Abstract
Cue-induced heroin seeking after prolonged withdrawal is associated with neuronal activation and altered gene expression in prefrontal cortex (PFC). However, these previous studies assessed gene expression in all neurons regardless of their activity state during heroin seeking. Using Fos as a marker of neural activity, we describe distinct molecular alterations induced in activated versus non-activated neurons during cue-induced heroin seeking after prolonged withdrawal. We trained rats to self-administer heroin for 10 days (6-h/day) and assessed cue-induced heroin seeking in extinction tests after 14 or 30 days. We used fluorescent-activated cell-sorting (FACS) to purify Fos-positive and Fos-negative neurons from PFC 90 min after extinction testing. Flow cytometry showed that Fos-immunoreactivity was increased in less than 10% of sparsely distributed PFC neurons. mRNA levels of the immediate early genes fosB, arc, egr1, and egr2, as well as npy and map2k6, were increased in Fos-positive, but not Fos-negative, neurons. In support of these findings, double-label immunohistochemistry indicated substantial co-expression of NPY- and Arc-immunoreactivity in Fos-positive neurons. Our data indicate that cue-induced relapse to heroin seeking after prolonged withdrawal induces unique molecular alterations within activated PFC neurons that are distinct from those observed in the surrounding majority of non-activated neurons.
Keywords: flow cytometry, self-administration, orbitofrontal, extinction, relapse, craving
Introduction
In former heroin addicts, environmental cues previously associated with heroin use can precipitate drug craving and relapse after prolonged withdrawal periods (Wikler 1973, O’Brien et al. 1992). The prefrontal cortex (PFC) is implicated in cue-induced heroin craving and relapse. In drug-free heroin addicts, cue-induced heroin craving is associated with PFC activation (Yang et al. 2009, Xiao et al. 2006), while in rats, cue-induced heroin seeking after prolonged withdrawal induces several immediate early genes (IEGs) (Koya et al. 2006, Kuntz et al. 2008, Fanous et al. 2012, Bossert et al. 2011, Bossert et al. 2012), including the neuronal activity marker Fos (Morgan & Curran 1991). These findings suggest similar activation of PFC in response to heroin cues in rats and humans. Pharmacological blockade of cue-induced neural activity or interference with cue-induced acute synaptic plasticity alterations in PFC attenuates context- and cue-induced relapse to heroin seeking (See 2009, Bossert et al. 2011, Van den Oever et al. 2008, Van den Oever et al. 2010a, Van den Oever et al. 2010b, Rogers et al. 2008, Fanous et al. 2012), reflecting a critical role for this region in heroin relapse. However, not all PFC neurons have identical roles in mediating cue-induced relapse to heroin seeking. Using our Daun02 inactivation procedure (Koya et al. 2009), we recently found that the small number of sparsely distributed activated Fos-expressing neurons have a particularly important causal role in context- and cue-induced relapse to heroin seeking following prolonged withdrawal (Bossert et al. 2011, Fanous et al. 2012).
The unique molecular or cellular alterations within these cue-activated Fos-positive neurons are unknown. Until recently, a methodology to study the unique molecular alterations in drug cue-activated neurons and compare them with the surrounding non-activated neurons was not available. Previous molecular studies measured gene expression or synaptic plasticity alterations found in either all neurons or specific neuronal cell-types, regardless of their activity during the tests for cue-induced heroin seeking (Koya et al. 2006, Kuntz et al. 2008, Kuntz-Melcavage et al. 2009, Van den Oever et al. 2008, Van den Oever et al. 2010a). In these studies, molecular or cellular changes induced in the activated neurons may be masked by a lack of alterations or even countered by opposing alterations in the surrounding majority of non-activated neurons.
To distinguish molecular alterations in activated versus non-activated neurons, we developed a procedure using fluorescence-activated cell sorting (FACS) to purify neurons from adult rats (Guez-Barber et al. 2012). We used this methodology to separate cocaine-activated striatal neurons from non-activated neurons using transgenic cfos-lacZ rats (Guez-Barber et al. 2011, Kasof et al. 1995). In the present study, we extended this FACS procedure to wild-type rats by using Fos as a marker of neuronal activity (Morgan & Curran 1991) to label and sort PFC (medial and orbital prefrontal cortex, mPFC and OFC, respectively) neurons activated during cue-induced heroin seeking, as assessed in an extinction test after prolonged withdrawal from the drug. We then assessed differential gene expression in Fos-positive versus Fos-negative neurons.
