
Statistical Analyses
Statistical analyses were performed using SigmaStat version 3.1 (Systat Software, San Jose, CA). Mean and standard error about the mean (SEM) are reported for all resulting data. Two hr and 24 hr ethanol consumption (g/kg), volume of ethanol intake (ml) and water intake (ml) after microinjection of CP-154,526 doses were analyzed using one-way analysis of variance (ANOVA) with repeated measures, followed by Bonferroni post hoc analyses if significant main effects were found (p < .05). Pearson’s correlation coefficient was calculated to compare baseline ethanol drinking behavior to the change of intake as a result of CP-154,526. Ethanol preference ratios were also calculated for baseline ethanol drinking, defined as volume of ethanol consumed (ml) divided by volume of total fluid consumed (ml) multiplied by 100 (%). Results for the two brain sites were analyzed separately. Since the rats generally demonstrated preference for water, ethanol drinking was analyzed separately from water drinking in all tests. Data for the two species were also analyzed separately because of the large differences in baseline ethanol preference between Long-Evans rats and B6 mice.
RESULTS
Baseline ethanol drinking
C57BL/6J Mice
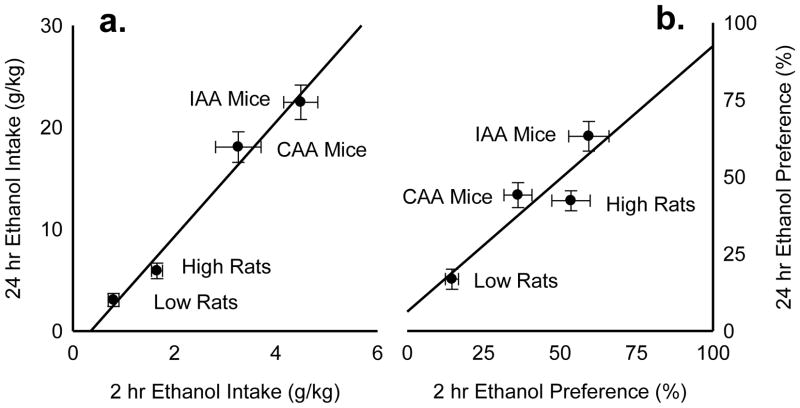
Male B6 mice (n=24) were given intermittent 24 hr access (IAA) to 20% ethanol and water for 4 weeks, or 12 2-bottle choice sessions. IAA mice acquired high ethanol drinking levels rapidly over the first week and maintained escalated ethanol consumption at 22.46 ± 1.69 g/kg/24h [Fig. 2a] and ethanol preference at 63.15 ± 4.82 % [Fig. 2b]. IAA mice also showed robust ethanol drinking levels during the initial 2 hr at 4.49 ± 0.34 g/kg [Fig. 2a] and ethanol preference at 59.41 ± 6.56 % [Fig. 2b]. After vehicle microinjection, IAA mice achieved average blood ethanol concentrations of 145.34 ± 27.20 mg/dl after 1 hr access of 2-bottle choice. Another group of mice (n=24) were given continuous 24 hr access (CAA) for 12 consecutive days. CAA mice also acquired high ethanol drinking during both the initial 2 hr access period and 24 hr overnight access period [Fig. 2a], but they consumed less alcohol than IAA mice. CAA mice showed less ethanol preference than IAA mice at 44.08 ± 4.05 % overnight and even less during the initial 2 hr access period at 36.24 ± 4.60 % [Fig. 2b]. After vehicle microinjection, CAA mice showed average blood ethanol concentrations of 79.37 ± 12.03 mg/dl after 1 hr ethanol and water access.
Fig. 2. Baseline ethanol drinking behavior.
Mice were given intermittent access to alcohol (IAA Mice; n=18) or continuous access to alcohol (CAA Mice; n=17) after vehicle microinjections into the VTA or DRN. High ethanol-preferring rats (High Rats; n=13) or low ethanol-preferring rats (Low Rats; n=15) were also assessed for fluid drinking behavior. Depicted is the correlation between mean 2 hr and 24 hr ethanol intake in grams per kilogram (g/kg) ± SEM (a) and the correlation between mean 2 hr and 24 hr ethanol preference (%) ± SEM (b).
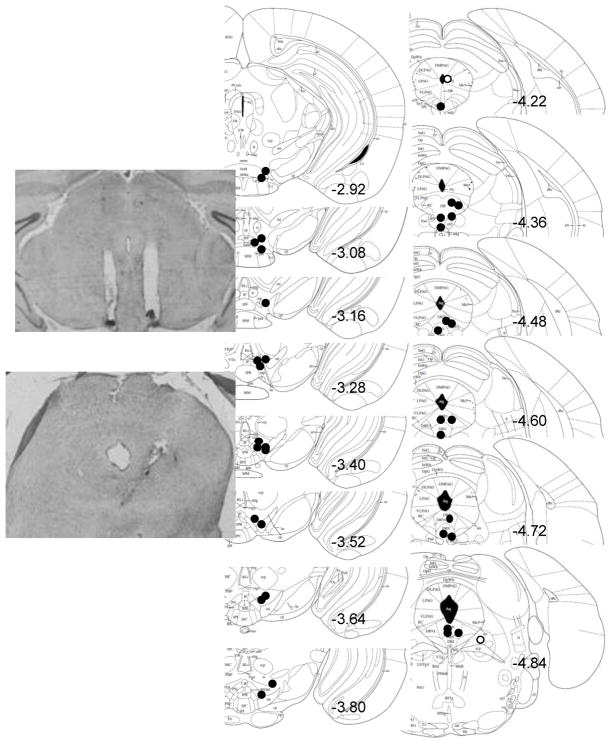
Of the 24 IAA mice and 24 CAA mice, half received VTA implants, and half received DRN implants. Two IAA and 3 CAA mice implanted intra-VTA did not survive the cannulation surgery. Two IAA and 4 CAA mice implanted intra-DRN were eliminated due to missed cannula placements or did not survive surgery. Schematics and photomicrographs of VTA and DRN placements in the mouse brain are shown in Fig. 3.
