Abstract
Mitochondrial dysfunction and synaptic damage have been described as early events in Alzheimer’s disease (AD) pathogenesis. Recent research using AD postmortem brains, and AD mouse and cell models revealed that amyloid beta (Aβ) and tau hyperphosphorylation are involved in mitochondrial dysfunction and synaptic damage in AD. Further, recent research also revealed that the protein levels of mitochondrial outer membrane protein, voltage-dependent anion channel 1 (VDAC1) are elevated in the affected regions of AD postmortem brains and cortical tissues from APP transgenic mice. In addition, emerging research using AD postmortem brains and AD mouse models revealed that VDAC1 is linked to Aβ and phosphorylated tau, blocks the mitochondrial permeability transition (MPT) pores, disrupts the transport of mitochondrial proteins and metabolites, impairs gating of VDAC, and causes defects in oxidative phosphorylation, leading to mitochondrial dysfunction in AD neurons. The purpose of this article is to review research that has investigated the relationship between VDAC1 and the regulation of MPT pores in AD progression.
Introduction
Alzheimer’s disease (AD) is a late-onset, progressive, age-dependent neurodegenerative disease, characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality [1–3]. Currently, 5.4 million Americans are living with AD – 5.2 million who are 65 years of age and older, and the remaining 0.2 million under the age of 65. Current estimates are that 16 million persons will have AD by 2050. Of Americans aged 65 years and older, 1 in 8 has AD, and nearly half of people aged 85 years and older have the disease [4]. With lifespan increasing in humans, AD is headed towards becoming the major health concern of elderly persons. In addition to the personal, social, and family hardships that AD creates, the numbers of expected AD patients will translate into extremely high health-care costs. In 2012, the direct costs of caring for those with AD or other dementias in the United States will total an estimated $200 billion [4].
AD is associated with the loss of synapses, synaptic function, mitochondrial abnormalities, inflammatory responses, and neuronal loss, in addition to 2 major pathological hallmarks: 1) intracellular neurofibrillary tangles and 2) extracellular amyloid beta (Aβ) deposits in the regions of the brain that are responsible for learning and memory. Genetic mutations in APP, PS1, and PS2 genes cause about 1–2% of all AD cases. Several factors contribute to late-onset AD: lifestyle, diet, environmental exposure, genetic variants in the sortilin-related receptor 1 gene clusterin, the complement component receptor 1, CD2AP, CD33, EPHA1, and MS4A4/MS4A6E genes and the ApoE 4/4 genotype [5].
Although AD pathogenesis involves multiple molecular and cellular events, 2 events that occur early in AD development are: 1) synaptic damage and 2) mitochondrial dysfunction [3,6–12]. These 2 events are likely caused by an age-dependent accumulation of Aβ and phosphorylated tau in neurons [9]. Recently, several studies reported mitochondrial abnormalities as additional molecular and cellular events in AD progression. These events include changes in mitochondrial DNA, decreased mitochondrial enzyme activities, abnormal mitochondrial gene expressions, increased mitochondrial fragmentation, and decreased mitochondrial fusion [13]. Recently, we reported Aβ and phosphorylated tau associated with mitochondrial membranes and causing mitochondrial dysfunction. Further, we found that the mitochondrial outer membrane protein, voltage-dependent anion channel 1 (VDAC1) directly interacts with Aβ and phosphorylated tau, and contributes to impairments in mitochondrial pore opening and closing [14. The purpose of this article is to review studies that are evaluating the role of VDAC1 in AD pathogenesis and the relationship between VDAC1 and phosphorylated tau in mitochondrial dysfunction that is known to occur in AD.
Mitochondrial permeability transition pore
Mitochondria, present in all eukaryotic cells, including neurons, are cytoplasmic organelles that are essential for cell survival and cell death [15]. The half-life of neuronal mitochondria is about one month [16]. A mitochondrion contains 2–10 copies of mtDNA [15]. The number of mtDNA copies and the number of mitochondria per cell are dependent on the energy demand of the cell. Mitochondria are controlled and regulated by mitochondrial and nuclear genomes. A mitochondrial genome is a 16.5-kb, double-stranded circular DNA molecule that is maternally transmitted [17]. mtDNA has 2 strands: a guanine-rich outer strand and cytosine-rich inner strand. mtDNA encodes 13 polypeptides participating in oxidative phosphorylation. mtDNA also encodes the 12S and 16S rRNA genes. The 22 tRNA genes are required for mitochondrial protein synthesis. Nuclear genes encode over 1000 mitochondrial proteins that participate in oxidative phosphorylation (OXPHOS) along with mitochondrial-encoded genes. Nuclear mitochondrial proteins are synthesized in the cytoplasm and are transported into mitochondria.