Methods
Animals and surgery
Male Sprague-Dawley rats (350–400 g, Charles Rivers Laboratories, total n=95) were maintained under a reverse 12-h light–dark cycle, with food and water freely available in home cages. Procedures followed the guidelines of the “Principles of Laboratory Care” (NIH publication no. 86–23, 1996) and were approved by the local Animal Care and Use Committee. The rats were allowed to habituate to their home cage for at least 7 days prior to surgery. On the day of surgery, rats were anesthetized with Equithesin (a mixture of chloral hydrate and sodium pentobarbital) and intravenous catheters were implanted as previously described (Bossert et al. 2009, Koya et al. 2009, Lu et al. 2009). The opiate analgesic buprenorphine (NIDA; 0.1 mg/kg, subcutaneous) was injected after surgery to decrease post-surgical pain. During recovery (7–10 days) and training, catheters were flushed every 24–48 h with sterile saline and the antibiotic Gentamicin (0.08 mg/ml) to prevent infections.
Heroin self-administration and extinction testing
For all experiments, rats underwent three experimental phases: heroin self-administration training, a withdrawal period, and extinction tests for cue-induced heroin seeking. During the training phase, rats were chronically housed in self-administration chambers located inside sound-attenuating cabinets and controlled by a Med Associates system (St. Albans, VT). Each chamber had two levers located 9 cm above the floor. Rats were trained to self-administer heroin (0.075 mg/kg per infusion over 3.2 s) during six daily 1-h sessions each separated by 5 min (fixed-ratio-1 with a 20-s timeout reinforcement schedule) (Airavaara et al. 2011, Fanous et al. 2012). At the beginning of the training sessions, catheters were connected via a modified cannula (Plastics One, Roanoke, VA) to liquid swivels (Instech, Plymouth Meeting, PA) with PE-50 tubing. Sessions started at the onset of the dark cycle and began with insertion of the active lever and illumination of a red house light that remained on during the sessions. Active lever presses activated the infusion pump and produced a 5-sec light cue. At the end of each 1-h session, the house light was turned off and the active lever retracted. Lever presses were recorded from both the active lever and a stationary inactive lever, but only active lever presses activated the infusion pump. The number of heroin infusions was limited to 100 per hour. The groups to be tested or not tested for cue-induced heroin seeking at the different withdrawal days were matched for their heroin intake during training. Food and water was freely available for all days of training. During the withdrawal phase, the rats were kept in their home cages for 13–15 days (14-day withdrawal group) or 28–31 days (30-day withdrawal group) and handled 3 times per week.
On test days, rats in the Extinction test groups were exposed to the heroin-related cues under extinction conditions, while rats in the No test groups remained in their home cages. For the Extinction test groups, 90-min extinction tests were conducted under the same experimental conditions as in training except that active lever presses were not reinforced with heroin. Tests started at the onset of the dark cycle and began with the insertion of the active lever and the illumination of the red house light that remained on for the duration of the session. Active lever presses during testing resulted in contingent presentations of the tone and light cue or the light cue alone that was previously paired with heroin infusions but not heroin.
Experiment 1: Gene expression in FACS-purified PFC neurons
A total of 88 rats were used in Experiment 1. All rats underwent behavioral training as described above. Extinction test rats were decapitated without anesthesia immediately after the extinction test, 90 min after the start of testing; No test rats were decapitated at the same time as the Extinction test rats. PFC tissue including OFC and mPFC was dissected within 2 min of decapitation. Subsequent cell dissociation, immunolabeling, and FACS purification were accomplished using the same procedures described in our previous studies (Guez-Barber et al. 2011, Guez-Barber et al. 2012), and repeated below with the exception of using Fos antibodies in the current study to label activated neurons and DAPI for labeling all cells.
Cells were incubated with primary antibodies against NeuN (1:1000 dilution; biotinylated NeuN antibody, MAB377B, Millipore) and Fos (1:1000 dilution; sc-52, Santa Cruz Biotechnologies, Santa Cruz, CA) for 30 min at 4°C followed by one PBS wash. Cells were re-suspended in 350 μl PBS and incubated with fluorescently-tagged streptavidin (1:1000 dilution; streptavidin-phycoerythrin, Life Technologies/Invitrogen, Grand Island, NY) and Alexa 488-labeled anti-rabbit IgG, 1:1000 dilution, Life Technologies/Invitrogen) and rotated 15 min end-over-end. After one PBS wash, cells were resuspended in 500–1000 μl PBS and kept on ice until sorting. Immunolabeled cells were sorted using a FACS Aria (BD Biosciences, Franklin Lakes, NJ) at the flow cytometry core facility on Johns Hopkins Bayview Campus. To verify labeling of intact cells after sorting, a small sample was incubated for 10 min with DAPI (4′,6-diamidino-2-phenylindole) reagent (Invitrogen/Life Technologies) and viewed under a microscope for Fos, NeuN, and DAPI-labeling. DAPI is a small fluorescent molecule that binds to DNA in the nucleus. For all experimental samples, Fos-positive or Fos-negative cells were sorted from rats from the Extinction test groups while all NeuN-positive cells were sorted from rats from the No test control groups.