Fig. 3. Histological verification of cannulae placement in C57BL/6J mice.
Correct (black circles) and incorrect (white circles) cannula placements are shown in representative coronal sections in mm from bregma surrounding the ventral tegmental area (VTA) or dorsal raphé nucleus (DRN, n=2 excluded). VTA placements are bilateral though only one of two sides is shown for clarity. DRN placements are unilateral. Photomicrographs of correct placements are shown after Nissl staining.
Long-Evans rats
In rats, intermittent access resulted in ethanol consumption (g/kg) steadily increasing over time, but we observed a distribution of individual differences for ethanol intake and preference. After correlational analyses, rats were characterized as high ethanol-preferring rats or low ethanol-preferring rats based on their baseline ethanol drinking behavior. High ethanol-preferring rats consumed 5.90 ± 0.76 g/kg/24hr [Fig. 2a] and showed a 42.23 ± 3.25 % ethanol preference over 24hr [Fig 2b]. High ethanol-preferring rats also displayed an even greater 53.59 ± 6.26 % ethanol preference during the initial binge period [Fig. 2b]. After vehicle microinjection, high ethanol preferring-rats had average blood ethanol concentrations of 112.28 ± 32.27 mg/dl after 1 hr of 2-bottle choice. In contrast, low ethanol-preferring rats demonstrated less than 1 g/kg ethanol intake during the 2 hr and 24 hr access periods [Fig. 2a]. This group also showed lower than 25 % ethanol preference during both 2 hr and 24 hr access periods. Low ethanol-preferring rats showed mean blood ethanol concentrations of 50.54 ± 4.41 mg/dl after 1 hr access.
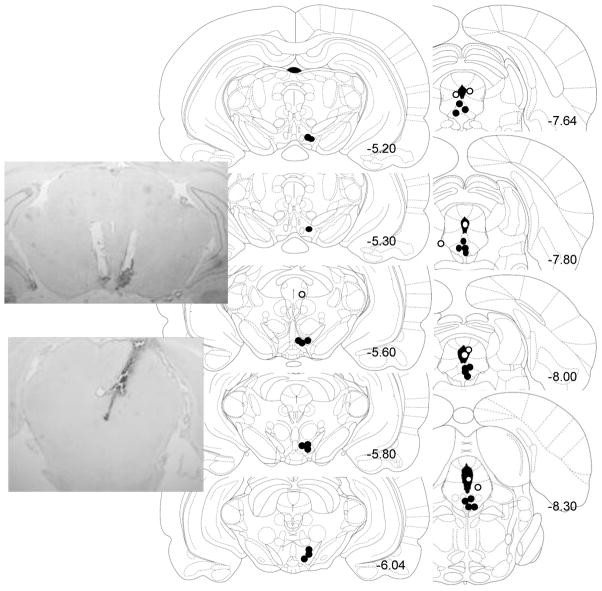
After recovery from stereotaxic surgery, animals maintained stable drinking levels. Sixteen high ethanol-preferring rats were implanted into the VTA (n=7) or DRN (n=9). Twenty-one low ethanol-preferring rats were implanted into the VTA (n=6) or DRN (n=15). One rat in the VTA group and 8 rats in the DRN group were eliminated due to missed cannula placements. Upon further analysis of missed DRN placements, there was a trend that cannula placements into the periaqueductal gray did not have an effect on drinking behavior. We also observed a trend for decreased alcohol drinking when cannula placements were located in the cerebral aqueduct. Placements targeting the VTA or DRN in the rat brain are depicted in Fig. 4. There were no differences in cannula placements between the high and low ethanol-preferring groups.
Fig. 4. Histological verification of cannulae placement in Long-Evans rats.
Correct (black circles) and incorrect (white circles) cannula placements are depicted in representative coronal sections in mm from bregma surrounding the ventral tegmental area (VTA, n=1 excluded) or dorsal raphé nucleus (DRN, n=7 excluded) for adult male Long-Evans rats. VTA placements are bilateral though only one of two sides is shown for clarity. DRN placements are unilateral. Photomicrographs of correct placements are shown after Nissl staining.