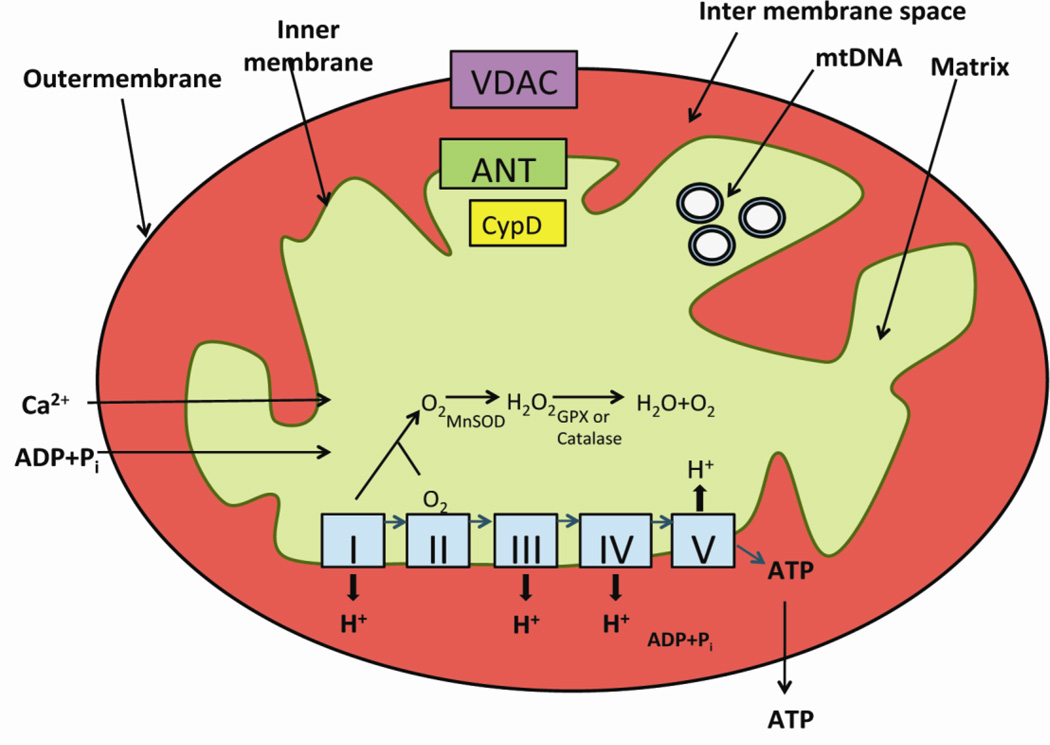
Mitochondria are the power houses of cells, performing several cellular functions, including intracellular calcium regulation, ATP production, the release of proteins that activate the caspase family of proteases, and the alteration of the reduction-oxidation potential of cells and free-radical scavenging. Structurally, mitochondria are compartmentalized into two biolipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane [16] (Fig. 1). The inner mitochondrial membrane houses the ETC and provides a highly efficient barrier to the flow of ions. The inner mitochondrial membrane covers the mitochondrial matrix, which contains tricarboxylic acid and beta-oxidation. The outer mitochondrial membrane is basically porous and allows the passage of low molecular-weight substances, between the cytosol and the mitochondrial intermembrane space (Fig. 2). These mitochondrial permeability transition (MPT) pores are formed by the mitochondrial outer membrane protein VDAC1, the mitochondrial inner membrane protein, the adenine nucleotide translocator (ANT), and the matrix protein cyclophilin D (CypD).
Figure 1.