RNA was extracted from sorted cells and analyzed with quantitative PCR using the same procedures described in our previous studies (Guez-Barber et al. 2011, Guez-Barber et al. 2012). Roche Universal Probe Library Assay Design Center (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=UP030000) was used to design the intron-spanning primer and probe combinations shown in Table 1. All qPCR reactions were performed using an Opticon Light Cycler (Biorad, Hercules, MD). After photobleaching with 20 plate reads at 50°C for photobleaching, reactions began with 5 min of denaturation at 95°C, and then 40 cycles of 94°C for 20 sec, 60°C for 1 min, and then a final plate read.
Table 1.
Primers and probes used for qPCR.
| Gene | Forward primer | Reverse primer | Probe | Efficiency |
|---|---|---|---|---|
| arc | gctgaagcagcagacctga | ttcactggtatgaatcactgctg | #79 | 1.94 |
| egr1 | cgaacaaccctacgagcac | gcgccttctcgttattcaga | #114 | 1.99 |
| egr2 | ctacccggtggaagacctc | tcaatgttgatcatgccatctc | #60 | 1.91 |
| fosB | tgcagctaaatgcagaaacc | ctcttcgagctgatccgttt | #109 | 1.87 |
| gad1 | tacaacctttggctgcatgt | tgagtttgtggcgatgctt | #77 | 2.01 |
| map2k6 | ttggagcctatagtggagctg | ctattaactgtggcccgtatcc | #77 | 2.09 |
| npy | caagctcattcctcgcaga | cattcgtttgttacctagcatca | #114 | 2.02 |
| pde10a | ctaacaatgcgagttgcttcc | tcaccttttcatccgtcaaa | #73 | 1.91 |
| sst | agcccaaccagacagagaac | cctcatctcgtcctgctca | #1 | 2.05 |
| vglut1 | ccttttgcggttcctatgc | ccaaaagatcccgaagctg | #20 | 2 |
Before running test reactions, efficiencies of qPCR reactions (Table 1) were determined by running standard curves for each gene. Relative levels of gene expression were calculated using efficiency^ΔCq where ΔCq = Cq(reference) − Cq(experimental), where Pde10a was the reference gene (Guez-Barber et al. 2011, Guez-Barber et al. 2012). We averaged technical triplicate Cq values before calculating ΔCq. To obtain sufficient mRNA for qPCR, 9–11 rats were pooled for the Fos-positive and Fos-negative Extinction test groups, and 4–10 rats were pooled for the NeuN-positive No test groups. Data from each pooled group represented one biological replicate. We averaged gene expression values across n = 3–6 of these biological replicates. Biological replicate values were averaged to obtain the mean and SEM.
Experiment 2. Fluorescent double-labeling immunohistochemistry of activated PFC neurons
A total of 7 rats were used in Experiment 2. Some of the brain sections collected from these rats were used for immunohistochemical analyses in (Fanous et al. 2012). All rats underwent behavioral training as described above for the Extinction test group that was tested after 14 withdrawal days. These rats were then perfused with paraformaldehyde immediately after the extinction test, 90 min after the start of testing. We used the same procedures for perfusions, double-labeling immunohistochemistry and image analysis as described in our previous studies (Guez-Barber et al. 2011, Guez-Barber et al. 2012, Fanous et al. 2012), and repeated below with the major exception of analyzing PFC brain regions and the addition of double labeling for Fos+NPY. PFC coronal sections were cut 40 μm thick between Bregma +3.7 and +2.7 (Paxinos & Watson 2005).
We used double-label fluorescent immunohistochemistry to characterize protein expression in Fos-expressing neurons in dorsomedial PFC (dmPFC), ventromedial PFC (vmPFC), and OFC. We double-labeled sections for Fos+Arc and Fos+neuropeptide Y (NPY) in order to compare protein expression to mRNA expression results obtained from our FACS and qPCR experiments. Arc and NPY are protein products of two genes whose mRNA expression was strongly altered in Fos-expressing neurons obtained from FACS purification (see Results). Primary antibodies were rabbit anti-Fos (1:400 dilution; sc-52, Santa Cruz Biotechnologies), biotinylated mouse anti-NeuN (1:2000 dilution; Millipore), mouse anti-Arc (1:100 dilution, sc-17839, Santa Cruz), or sheep anti-NPY (1:500 dilution; AB1583, Millipore). Secondary antibodies were Alexa 488-labeled donkey anti-rabbit antibody (1:200 dilution; A-21206, Invitrogen) or Alexa 568-labeled donkey anti-rabbit antibody (1:200 dilution; A-10042, Invitrogen) to label Fos, Alexa 568-labeled donkey anti-mouse antibody (1:200 dilution; A-21202, Invitrogen) to label Arc, and Alexa 488-labeled donkey anti-sheep antibody (1:200 dilution; A-11015, Invitrogen) to label NPY. After labeling and final washes in PBS, sections were mounted and coverslipped onto chromalum-gelatin-coated slides.