Intra-VTA modulation of ethanol drinking
IAA mice
IAA mice receiving intra-VTA microinfusions (n=10) showed an acute suppression of ethanol drinking (g/kg) behavior in the initial 2 hr [F(2,9) = 17.33, p < .001; not shown]. Both doses significantly decreased ethanol drinking (g/kg) compared to vehicle [aCSF vs. 0.3 μg: t = 4.60, p < .001; aCSF vs. 0.6 μg: t = 5.48, p < .001]. CP-154,526 microinjections also reduced the volume of ethanol consumed (ml) in a similar fashion [F(2,9) = 17.40, p < .001; Fig. 5a]. Ethanol intake (ml) was lower after both doses compared to the vehicle treatment [aCSF vs. 0.3 μg: t = 4.57, p < .001; aCSF vs. 0.6 μg: t = 5.51, p < .001]. This suppression appeared to be dose-dependent as indicated by a linear trend analysis [r2 = 0.87]. Two hr water intake (ml) was not affected [p = .46]. Intra-VTA CP-154,526 continued to reduce ethanol intake (g/kg/24h) overnight [F(2,9) = 10.08, p < .01; not shown], specifically at the 0.6 μg drug dose [t = 4.04, p < .05]. Microinjections also suppressed volume of ethanol consumed (ml) over 24 hr [F(2,9) = 10. 09, p < .01] also at the highest dose [t = 3.63, p < .01]. Overnight water drinking (ml/24hr) in mice was not affected by intra-VTA microinjections of the CRF-R1 antagonist [p = 0.34].
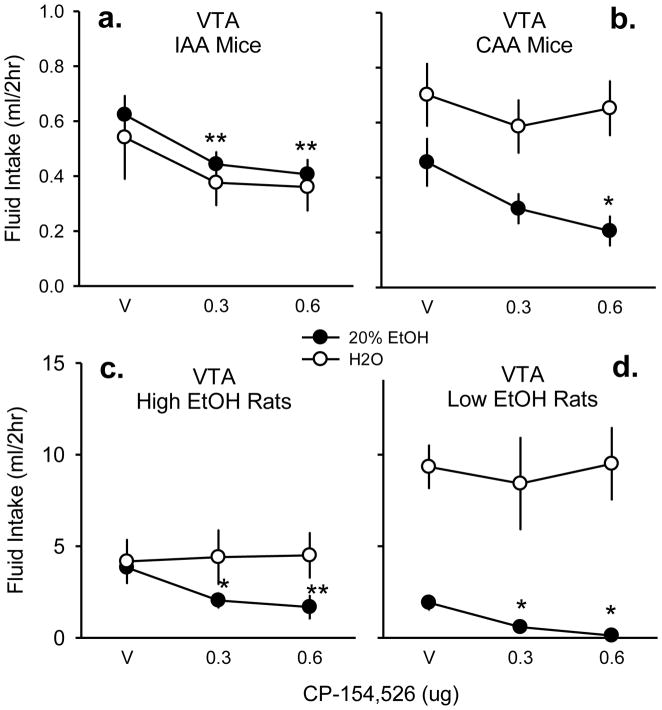
Fig. 5. Fluid drinking affected by CRF-R1 antagonist in VTA.
Vehicle (v) aCSF, 0.3, and 0.6 μg CP-154,526 were microinjected into the ventral tegmental area (VTA) of mice given intermittent access (a, n=10) or continuous access to alcohol (b, n=9). High ethanol-preferring Long-Evans rats (c, n=6) or low ethanol-preferring rats (d, n=6) were also tested for intra-VTA fluid drinking. Mean fluid intake in milliliters (ml) over the initial 2 hr access period is displayed ± SEM. Both 20% ethanol (black circles) and water (white circles) intake were measured in the two-bottle choice procedure. * p < .05 compared to vehicle, ** p < .001 compared to vehicle.
CAA Mice
Like the IAA mice, intra-VTA CP-154,526 microinjections reduced 2 hr ethanol intake (g/kg) in CAA mice (n=9) [F(2,8) = 5.67, p < .05] confirmed at the highest dose [t = 3.31, p < .05]. Drug treatment similarly reduced volume of ethanol consumed (ml/2hr) [F(2,8) = 5.38, p < .05; Fig. 5b] also at the 0.6 μg dose [t = 3.22, p < .05] without significantly altering acute water consumption (ml/2hr) [p = .80]. Linear trend analysis confirmed an apparent dose-dependent suppression [r2 = 0.96]. For 24 hr ethanol drinking, intra-VTA microinjections into CAA mice did not affect ethanol consumption per body weight (g/kg) [p =.71], volume of ethanol intake (ml) [p = .48] or volume of water intake (ml) [p = .48] overnight.
High ethanol-preferring rats
High ethanol-preferring rats (n=6) also demonstrated an acute attenuation of ethanol drinking (g/kg/2h) [F(2,5) = 15.95, p < .01], caused by both doses of intra-VTA CP-154,526 [aCSF vs. 0.3 μg: t = 3.38, p < .05; aCSF vs. 0.6 μg: t = 5.58, p < .001]. CP-154,526 also decreased the volume of ethanol drinking (ml) [F(2,6) = 21.61, p < .001; Fig. 5c] resulting from differences in both drug doses [aCSF vs. 0.3 μg: t = 4.04, p < .01; aCSF vs. 0.6 μg: = 6.47, p < .001], compared to no significant change in water drinking (ml) [p = 0.86] in the first 2 hours of access to 2-bottle choice. Linear trend analysis confirmed the apparent dose-dependent suppression [r2 = 0.87]. By the 24 hr time point, intra-VTA infusions of CP-154,526 did not alter ethanol consumption per body weight (g/kg) [p = .86], or total volume of ethanol (ml) [p = 0.61] or water consumed (ml) [p = .13].