The structure of mitochondria. Mitochondria are compartmentalized into two biolipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane. The inner mitochondrial membrane houses the mitochondrial respiratory chain or electron transport chain (ETC) and provides a highly efficient barrier to ionic flow. In the ETC - complexes I, III leak electrons to oxygen, producing primarily superoxide radicals, and superoxide radicals are dismutated by manganese superoxide dismuase and produce H2O2. Further, ETC involves the reduction of H2O2 to H2O and O2 by catalase or glutathione peroxidase accepting electrons donated by NADH and FADH2 and then yielding energy for the generation of ATP from adenosine diphosphate and inorganic phosphate.
Figure 2.
Schematic representation of mitochondrial permeability transition pore opening and closure. In an open state of mitochondrial permeability transition pore, metabolites, including ADP/ATP, inorganic phosphate, pyruvate and other substrates and ions, enter and leave mitochondria after passing through the outer mitochondrial membrane. In a closed state, VDAC does not allow the regular flow of ADP/ATP through the outer membrane.
The cross talk and transport of metabolites and proteins between mitochondria and the rest of cell are important to complete OXPHOS and to produce mitochondrial ATP. Increasing evidence suggests that mitochondrial pore opening and pore closure of VDAC are impaired in mitochondria, in brain tissues from patients with neurodegenerative diseases, with inherited mitochondrial diseases, stroke, cancer, and ischemia [18–21].
VDAC structure and expression
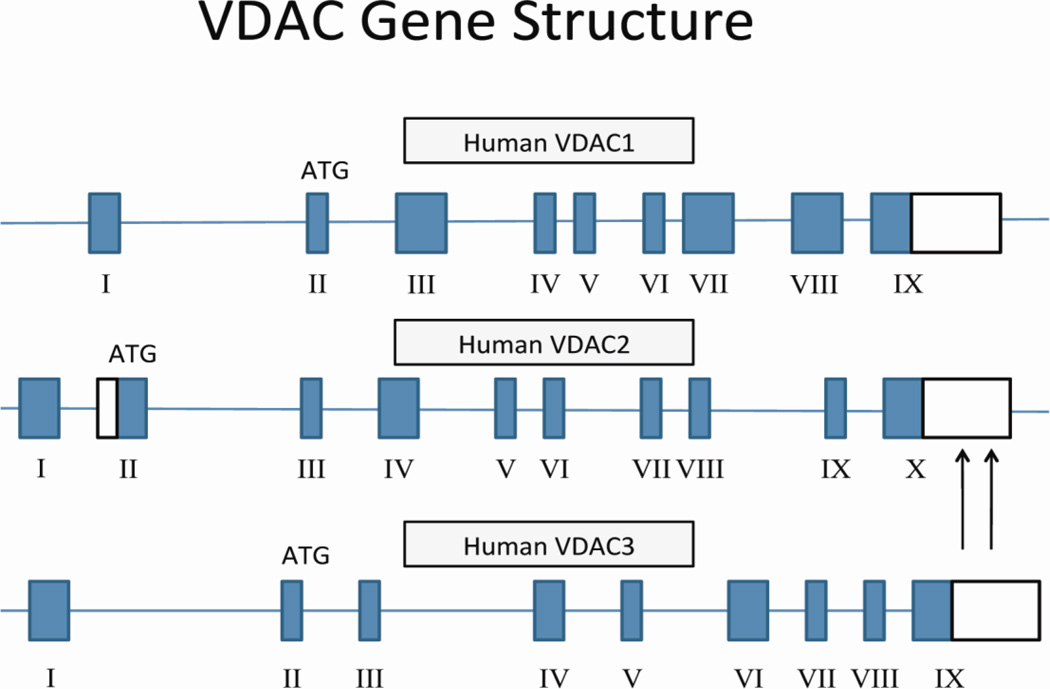
VDACs, also known as mitochondrial porins, have been found in the outer mitochondrial membrane of paramecium tetraurelia [22] and in the outer mitochondrial membrane of mitochondria in mammals, including humans [23–24]. Studies have found that VDACs are highly conserved [25–28]. Three isoforms of VDAC: VDAC1 and VDAC3 are reported to have 9 exons and VDAC2, 10. The additional exon in VDAC2 is believed to encode part of the 5′-UTR region [29–30] (Fig. 3). VDAC1 has 2 splice variants, and VDAC2 and VDAC3 each have one splice variant.
Figure 3.