All fluorescent images of dmPFC, vmPFC, and OFC (±3.2 mm anterior to Bregma) were obtained using a Coolsnap Photometrics (Roper Scientific) camera on a Zeiss Axioskop 2 microscope. Double-labeled images were captured at 400x magnification. Numbers of Fos-labeled and double-labeled cells in dmPFC, vmPFC, and OFC were counted from one section per rat. For this, we used IPLab software, version 3.9.4 r5 for MacIntosh (Scanalytics Inc).
Statistical analyses
We used a mixed two-factor ANOVA to analyze active and inactive lever-presses during the extinction tests (between-subjects factor-Withdrawal Day; within-subjects factor-Lever [active or inactive]). We also analyzed active lever-presses over time during the extinction test (between-subjects factor-Withdrawal Day; within-subjects factors-Session minute). In Experiment 1, qPCR data were not normally distributed, and were thus analyzed using Kruskal-Wallis non-parametric ANOVAs for the three cell groups (Extinction test NeuN-positive/Fos-negative, Extinction test NeuN positive/Fos-positive, No test NeuN-positive) followed by Mann-Whitney tests for post-hoc analysis.
Results
Cue-induced heroin seeking after withdrawal
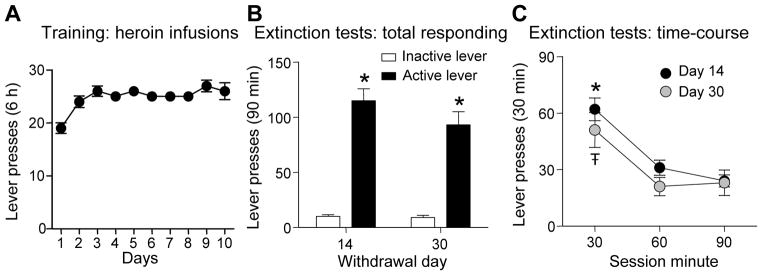
Rats demonstrated reliable heroin self-administration (Figure 1A, final n=85). The mean ± SEM daily heroin intake (infusions per 6 h) over 10 days was 24±1.4. Three rats were excluded because of failure to reach a mean of 10 infusions per day.
Figure 1. Rats self-administer intravenous heroin and show cue-induced heroin seeking after prolonged withdrawal from the drug.
(A) Rats learn to self-administer heroin over 10 days of training. Data represent mean ± SEM heroin infusions per day (total n=85). (B) Cue-induced heroin seeking in the extinction test. During the extinction tests, active lever-presses resulted in delivery of light cue previously paired with heroin infusions but not heroin; inactive lever-presses had no consequences. Total number of lever presses during extinction tests (mean ± SEM; *p<0.05 vs. inactive lever presses for corresponding withdrawal day). (C) Time course of active lever-presses during the extinction tests (mean ± SEM; *Ŧp<0.05 vs. lever presses at 60-min and 90-min time points for corresponding withdrawal day).
Cue-induced heroin seeking was operationally defined as the number of non-reinforced active lever-presses during the extinction tests after 14 (n=41) or 30 days (n=9) of withdrawal. The number of active lever presses during extinction tests were similar for rats withdrawn for 14 and 30 days (Figure 1B and 1C), and were significantly higher than inactive lever presses. The ANOVA for total responding during 90 min on the active lever (Figure 1B), which included the between-subjects factor of Withdrawal Day and the within-subjects factor of Lever, indicated a significant effect of Lever (F1,48=71.9, p<0.001) but not Withdrawal Day or an interaction between the two factors (p values >0.05). Likewise, the ANOVA of the within session time course active lever-presses (Figure 1C), which included the between-subjects factor of Withdrawal Day and the within-subjects factor of Session Time in 30-min intervals, indicated a significant effect of Session Time (F1,47 = 18.7, p < 0.001) but no effects of Withdrawal Day or interaction between the two factors (p values >0.05).