Low ethanol-preferring rats
Intra-VTA CP-154,526 significantly reduced ethanol consumption in the initial 2 hr access (g/kg) of low ethanol-preferring rats (n=6) [F(2,5)=13.59, p < .05]. Both drug doses were significantly different from vehicle [aCSF vs. 0.3 μg: t = 3.78, p < .05; aCSF vs. 0.6 μg: t = 5.00, p < .01]. There was also a similar suppression in volume of ethanol intake (ml/2hr) [F(2,5) = 13.06, p < .01; Fig. 5d] at both the 0.3 μg dose [t = 3.66, p < .05] and the 0.6 μg dose [t = 4.92, p < .01]. Volume of water intake during the initial 2-bottle choice period was not affected by drug treatment [p = .83]. During the 24 hr access period, neither ethanol consumed per units of body weight (g/kg) [p = .09], ethanol volume (ml) [p = .08], or water volume (ml) [p = .49] were affected by CP-154,526 intra-VTA in low ethanol-preferring rats.
Intra-DRN modulation of ethanol drinking
IAA mice
Like intra-VTA microinjections of IAA mice, intra-DRN microinjections of IAA mice (n=8) displayed a significant reduction of ethanol drinking (g/kg) behavior in the initial 2 hr after CP-154,526 [F(2,7) = 7.33, p < .01], specifically at the highest dose compared to vehicle [t = 3.62, p < .01]. As shown in the reduction of ethanol drinking behavior, intra-DRN CP-154,526 similarly reduced volume of ethanol consumed (ml) [F(2,7) = 7.38, p < .01; Fig. 6a]. This effect was largest at the highest dose compared to vehicle [t = 3.68, p < .01]. Linear trend analysis confirms this apparent dose-dependent decrease [r2 = 0.92]. Water drinking (ml) seemed to be attenuated by CP-154,526 in the mouse DRN, but this effect was not statistically significant [p = .18; Fig. 7a]. After 24 hr 2-bottle choice, mice showed no significant changes in ethanol drinking (g/kg) [p = .44] or in fluid consumption (ml) due to drug microinjections in the DRN [ethanol: p = 0.39; water: p = .70].
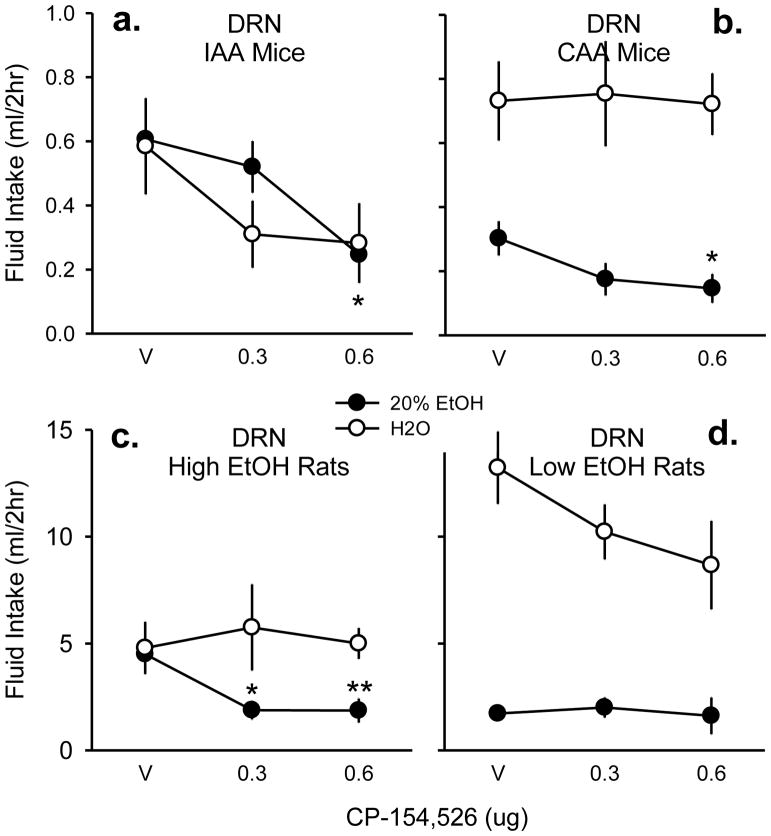
Fig. 6. Fluid drinking affected by CRF-R1 antagonist in DRN.