The structure of human VDAC gene. The human VDAC gene has 3 isoforms; isoform 1 or VDAC1 has 9 exons with a start codon, ATG in exon 2 and a long polyadenylation site. Isoform 2 and VDAC2 has 10 exons with multiple polyadenylation sites, and isoform 3 has 3 has 9 exons. VDAC1 has 2 splice variants, and VDAC2 and VDAC3 each have one splice variant.
VDAC1 is believed to be youngest of the 3 isoforms, and VDAC3, the oldest. VDAC1/2 is estimated to have diverged from VDAC3 about 365 60 MY ago, and VDAC1 and VDAC2, about 289±63 MY ago [31–33]. The human VDAC1 has been mapped to chromosome 5, and the human VDAC2, to chromosome 10. The mouse VDAC1 has been mapped to chromosome 11; the mouse VDAC2, to chromosome 14; and the mouse VDAC3, to chromosome 8. Both VDAC1 and VDAC2 cDNA sequences in humans and mice have 90% homology, in contrast to the VDAC3 cDNA sequences, which have 68% homology between humans and mice (NCBI database).
Of the 3 VDAC isoforms, in mammals, VDAC1 is the most widely expressed, followed by VDAC2 and then VDAC3 [34–35]. VDAC1 and VDAC2 are expressed in the heart, liver, and skeletal muscles, and in the brain. VDAC1 is also expressed, but in very low levels, in the testes [29–30]. VDAC3 is expressed in the testes, liver, ovary, adrenal, lung, spleen, and kidney muscles [36].
VDAC function and physiology
VDAC proteins perform several important functions in the cell, including regulating cell survival, growth, and fertility; maintaining synaptic plasticity through mitochondrial permeability in the transition pore; regulating calcium transport, regulating ATP transport, regulating mitochondrial shape and structural changes; regulating hexokinase interactions with mitochondria; and regulating apoptosis signaling [37–39] (Fig. 4). These functions have been found to be altered in cells from patients with neurodegenerative and mitochondrial diseases, leading to mitochondrial dysfunction. Further, increasing evidence suggests that VDAC interacts with several cytoplasmic proteins, alters channel activity, VDAC closure and reduces VDAC channel conductance [18–21].
Figure 4.
VDACs perform multiple functions – 1) regulating cell survival and cell growth, 2) fertility; 3) maintaining synaptic plasticity through MPT pore, 4) regulating calcium transport, 5) regulating ATP transport, 6) regulating mitochondrial shape and structural changes, 7) regulating hexokinase interactions with mitochondria and 8) regulating apoptosis signaling.
VDAC1 and VDAC2 form pores in the mitochondrial outer membrane, specifically in the biolipid layers of the mitochondrial outer membrane, and there is evidence suggesting that recombinant VDAC3 is not able to open the pores [40]. VDACs exhibit voltage dependence, and VDAC1 also closes the mitochondrial pores, when the transmembrane voltage exceeds 20–30 mV [41–42]. In a normal and open state of VDAC, metabolites, including ADP, ATP, inorganic phosphate and other substrates, enter and leave mitochondria after passing through the outer mitochondrial membrane. In a closed state, VDAC does not allow the regular flow of ADP/ATP through the outer membrane (Fig. 2). It is believed that VDAC is constantly open in metabolic state. However, recent evidence suggests that VDAC closes clearly during apoptosis in diseased neurons. Consequently, with its pores closed, mitochondria may not be able to release ATP into the cytoplasm and/or to uptake ADP, and inorganic phosphate and respiratory substrates from the cytoplasm [43–44]. The anti-apoptotic protein Bcl2-XL has been found to prevent VDAC closure [44], whereas tBid, a pro-apoptotic member, has been found to promotes the pore closure [45]. VDAC appears to be involved in both pro- and anti-apoptosis aspects of mitochondria.