Experiment 1: FACS purification and gene expression in activated prefrontal cortex neurons
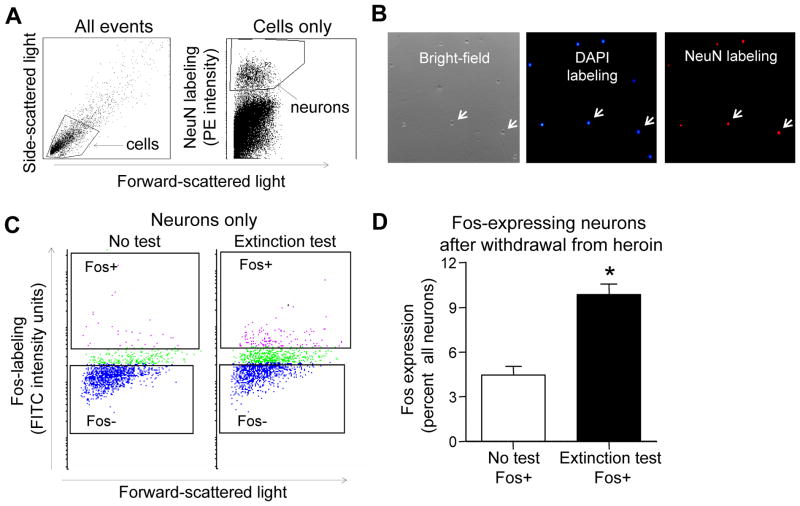
Since behavioral results for the Extinction test groups and the No test groups were similar after 14 and 30 withdrawal days, gene expression results from these two withdrawal times were combined. Prefrontal cortex tissue containing OFC and mPFC was obtained from rats immediately after the 90-min extinction tests (Extinction test group) or immediately after transport from the housing facility (No test group). The majority of dissociated cortical cells were found in the lower left quadrant of the forward and side light-scattergrams (Figure 2A, left panel), similar to neurons obtained in (Guez-Barber et al. 2011, Guez-Barber et al. 2012). Events in this quadrant were selected for further examination using a gate encompassing this quadrant and incubating them with DAPI (Figure 2B). Microscopy indicated most events were round DAPI-labeled cell bodies devoid of processes.
Figure 2. FACS of Fos-labeled neurons from mPFC and OFC after prolonged withdrawal from heroin self-administration.
(A) Left panel: representative light scatter plot in which each dot represents one event (cell or debris). Forward scatter is a measure of size; side scatter is a measure of granularity. The ‘gate’ indicated by the solid-line box encompasses intact cells. Right panel: representative light scatter and fluorescence plot showing only events from the “cells” gate in the left panel scatter plot. Events high on the Y-axis represent neurons. A second gate indicated by the solid-line box labeled “neurons” encompasses all events analyzed on subsequent fluorescence plots. These gates allow selective analysis of neurons and exclusion of other cells and debris. (B) Photomicrographs of FACS-purified cells labeled with DAPI. Left panel: Bright-field image of intact cells indicated by arrows. Middle panel: fluorescence image of blue DAPI-labeling of intact cells indicated by arrows. Right panel: fluorescence image of red NeuN-labeling of neurons indicated by arrows. (C) Representative light scatter and fluorescence plots from the Extinction test and No test rats indicate degree of Fos-labeling of neurons selected by event gates in Figure 3A. Events high and low on the Y-axis represent activated neurons (Fos+) and non-activated neurons (Fos−). (D) Percentage of activated (Fos+) neurons in the Extinction test and No test conditions (mean ± SEM, *p < 0.05, n=3–5 pooled samples (n=46–60 rats) per experimental condition).
After selection of cells encompassed by the first gate from forward and side light-scattergrams, a second forward gate was applied to include only cells labeled for the neuronal marker NeuN (Figure 3A, right panel). Cells within this second gated region (neurons) were then sorted for activation state according to their level of immunolabeling with Fos antibodies (Figure 2C).
Figure 3. Quantitative PCR indicates different gene expression profile for Fos-positive mPFC and OFC neurons compared to Fos-negative neurons from the same Extinction test rats, or compared to all neurons from No test rats.
(A) mRNA levels for the immediate early genes arc, fosB, egr1 and egr2 are increased in Fos-positive neurons from the Extinction test rats. Dotted line represents gene expression level in all neurons from the No test rats (set at 1); *p < 0.05 vs. Fos-negative neurons from same Cue rats; Ŧp < 0.05 vs. all neurons from No cue rats. (B) mRNA levels for npy and map2k6 are increased in Fos-positive neurons; *p ≤ 0.05 vs. Fos-negative neurons from the same Extinction test rats. Ŧp < 0.05 vs. all neurons from No test rats. Data are mean ± SEM; n=3–5 pooled samples (n=46–60 rats) per experimental condition.