Vehicle (v) aCSF, 0.3, and 0.6 μg CP-154,526 were microinjected into the dorsal raphé nucleus (DRN) of mice given intermittent access (a, n=8) or continuous access to alcohol (b, n=8). High ethanol-preferring Long-Evans rats (c, n=7) or low ethanol-preferring rats (d, n=9) were also tested for intra-DRN modulations of fluid drinking. Mean fluid intake in milliliters (ml) over the initial 2 hr access period is displayed ± SEM. Both 20% ethanol (black circles) and water (white circles) intake were measured in the two-bottle choice procedure. * p < .05 compared to vehicle, ** p < .001 compared to vehicle.
Fig. 7. Change in ethanol drinking according to baseline ethanol preference.
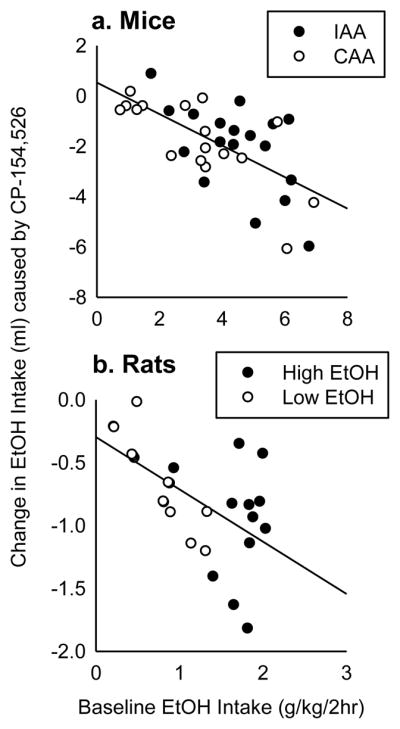
Baseline ethanol intake (g/kg/2h) is correlated with the absolute change in 2 hr ethanol drinking (ml) caused by 0.6 μg CP-154,526 into either the VTA or the DRN of mice (a, n=35) or rats (b, n=28). High ethanol drinking animals (IAA mice and High ethanol-preferring rats) are shown in black circles. Lower ethanol drinking animals (CAA mice and Low ethanol-preferring rats) are shown in white circles.
CAA Mice
In CAA mice (n=8), intra-DRN microinjections reduced ethanol drinking (g/kg) but to a lesser degree than intra-VTA CAA mice [F(2,7) = 5.69, p < .05]. Only the highest drug dose was effective at reduced CAA in the DRN compared to vehicle [t = 3.17, p < .05]. Similarly, CP-154,526 acutely decreased volume of ethanol intake (ml) [F(2,7) = 6.09, p < .05; r2 = 0.88] without altering water intake (ml) [p = .98; Fig. 6b]. Intra-DRN drug treatment did not alter 24hr ethanol drinking per body weight (g/kg) [p = .44] or volume of ethanol intake (ml) [p = .37]. However, total water consumed overnight was significantly decreased after CP-154,526 in CAA animals [F(2,7) = 5.26, p < .05] specifically at the 0.6 μg dose compared to vehicle [t = 2.94, p < .05].
High ethanol-preferring rats
In high ethanol-preferring rats (n=7), intra-DRN microinjections significantly attenuated 2 hr ethanol drinking (g/kg) [F(2,6) = 23.41, p < .001]. This drug effect was evident at both the moderate drug dose [t = 4.41, p < .01] and the highest drug dose compared to the vehicle condition [t = 6.56, p < .001]. CP-154,526 also reduced volume of ethanol (ml) in the first 2 hours of fluid access [F(2,6) = 20.36, p < .001; Fig. 6c] at both drug doses compared to vehicle [aCSF vs. 0.3 μg: t = 3.98, p < .01; aCSF vs. 0.6 μg: t = 6.16, p < .001]. Acute water drinking was not affected by drug treatment [p = .92]. Intra-DRN infusions of CP-154,526 did not alter total 24 hr ethanol drinking (g/kg) [p = .59] or fluid consumption (ml) [ethanol: p = 0.91; water: p = 0.51] in the high ethanol-preferring rats.
Low ethanol-preferring rats
In contrast to the high ethanol-preferring group, low preferring rats (n=9) were not significantly affected by intra-DRN CP-154,526 at the 2 hr time point [p = .89]. The lack of the drug effect was also shown in no alterations of volume of ethanol intake (ml) [p = .84; Fig. 6d]. However, there was a nonsignificant reduction in acute water intake [p = .052; Fig. 6d], but this trend did not last over the 24 hr access period [p = .27]. Similarly, intra-DRN microinjections did not affect 24 hr ethanol consumption (g/kg) [p = .30] or volume of ethanol intake (ml) [p = .31].
Change in ethanol drinking according to baseline ethanol preference
Since CRF-R1 antagonists have been reported to affect ethanol drinking in dependent rodents more than non-dependent rodents, we expected that animals which show higher baseline drinking behavior would also show greater reductions in ethanol drinking caused by CP-154,526. In both IAA and CAA mice, we observed a moderate, but significant correlation between baseline 2 hr ethanol intake (g/kg) and the absolute change in milliliters of ethanol consumed after the highest dose of CP-154,526 [r = -0.65, p < .001; Fig. 7a]. We also observed a similar correlation across high ethanol-preferring rats and low ethanol-preferring rats, though the rat drinking levels were overall less than the mice in 2 hr [r = -0.58, p < .01; Fig. 7b]. Still, individuals that had high baseline binge drinking behavior were more affected by the CRF-R1 antagonist while individuals with low baseline drinking behavior were less affected.