VDAC channel conductance may be impaired in a couple different ways: 1) In neurons from mitochondrial diseases, VDAC may interacts with mutant and cytoskeletal proteins that may have accumulated during disease progression and may have blocked the mitochondrial pores 2. Phosphorylated VDAC may also interact with cytoplasmic proteins, leading to the blockade of mitochondrial pores. In both scenarios, channel conductance would be impaired and may lead to reduced mitochondrial respiration and to mitochondrial dysfunction. In support of VDAC inhibition and subsequent impairment in VDAC channel conductance by cytoskeletal protein, tubulin, Rostovtseva and colleagues [46] reported an abnormal interaction between VDAC and tubulin, resulting in the blockage of mitochondrial pores, disruption in the flux of metabolites between mitochondria and cytoplasm, and the inhibition of mitochondrial respiration [46]. Recently, in a study of brain tissue from postmortem brains of patients with AD, Manczak and Reddy [14] found that VDAC interacted with mutant AD proteins (including Aβ and phosphorylated tau), which in turn blocked mitochondrial pores and interrupted the flow of ATP, ADP, inorganic phosphate substrates, and respiratory substrates between mitochondria and the cytoplasm, ultimately leading to mitochondrial dysfunction [14].
In addition, recent studies revealed that VDAC proteins and their binding partners are modified post-translationally due to VDAC hyperphosphorylation and are involved in the impairment of channel conductance and malfunction of VDAC [21–47–48]. VDAC protein consists of multiple phosphorylation sites, some of which are reported to undergo phosphorylation [21]. VDAC1 is phosphorylated by protein kinase C, leading to a decreased single channel current and open probability [49]. GSK3β protein phosphorylates VDAC1 at Thr51, which in turn disrupts the binding of HK-II to VDAC [50]. Recently, Manczak and Reddy [14] reported phosphorylated tau interacts with VDAC1, leading to pore closure and to reduced mitochondrial function in neurons from APP transgenic mice.
Overall, VDAC performs several functions VDAC channel conductance is regulated by multiple factors. Further research is needed to understand the mechanistic links between VDAC and phosphorylated tau and between VDAC and Aβ.
VDAC knockout mouse models
In studies aimed at elucidating the function of VDAC proteins, William Craigen’s research group generated VDAC1, VDAC2, and VDAC3 heterozygote (+/−) knockout embryonic stem (ES) cells studied them for mitochondrial respiration and mitochondrial enzymatic activity [51]. The ES cells that were deficient in VDAC1, VDAC2, and VDAC3 were viable but showed a 30% reduction in oxygen consumption and reduced cytochrome oxidase activity. The VDAC3-deficient cells did not show any change in cytochrome oxidase activity relative to wild-type VDAC3 cells. These results indicated that each mouse VDAC isoform is not essential for cell viability [51]. Heterozygote knockout VDAC1, VDAC2, and VDAC3 ES cells were used to produce both hetero- and homozygous knockout mice for VDAC1, VDAC2, and VDAC3, but only VDAC1 and VDAC3 homozygous knockout mice were able to be generated. Further genetic analysis revealed that mutant and wild-type alleles of the VDAC3 locus were transmitted in the expected Mendelian ratios, but VDAC1−/− mice were bred in less-than-expected numbers, suggesting partial embryonic lethality of VDAC1−/−, in particular between embryonic days 10.5 and 11.5. The surviving VDAC1−/− mice were fertile, but were mildly retarded in growth, while the VDAC3−/− male mice were infertile [52]. However, the VDAC heterozygote knockout (VDAC1+/−) and VDAC3+/− mice [52] were fertile and had a normal lifespan.
Using cell culture studies, Cheng et al. [53] studied the role of VDAC2 in the mitochondrial pathway of apoptosis. They found BAK complexed with VDAC2, a VDAC isoform present in low abundance that interacts specifically with the inactive conformer of BAK. Cells deficient in VDAC2 exhibited enhanced BAK oligomerization and were more susceptible to apoptotic death. The overexpression of VDAC2 selectively prevented BAK activation and inhibited the mitochondrial apoptotic pathway. Thus, VDAC2, an isoform restricted to mammals, may regulate the activity of BAK and may provide a connection between mitochondrial physiology and the core apoptotic pathway. Findings from this study may partially explain why VDAC2−/− mice were not able to produce.