From Extinction test rats, 9.9% of NeuN-labeled neurons had Fos expression levels above threshold (Figure 2C and D). We obtained approximately 62,500 Fos-labeled neurons from each rat. All cells labeled for Fos also expressed NeuN; thus only neurons expressed Fos. In contrast, only 4.4% of NeuN-labeled neurons had Fos expression levels above threshold. Overall, heroin cue exposure in the extinction tests induced significantly more Fos-labeled cells than in the No test group (t(11) = 6.43, p < 0.05), which supports Fos immunoreactivity as a marker of neural activation.
We collected Fos-positive and Fos-negative neurons from the Extinction test group for subsequent qPCR analyses. In contrast, for control samples, we collected all NeuN-labeled cells from the No test rats for subsequent qPCR analyses, without separating Fos-positive from Fos-negative neurons. Approximately 4.5% of all events from FACS analysis of No test samples were NeuN-labeled, which corresponded to approximately 302,000 cortical neurons per rat. Our rationale for this control condition was that most heroin cue-activated neurons in the extinction tests are not activated prior to testing, thus any Fos-positive neurons in the No test neuron population are not related to the extinction test. Thus gene expression in Fos-positive neurons from the No test group likely do not represent basal gene expression for other neurons that will eventually be activated during the extinction tests. Any gene expression alterations within Fos-expressing neurons in the No test samples, which are not associated with the extinction tests, would be diluted 20–25-fold and thus have a minimal effect on overall gene expression in these control samples.
Overall, we used gene expression in all neurons from the No test groups to represent a hypothetical baseline gene expression for all neurons prior to heroin cue exposure in the extinction tests. We then calculated gene expression alterations in Fos-positive and Fos-negative neurons from the Extinction test group relative to gene expression in all neurons from the No test group.
For qPCR, Kruskal-Wallis non-parametric tests were performed across three groups of samples: (1) Fos-positive and (2) Fos-negative neurons from the Extinction test rats and (3) all neurons from No test rats (n=3–5 samples for each condition; each sample represented 10–11 rats for the Extinction test group and 4–9 rats for the No test group). IEG expression was increased in only Fos-positive neurons obtained from the Extinction test group. mRNA levels for arc, fosB, egr1, and egr2 were elevated in Fos-positive neurons compared to Fos-negative neurons obtained from the same rats (Figure 4, χ2(2) = 9.6, 9.4, 6.0, and 8.7, respectively, p ≤ 0.05). Levels of arc, fosB, and egr2 mRNAs in Fos-positive neurons, but not Fos-negative neurons, were also elevated relative to all NeuN-labeled neurons obtained from the No test group (p < 0.05). Expression levels for npy and map2k6 were elevated in Fos-positive neurons compared to Fos-negative neurons obtained from the Extinction test rats and compared to all neurons obtained from No test rats (χ2(2) = 6.5 and 6.4, respectively, p < 0.05). In contrast, npy expression in Fos-negative neurons from Extinction test rats was decreased relative to all NeuN-labeled neurons from No test rats (p < 0.05).
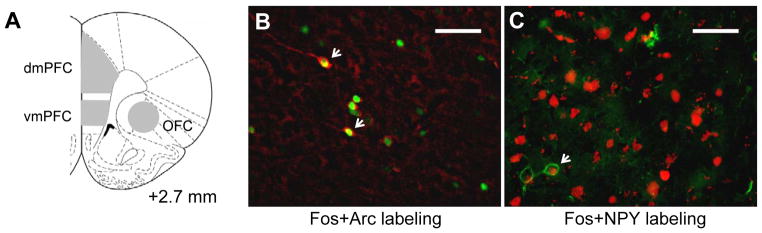
Figure 4. Immunohistochemical characterization of Fos-expressing neurons in mPFC and OFC after cue-induced heroin seeking on withdrawal day 14.
(A) Schematic showing regions analyzed using fluorescent immunohistochemistry (Paxinos & Watson 2005). Representative images: (B) Fos+Arc double-labeling in dmPFC, scale bar 50μm; Arc-labeling in red, Fos-labeling in green, examples of double-labeled neurons in red and green indicated by white arrows. (C) Fos+NPY double-labeling in OFC, scale bar 50μm; NPY-labeling in green, Fos-labeling in red, example of double-labeled neuron in red and green indicated by white arrow.
Experiment 2: Fluorescent immunohistochemistry
To confirm increased expression of npy and arc in Fos-positive neurons, we assessed co-expression of NPY and Arc protein with Fos in histochemical sections of dmPFC, vmPFC, and OFC (Table 2). We found 37% of all Fos-labeled neurons co-expressed Arc (Figure 4B), and 7.5% of all Fos-labeled neurons co-expressed NPY (Figure 4C). This represents a preferential activation of NPY-containing neurons, because NPY-containing neurons comprise only 10% of all GABA neurons in the cortex (Kubota et al. 2011), and GABA neurons comprise ~25% of cortical neurons (Tamminga et al. 2004); thus, NPY-containing neurons only comprise 2.5% of all cortical neurons but comprise 7.5% of all Fos-labeled neurons in our Extinction test rats. This indicates that Fos-positive neurons are 3 times more likely to contain NPY than the overall distribution of NPY-containing neurons in cortex.