DISCUSSION
In a cross-species comparison, this set of experiments investigated high alcohol drinking that was particularly sensitive to CRF-R1 intervention. Intermittent access to 20% ethanol was a successful method to escalate voluntary alcohol consumption over time compared to continuous access. Outbred Long-Evans rats given intermittent access demonstrated individual differences for ethanol drinking behavior. Some rats displayed low to moderate ethanol drinking, while some individuals consistently displayed high ethanol preference, both in the initial 2 hr access period and in the overnight access period, mimicking high ethanol preference in B6 mice. Overall, CP- 154,526 in the VTA reduced alcohol consumption in all animals, whereas only the high alcohol preferring animals, the B6 mice and high-preferring rats, were responsive to CRF-R1 antagonism in the DRN. Thus, the data suggest differences between brain sites in how selective the prototypic CRF-R1 antagonist CP-154,526 can decrease high ethanol drinking in some individuals but not others.
The present investigation found that microinfusion of CRF-R1 antagonist into the VTA selectively decreased ethanol binge drinking without altering water drinking. Many classic studies have reported the impact of ethanol on the mesolimbic dopamine system (Gessa et al. 1985; Pfeffer and Samson 1986; Carlsson et al. 1974; Gatto et al. 1994). Recent work suggests an essential role for synaptic plasticity in the VTA in the early behavioral responses following initial drug exposures, as well as in triggering long-term adaptations in regions innervated by DA neurons of the VTA (Kauer 2004; Kauer and Malenka 2007). This set of pharmacological experiments is consistent with previous electrophysiological findings that CRF potently influences DA neurons in the VTA, which may shape an individual’s likelihood for relapse (Wang et al. 2005). In addition to altered DA neurotransmission during relapse, withdrawal from chronic ethanol leads to substantial decrements in DA firing in the VTA (Diana et al. 1992; Shen and Chiodo 1993) and extracellular NAcc DA levels (Rossetti et al. 1992; Weiss et al. 1996). Acute treatment with CRF-R1 antagonist CRA-0450 in the VTA significantly increases DA neuronal activity in the NAcc and reduced cocaine-stimulated DA overflow in the NAcc (Lodge and Grace 2005). The current study supports the crucial role of the VTA in ethanol drinking since acute blockade of CRF-R1 potently reduced alcohol intake in all animals. These results are consistent with the hypothesis that long-term drug taking may decrease stress-enhanced DA transmission. We speculate that CP-154,526 acts on hyporesponsive DA neurons to increase DA flow thereby reducing alcohol drinking behavior, which is a focus of future study.
We found that CP-154,526 infusion in the rat DRN produced differential effects on high ethanol-preferring rats compared to low ethanol-preferring rats. High-preferring rats, in a similar fashion as high-preferring B6 mice, showed a suppression of ethanol drinking. In contrast, CP-154,526 infused intra-DRN of low-preferring rats had no effect on ethanol drinking. This interaction was only apparent at the level of the rat DRN, as identical doses in the VTA significantly suppressed ethanol intake in all individuals. A change in CRF receptor signaling in the DRN may occur during the intermittent access procedure since increased drinking may result from the repeated, stress-inducing cycles of binging and withdrawal. One interpretation is that during acute stress, CRF acts on CRF-R1 subtypes, decreasing 5-HT impulse flow and release (Valentino and Commons 2005). A limitation was that intra-DRN microinjections tended to suppress water drinking acutely or overnight in several groups of animals. CRF has been reported to decrease water intake in CRF-R1 deficient mice (Contarino et al. 2000), which may explain the decrease in water drinking after application of a CRF-R1 antagonist. CRF, or more likely urocortin 1, acts also on CRF-R2 since CRF-R2 is more abundant in the DRN than CRF-R1 (Chalmers et al. 1995). CRF-R2 activation has also been associated with decreases in feeding (Spina et al. 1996; Pelleymounter et al. 2000). We also cannot discount the role of urocortin 1 in the centrally-projecting Edinger-Westphal nucleus (Giardino et al. 2011) that may have terminals on VTA and DRN neurons. Urocortin 1 neurons have been known to interact in the DRN to regulate fluid consumption, as well (Weitemier and Ryabinin 2006).
Furthermore, future studies need to isolate which specific CRF receptor populations within the brain are involved in the regulation of excessive drinking. Since the posterior vs. anterior VTA (pVTA, aVTA) differ in efferent DA neurons, the two subregions may be differentially involved in binge drinking (Rodd et al. 2004; Melon and Boehm 2011). In the rats, most placements were in the pVTA, which is consistent with the past literature implicating the pVTA and alcohol seeking (Rodd et al. 2004; Hauser et al. 2010). In the mice as illustrated in Fig. 3, 6 placements were in the aVTA. Mice with placements in the aVTA still showed reductions in drinking though the decreases appeared to be less substantial than reductions in drinking from mice in the pVTA. Further methodological refinements are required in order to localize microinjections in the anterior versus posterior VTA of mice. Likewise, 5-HT receptor densities differ according to subregions of DRN (Graeff et al. 1996; Kirby et al. 2000). Monoaminergic impulse flow to the forebrain could vary according to subregions. Again, microdialysis studies would be useful to measure DA and 5-HT concentrations as a function of CRF-R1 blockade from different CRF receptor populations.