To determine the role of VDACs in mitochondrial permeability pore transition, Baines et al. [49] generated simultaneous ablation of VDAC1, VDAC2, and VDAC3 proteins by combining gene deletion and the silencing approach. They found that mitochondria from VDAC 1-, VDAC 3-, and VDAC 1– VDAC 3 null mice exhibited Ca2+- and oxidative stress-induced MPT pores that were indistinguishable from those in wild-type mitochondria. Similarly, Ca2+- and oxidative-stress-induced MPT pores and cell death were unaltered in fibroblasts lacking VDAC1, VDAC 2, VDAC 3, VDAC 1– VDAC 3, and VDAC 1– VDAC 2– VDAC 3. Wild-type and VDAC-deficient mitochondria also exhibited equivalent cytochrome c release, caspase cleavage, and cell death in response to the pro-death Bcl-2 family members Bax and Bid. These results indicate that VDACs are critical for both MPT and Bcl-2 family member-driven cell death.
Overall, these knockout VDAC mouse studies suggest that VDAC2 is important for cell survival, but that VDAC1 and VDAC 2 are also essential for cell death, MPT pores, and mitochondrial functions, but that cells are able to survive with a reduction in VDAC1 and VADC3.
Mitochondrial dysfunction in Alzheimer’s disease
Mitochondrial dysfunction and synaptic damage have been identified as early events in AD pathogenesis, but their underlying mechanisms are not completely understood. Mitochondrial dysfunction has been described in AD postmortem brains [54–60], AβPP transgenic mice [61–67], and cells that express mutant APP and cells treated with Aβ [68–77]. Recently, several groups investigated mitochondrial gene expressions in AD postmortem brains [59–60,78–80] and in AβPP mice [61]. They found mitochondrial-encoded genes abnormally expressed in the brains from the AD patients and the AβPP mice. These abnormal mitochondrial gene expressions may be compensatory responses to mitochondrial dysfunction that Aβ may induced. Several studies found increased free radical production, lipid peroxidation, oxidative DNA and protein damages, and reduced ATP production in brains from AD patients compared to control subjects [54–56,81–83]. In addition, studies of mitochondrial structure in AD postmortem brains and neuronal cells expressing the mutant APP found that Aβ fragments mitochondria and causes structural changes in neurons [57,60,68–71].
APP, Aβ and their association with mitochondria in Alzheimer’s disease
As discussed above, mitochondria dysfunction and oxidative stress have been extensively reported. However, the precisely molecular link between mitochondrial dysfunction and AD pathogenesis described recently. Using biochemical, molecular, and electron microscopy studies, and AD postmortem brains and brains from AβPP mice, several groups studied the connection between Aβ and mitochondria, and found that A is associated with mitochondria and is responsible for generating increased free radicals and mitochondrial dysfunction [62,65–67,84–87]. Further, a recent study found that Aβ is transported into the inner mitochondrial membrane via the translocase of the outer membrane machinery [85].
Shi Du Yan’s group reported on the interaction between Aβ and the mitochondrial matrix proteins ABAD and CypD, which was found to lead to increased ROS production, mitochondrial dysfunction, and cognitive damage in APP mice (J20 line) [62–84]. They also found reduced interaction between Aβ and ABAD, and between Aβ and CypD. They found that reduced ABAD and CypD protects against Aβ toxicity in AD neurons [84,88].
Several studied found APP in mitochondrial membranes, in neurons affected by AD [55,89–91]. These studies also found mitochondrial APP in the N-terminal, inside the mitochondria, and in the C-terminal of the protein that faces the cytosolic side [89,91]. Recently, Devi and colleagues found that full-length APP and the C-terminal truncated APP without the Aβ domain accumulate progressively in mitochondria, in patients with mild, moderate, and severe AD, but not in age-matched subjects without AD [55].
Overall, these studies suggest that APP and Aβ are associated with mitochondrial membranes and are critically involved in mitochondrial dysfunction and neuronal damage in AD progression.
Phosphorylated tau and its association with mitochondria in Alzheimer’s disease
Several groups recently investigated the relationship between tau and mitochondria, and found that N-terminal tau is associated with mitochondrial membranes [92–94]. Amadoro et al. [92] reported that a 20–22 kDa NH2-truncated tau fragment was largely enriched in human mitochondria from synaptosomes of AD brains and that the amount of tau in the terminal fields correlated with pathological synaptic changes and with organelle functional impairment.