Table 2.
Characterization of Fos-positive neurons in PFC of rats exposed to heroin cues in the extinction tests (n = 4–7 rats).
| dmPFC | vmPFC | OFC | Overall | |
|---|---|---|---|---|
| Percent Fos-positive neurons containing NPY: | 3.85 ± 3.4 | 4.29 ± 4.8 | 14.38 ± 7.2 | 7.51 ± 2.7 |
| Percent of Arc-positive neurons containing Fos: | 53.32 ± 7.2 | 44.58 ± 10.8 | 49.86 ± 9.2 | 49.59 ± 2.3 |
| Percent of Fos-positive neurons containing Arc: | 37.02 ± 5.1 | 22.85 ± 6.7 | 32.47 ± 7.3 | 31.35 ± 1.7 |
Discussion
We used FACS to purify a small proportion of PFC neurons selectively activated during extinction tests after prolonged withdrawal from self-administered heroin. This is the first FACS purification of activated neurons from wild-type rats and the first to use FACS following cue-induced drug seeking in an animal model of drug relapse and craving (Shaham et al. 2003, Epstein et al. 2006). Cue-induced heroin seeking in the extinction tests produced unique gene expression patterns in activated neurons that were distinct from those observed in non-activated neurons obtained from the same tissue. IEG expression in particular was increased in activated neurons while expression was unaltered in non-activated neurons. These results indicate that very different patterns of gene expression are induced in activated neurons versus non-activated neurons in the extinction tests. These changes are likely to be important for relapse behavior because activated ventral mPFC neurons play a causal role in context-induced heroin seeking after extinction (Bossert et al. 2011, Bossert et al. 2012) and activated lateral OFC neurons play a causal role in cue-induced heroin seeking (assessed in extinction tests) after prolonged withdrawal (Fanous et al. 2012).
FACS and double-labeling data indicated very different patterns of IEG expression between Fos-positive and Fos-negative neurons. Cue-induced heroin seeking was associated with increased mRNA expression of arc, fosB, egr1, and egr2 (4.5 to 13.6-fold) in only Fos-positive PFC neurons but not in Fos-negative neurons obtained from the same tissue. Previously, cue-induced heroin seeking was shown to increase mRNA expression of arc, egr1, and egr2 (maximally 2.5-fold) in PFC homogenates (Koya et al. 2006, Kuntz et al. 2008). Lower degrees of increased gene expression in these studies are probably due to dilution of IEG induction in the small number of Fos-expressing neurons by the majority of Fos-negative neurons in the homogenates. Heroin cue-induced expression of multiple IEGs in only the Fos-positive neurons further supports the idea that Fos is an endogenous marker of neural activity (Morgan & Linnoila 1991). Graybiel and colleagues used induction of a similar cassette of IEGs to validate neural activation within c-fos-expressing neurons (Moratalla et al. 1996, Moratalla et al. 1992). It should be noted that many of these IEGs are transcription factors capable of regulating downstream waves of gene expression (Nestler 2001, Russo et al. 2010, Wolf et al. 2004). Thus subsequent gene expression patterns in activated Fos-expressing neurons will likely be very different from those in the less activated majority of Fos-negative neurons.
Sparse cue-induced neuronal activation is consistent with the larger hypothesis that these activated neurons are part of a neuronal ensemble encoding the learned association between environmental cues and heroin, and reactivated on test day by heroin-related cues (Bossert et al. 2011, Bossert et al. 2012). Using our Daun02 inactivation procedure (Koya et al. 2009), we have shown that neuronal ensembles in the mPFC and OFC activated in the heroin self-administration environment mediate context-induced reinstatement heroin seeking (Bossert et al. 2011) and enhanced cue-induced heroin seeking (incubation of heroin craving) after prolonged withdrawal (Fanous et al. 2012). In our previous FACS purification studies, we showed similar patterns of IEG expression in activated versus non-activated striatal neurons obtained from c-fos-lacZ transgenic rats following context-specific sensitization of cocaine-induced locomotion (Guez-Barber et al. 2011). Likewise, in this behavioral model, Daun02 inactivation demonstrated a critical role for these neuronal ensembles in the learned associations between drug injections and environmental cues associated or paired with exposure to the drug (Koya et al. 2009). Overall, we speculate that unique alterations of gene expression within these activated neurons are likely to play a more important role in conditioned drug effects than gene expression changes in the non-activated majority of neurons.