This cross-species comparison confirmed that blockade of CRF receptors can reduce escalated ethanol drinking in high drinking rodents, but has less of an effect on ethanol consumption in non-dependent rodents (Chu et al. 2007; Finn et al. 2007; Funk and Koob 2007; Funk et al. 2007; Gehlert et al. 2007; Valdez et al. 2002). This implies that intermittent access to alcohol may generate withdrawal-related binge drinking that causes changes in the CRF system in some animals. Our results are consistent with research linking CRF-R1 antagonists to reduce binge-like drinking, but not non-binge-like drinking (Lowery-Gionta et al. 2012; Sparta et al. 2008). In line with this hypothesis, even though rats bred for high ethanol preference may be innately high consumers, these animals do not show reduced ethanol seeking due to CRF-R1 antagonism if they are not rendered dependent via exposure to alcohol vapor (Sabino et al. 2006; Gilpin et al. 2008). B6 mice are known for their high voluntary ethanol consumption (Fuller 1964; McClearn and Rodgers 1959), and in the current procedure exhibited consistently escalated drinking at greater than 20 g/kg per day (Hwa et al. 2011) as well as blood ethanol concentrations between 100–200 mg/dl. Intermittent access generated increased daily drinking in outbred rats, confirming similar values in previous studies (Cippitelli et al. 2012; Simms et al. 2008; Wise 1973). Unlike the consistently high alcohol-preferring mice, the Long-Evans strain of rat provided the opportunity to also study large individual differences in episodic drinking. Using intermittent access to escalate voluntary drinking, CRF may play an important role in certain extrahypothalamic sites in animals undergoing withdrawal stress. There may be a dysregulation of CRF-R1 receptors or an accumulation of CRF in these sites that increase the efficacy of the CRF-R1 blockade. Quantifying CRF-R1 mRNA or CRF levels in the brain would help clarify this hypothesis. Still, the current preparation does not adequately assess an animal’s motivation for alcohol, so experiments using progressively higher behavioral demands to obtain alcohol reinforcement would be useful to further confirm the CRF-escalated drinking hypothesis.
The current study adds to the substantial preclinical evidence supporting CRF-R1 antagonists as treatment of alcohol abuse disorders, but the successful replications in various experimental preparations and species have failed to work in the clinic (recently reviewed by Zorrilla and Koob 2010). Most notably, pharmaceutical development of nonpeptide CRF-R1 antagonists has been hindered because most compounds were either undesirably hydrophobic or difficult to deliver (Zorrilla and Koob 2004; Chen 2006). CRF also modulates numerous neurobiological systems, including feeding, anxiety and depression, and HPA axis signaling (Hauger et al. 2006; Heinrichs and Richard 1999; Smith and Vale 2006; Menzaghi et al. 1994; Kehne 2007; Ryabinin and Weitemier 2006). Chronic dampening of the CRF system may render unwanted side effects on vital systems outside of the specific pathways implicated in ethanol abuse, for example peripheral or hypothalamic CRF receptors.
In conclusion, the present findings suggest that intermittent access to ethanol is based on CRF mechanisms within discrete neural circuits. Ongoing studies are further confirming the role of CRF-R1 within the DRN by testing their interaction with mu opioid receptors (Hwa et al. meeting abstract). Co-infusions of intra-DRN CP-154,526 and naltrexone may exert a more effective suppression of intermittent ethanol drinking via receptor disinhibition on serotonin neurons. The present results show acute drug effects that are limited to the initial 2 hr access period. In nearly all treatment groups, animals compensated over the 24 hr access period to return to overnight intake levels before any drug treatment. This may represent a significant limitation with microinjections, as drug effects in the clinic need to be long-lasting. Current experiments hope to address this problem by implementing chronic i.c.v. dosing of CP-154,526 delivered via osmotic minipump. Central CP-154,526 also effectively blocks intermittent access ethanol consumption for more than 72 hrs when administered chronically (Hwa et al. unpublished results). Again, focusing on regulation of extrahypothalamic CRF seems crucial to understanding the vulnerability to alcohol-use disorders. These pathways may play a part in determining the neurobiological differences between individuals who consume alcohol in excess and those who do not.
Acknowledgments
This work was supported by funding from NIH R01 AA013983 awarded to KAM. The authors would like to thank Tufts University research assistants Rock Hwang, Monita Wong, and Shaina Kaye as well as Tufts Sackler School of Biomedical Sciences postdoctoral fellow Sally McIver for her technical expertise. Lastly, thanks to Tufts Professor of Biology L. Michael Romero for his feedback.