Atlante et al. [93] studied the relationship between overexpressed N-terminal tau fragments (1–25 aa and 26–44 aa) and mitochondrial dysfunction. They tested both fragments for ATP synthesis, membrane potential and ANT activity. They found that oxidative phosphorylation was not affected by the N-terminal fragment 1–25 aa tau fragment, but was dramatically impaired by the N-terminal 26–44 aa tau fragment. Both cytochrome c oxidase and the ANT are targets of the N-terminal 26–44 tau fragment, but the ANT is a unique mitochondrial target, responsible for impairment of oxidative phosphorylation by the N-terminal 26–44 tau fragment, which exerts deleterious effects on cellular availability of ATP when the ATP is synthesized in the inner mitochondrial membrane.
Quintanilla et al. [94] reported that the expression of tau that they induced at Asp-421 to mimic caspase cleavage (T4C3) was toxic to immortalized cortical neurons compared with a full-length tau isoform (T4). T4C-expressing cells induced mitochondrial fragmentation and elevated oxidative stress levels, in comparison with T4-expressing cells. Thapsigargin treatment of T4 or T4C3 cells, which causes an increase in intracellular calcium levels, resulted in a significant decrease in mitochondrial potential. It also resulted in the loss of mitochondrial membrane integrity in T4C3 cells when compared with cells expressing T4. Mitochondrial fragmentation and membrane damage were ameliorated in T4C3 cells when they were pretreated with cyclosporine A or FK506, indicating that the calcium-dependent phosphatase calcineurin in these pathogenic events.
Overall, findings from these studies suggest that the N-terminal fragment of tau may cause mitochondrial dysfunction and defects in oxidative phosphorylation, in AD neurons.
Elevated VDAC1 levels in AD brains and AD transgenic mice
To our knowledge, very little published evidence is available addressing the involvement of VDAC1 in the progression of disease in AD. To determine the role of VDAC1 in AD progression and pathogenesis, using quantitative real-time RT–PCR with Sybr-Green chemistry, we measured mRNA fold changes for VADC1 in postmortem brain specimens from patients in early and late stages of AD and in postmortem brain specimens from patients without AD [65]. Increased mRNA expression was found in AD brains relative to brains from control subjects. Further, using immunoblotting analysis and quantitative densitometry, VDAC1 protein levels were quantified in frontal cortical tissues from all AD patients and control subjects. Significantly increased VDAC1 was found in AD patients relative to control subjects [14]. These findings suggest that VDAC1 levels progressively increase as AD progresses and may be implicated in AD progression.
To determine whether VDAC1 increases with age in Aβ-overexpressed APP transgenic mice, we also quantified VDAC1 protein levels in cerebral cortex tissues from 6-, 12-, and 24-month APP mice and age-matched non-transgenic wild-type mice. We found significantly increased levels of VDAC1 in the 12- and 24-month-old APP mice, relative to the 6-month-old APP mice, indicating an age-dependent increase of VDAC1 in the cerebral cortex of APP mice. To determine whether mutant APP and/or Aβ elevates VDAC1, we compared VDAC1 immunoblotting and densitometry data with VDAC1 data from the wild-type mice. We found significantly increased levels of VDAC1 in the 6- and 12-month-old APP mice, relative to the 6- and 12-month-old non-transgenic wild-type mice. To determine if aging plays a role in VDAC1 expression, we compared immunoblotting data from the 6-, 12-, and 24-month-old wild-type mice. We found significantly increased levels of VDAC1 in the 12- and 24-month-old non-transgenic wild-type mice, relative to 6-month-old wild-type mice, indicating an age-dependent increase in VDAC1, in the cerebral cortex of the wild-type mice.
Ren and colleagues [95] studied the effect of the Aβ peptide 25–35 on mitochondrial structure and function and on the expression of proteins associated with the mitochondrial permeability transition pore in rat hippocampal neurons. They injected Aβ peptide into hippocampal area CA1. Normal saline was injected as a control to assess the hippocampal structure by transmission electron microscopy. ATPase activity, intracellular Ca2+, and mitochondrial membrane potential were measured. The expression of genes associated with the MPTP, including VDAC1, ANT, and CypD, were evaluated. They found that the Aβ injection damaged the mitochondrial structure of hippocampal neurons, decreased ATPase activity and mitochondrial membrane potential, and increased intracellular Ca2+. The expression levels for VDAC, ANT, and CypD in all groups were significantly higher than those in the normal control group injected after Aβ25–35 peptide. These results indicate that Aβ-25–35 damages mitochondria in rat hippocampal neurons and affects mitochondrial dysfunction, as well as increases the expression of genes associated with MPT pores. Mitochondrial dysfunction may result in increased MPTP gene expression, leading to neurodegenerative effects.