FACS and qPCR results also indicate increased npy mRNA in activated PFC neurons and decreased npy in non-activated neurons from the Extinction test group. In contrast, exposure to heroin cues in extinction tests decreases npy mRNA in homogenates of PFC tissue (Kuntz-Melcavage et al. 2009); this latter effect is likely due to decreased npy expression in the non-activated majority of neurons, which masked increased npy expression in only the small number of activated neurons. This contrast exemplifies the potential masking of gene expression in selectively activated neurons when homogenates are used to measure gene expression changes induced by learned behaviors. PFC Fos-positive neurons activated by heroin cues in the extinction tests also had enhanced map2k6 mRNA, a gene which activates the p38 MAP kinase signaling pathway (Raingeaud et al. 1996) and is involved in synaptic plasticity and learning and memory (Bolshakov et al. 2000). Thus, enhanced signaling selectively in neurons activated during the extinction tests may reflect prior repeated activation of these neurons during heroin self-administration training.
Concluding remarks and future directions
We used a novel FACS-based technique to isolate PFC neurons activated during tests for cue-induced heroin seeking after prolonged withdrawal. PFC neurons were activated by heroin cues in the extinction tests and had very different gene expression than non-activated neurons, particularly of IEGs. We propose that this methodology has wide-scale applicability for identifying unique molecular alterations in activated neuronal ensembles encoding other learned behaviors. An important future direction of the FACS-based approach is to further refine the methodology to be able to use small amounts of tissue from PFC sub-regions and from single rats to avoid pooling multiple subjects for each qPCR sample. Finally, the causal roles of unique neuroadaptations found only in activated neurons in neural plasticity and behavior must eventually be examined by manipulating gene expression selectively in activated neurons, although techniques to achieve this goal have yet to be developed.
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program. D.G.B. was supported by National Institutes of Health Medical Scientist Training Program TG 5T32GM07205, Award Number F30DA024931 from the National Institute on Drug Abuse. We would like to thank Joe Chrest for his excellent technical assistance with FACS, and Jennifer Bossert for advice on technical matters in behavioral experiments.
Footnotes
The authors have no conflict of interests.
Author contributions: Behavioral experiments were planned by S.F., B.T.H, and Y.S., and carried out by S.F., R.S., E.M.G., and F.T. FACS and PCR experiments were planned by S.F. D.G.B. and B.T.H., and carried out by S.F. and D.G.B. S.F. and E.M.G performed immunohistochemistry experiments. S.F. wrote and B.T.H, D.G.B and YS edited the manuscript.
References
- Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, Liu QR, Hoffer BJ, Shaham Y. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F. Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci. 2000;3:1107–1112. doi: 10.1038/80624. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FRM, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. Journal of Neuroscience. 2012;32:11600–11609. doi: 10.1523/JNEUROSCI.1914-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Golden SA, et al. FACS Identifies Unique Cocaine-Induced Gene Regulation in Selectively Activated Adult Striatal Neurons. J Neurosci. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez-Barber D, Fanous S, Harvey BK, Zhang Y, Lehrmann E, Becker KG, Picciotto MR, Hope BT. FACS purification of immunolabeled cell types from adult rat brain. J Neurosci Methods. 2012;203:10–18. doi: 10.1016/j.jneumeth.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasof GM, Mandelzys A, Maika SD, Hammer RE, Curran T, Morgan JI. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J Neurosci. 1995;15:4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective Coexpression of Multiple Chemical Markers Defines Discrete Populations of Neocortical GABAergic Neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav. 2008;90:349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz-Melcavage KL, Brucklacher RM, Grigson PS, Freeman WM, Vrana KE. Gene expression changes following extinction testing in a heroin behavioral incubation model. BMC Neurosci. 2009;10:95. doi: 10.1186/1471-2202-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morgan PF, Linnoila M. Regional induction of c-fos mRNA by NMDA: a quantitative in-situ hybridization study. Neuroreport. 1991;2:251–254. doi: 10.1097/00001756-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam: 2005. [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Tamminga C, Hashimoto T, Volk DW, Lewis DA. GABA neurons in the human prefrontal cortex. Am J Psychiatry. 2004;161:1764. doi: 10.1176/ajp.161.10.1764. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Lubbers BR, Goriounova NA, et al. Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology. 2010a;35:2120–2133. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010b;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Lee T, Zhang JX, Wu Q, Wu R, Weng X, Hu X. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug Alcohol Depend. 2006;83:157–162. doi: 10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xie J, Shao YC, Xie CM, Fu LP, Li DJ, Fan M, Ma L, Li SJ. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum Brain Mapp. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]