Footnotes
DISCLOSURE / CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PEM, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharm. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Cannon DS. The effect of prior ethanol experience on ethanol-induced saccharin aversions. Physiology & Behavior. 1974;12:1041–1044. doi: 10.1016/0031-9384(74)90152-8. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administraiton by CRF-R1 antagonist in the rat VTA. Psychopharmacology. 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchey L, Shaw S, Lieber CS. Ethanol impairs tryptophan transport into the brain and depresses serotonin. Life Sci. 1981;29:2751–2755. doi: 10.1016/0024-3205(81)90534-8. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Carlsson A, Engel J, Strombom U, Svensson TH, Waldeck B. Suppression by dopamine-agonists of the ethanol-induced stimulation of locomotor activity and brain dopamine synthesis. Naunyn Schmiedebergs Arch Pharmacol. 1974;283:117–128. doi: 10.1007/BF00501138. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotroin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Recent advances in small molecule antagonists of the corticotropin-releasing factor type-1 receptor-focus on pharmacology and pharmacokinetics. Curr Med Chem. 2006;13:1261–1282. doi: 10.2174/092986706776873014. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale WW, et al. Dissociation of locomotor activation and suppression of food intake induced by CRF in CRFR1-deficient mice. Endocrinology. 2000;141:2698–2702. doi: 10.1210/endo.141.7.7653. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Bean AJ, Bissette G, Nemeroff CB, Robbins RJ, Roth RH. Stress-induced alterations in neurotensin, somatostatin and corticotropin- releasing factor in mesotelencephalic dopamine system regions. Brain Res. 1987;417:350–354. doi: 10.1016/0006-8993(87)90462-8. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- D’Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J) J Chem Neuroanat. 2007;33:67–74. doi: 10.1016/j.jchemneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Diana M, Gessa GL, Rossetti ZL. Lack of tolerance to ethanol-induced stimulation of mesolimbic dopamine system. Alcohol Alcohol. 1992;27:329–333. [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Measurement of alcohol preference in genetic experiments. J Comp Physiol Psychol. 1964;57:85–88. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS ONE. 2011;6:e26997. doi: 10.1371/journal.pone.0026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Kakihana R. Circadian rhythms of ethanol consumpton in mice: a simple computer analysis for chronopharmacology. Psychopharmacology. 1977;52:41–45. doi: 10.1007/BF00426598. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Nat Acad Sci. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalson JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Richard D. The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides. 1999;33:350–359. doi: 10.1054/npep.1999.0047. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Kayyali T, DeBold JF, Miczek KA. Excessive alcohol drinking in C57BL/6J mice due to intermittent access to alcohol: influence of CRF and opiod systems. Research Abstract for Res Soc Alcoholism annual scientific meeting. Alcohol Clin Exp Res. 2011;6(Suppl):69A. [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kehne JH. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets. 2007;6:163–182. doi: 10.2174/187152707780619344. [DOI] [PubMed] [Google Scholar]
- Keifer SW, Bice PJ, Badia-Elder N. Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcohol Clin Exp Res. 1994;18:555–559. doi: 10.1111/j.1530-0277.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek KA, et al. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Foundation Symposium. 1992;172:277–295. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal ML. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–59. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: Findings of animal studies. Biol Psychiatry. 1999;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pliel KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist J, Chai Z, Teheranian R, Hasanvan H, Bartfai T, Jenck F, et al. A non-peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur J Pharmacol. 1996;309:195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL. GABAA receptors in the posterior, but not anterior, ventral tegmental area mediate Ro15–4513-induced attenuation of binge-like ethanol consumption in C57BL/6J female mice. Behav Brain Res. 2011;220:230–237. doi: 10.1016/j.bbr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a novel and poten corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- Merlo-Pich PE, Lorang M, Yeganeh M, Rodriguez dF, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: What their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; San Diego: 2003. [Google Scholar]
- Paxinos G, Watson C. The rat brian in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, et al. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrom induced by CRF. 2000 [PubMed] [Google Scholar]
- Pfeffer AO, Samson HH. Effect of pimozide on home cage ethanol drinking in the rat: Dependence on drinking session length. Drug Alcohol Depend. 1986;17:47–55. doi: 10.1016/0376-8716(86)90035-9. [DOI] [PubMed] [Google Scholar]
- Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976;16:693–698. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LJ, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Hwa LS, Shimamoto A, Carlson J, DeBold JF, Miczek KA. Prevention of alcohol-heightened aggression by CRF1 receptor antagonists: critical role for the DRN-PFC serotonin pathway. doi: 10.1038/npp.2014.139. in prep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxicaiton in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, et al. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, et al. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Phillips TJ. Central urocortin 3 administraiton decrases limited access ethanol intake in non-dependent mice. Behav Pharmacol. 2009;20:326–351. doi: 10.1097/FBP.0b013e32832f01ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Chiodo LA. Acute withdrawal after repeated ethanol treatment reduces the number of spontaneously active dopaminergic neurons in the ventral tegmental area. Brain Res. 1993;622:289–293. doi: 10.1016/0006-8993(93)90831-7. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current studies. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF. Inhibition of the transport of 5-hydroxyindoleacetic acid from brain by ethanol. J Neurochem. 1975;24:1043–1051. doi: 10.1111/j.1471-4159.1975.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesocortical DA system by stress. Nature. 1976;263:242–243. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Tomie A, Miller WC, Dranoff E, Pohorecky LA. Intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol Alcohol. 2006;41:225–230. doi: 10.1093/alcalc/agl002. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administraiton in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]