Cuadrado-Tejedor and colleagues [96] studied whether VDAC1 is involved in the release of apoptotic proteins in AD. Through proteomic analysis followed by immunoblotting blotting and immunohistochemical analyses, they found that VDAC1 is overexpressed in the hippocampus from amyloidogenic AD transgenic mice. VDAC1 was also overexpressed in postmortem brain tissue from AD patients at an advanced stage of disease progression. Interestingly, Aβ soluble oligomers were able to induce the up-regulation of VDAC1 in a human neuroblastoma cell line, further supporting a correlation between Aβ levels and VDAC1 expression. In hippocampal extracts from transgenic mice, a significant increase was observed in the level of VDAC1 that was phosphorylated at an epitope susceptible to phosphorylation by glycogen synthase kinase-3β, whose activity was also increased. The levels of hexokinase I (HXK1), which interacts with VDAC1 and affects its function, were decreased in mitochondrial samples from AD models, indicating that reduced HXKI levels favor a VDAC1 involvement in disease progression of AD [96].
Overall, these studies suggest that VDAC1 increases with the progression of AD. Further, VDAC1 also increases in an age-dependent manner, likely an important factor in better understanding AD progression.
Elevated VDAC1 and its interaction with Aβ in neurons from AD tissues
As discussed above, in AD neurons, we found large amounts of Aβ associated with the outer mitochondrial membrane [65]. Further to determine, if Aβ is associated with outer mitochondrial membrane, immunoprecipitation analysis was conducted, using cortical protein lysates from brains of AD patients at different stages of disease progression, and from brains of APP and APP/PS1 transgenic mice and of age-matched wild-type mice. These histologic studies involved the use of Aβ (6E10 monoclonal A11 oligomeric Aβ) and VDAC1 antibodies [14]. A 4 kDa Aβ and a 100 kDa full-length APP were found in VDAC1 immunoprecipitation elutes from definite and severe AD patients and from 20-month-old APP and APP/PS1 mice. Similar to monomeric Aβ, oligomeric Aβ was also found in immunoprecipitation elutes from definite and severe AD patients and from APP and APP/PS1 mice. Findings from these studies, clearly suggest that both monomeric and oligomeric Aβ interact with VDAC1 and blocks the mitochondrial pores, interrupting the transport of metabolites and proteins between mitochondria and the rest of the cell.
Thinnes and colleagues [97] proposed that GxxxG motif of N-terminal part of VDAC1 might interact with GxxxG motif of C terminal part of Aβ peptide. The GxxxG motifs are established as aggregation/membrane perturbation motifs. Aβ, a C-terminal cleaved product from APP by beta-secretase BACE1 and gamma-secretase, has been insinuated to induce AD via apoptosis by opening type-1 porin/VDAC in cell membranes of hypometabolic neuronal cells. Considering the ubiquitous expression modus of APP, beta- and gamma-secretases and type-1 VDAC/eukaryotic porin a basic model of apoptosis might be given.
Ramirez and colleagues [98] studied VDAs involvement in AD pathogenesis using affected regions, including frontal cortex and hippocampus, from AD patients and controls subjects. They found pl-VDAC and mERα present in the caveolae tissues from the human cortex and hippocampus, in a complex with scaffolding caveolin-1 which likely provides mERα stability at the plasma membrane. In AD brains, VDAC was accumulated in the caveolae and in dystrophic neurites of senile plaques, whereas mERα was expressed in astrocytes surrounding the plaques. Together with previous data on murine neurons demonstrating the participation of pl-VDAC in Aβ-induced neurotoxicity, Ramirez et al. data suggest that the mitochondrial channel may be involved in membrane dysfunction similar to that observed in AD neuropathology.
Overall, findings from these studies suggest that VDAC1 normally interacts with Aβ, and this abnormal interaction increases with AD progression. Further, there is evidence to support pl-VDAC being linked with the caveolae of the cortex and hippocampus, and that VDAC1 is heavily localized in dystrophic neurites of senile plaques.
Elevated VDAC1 and its interaction with phosphorylated tau in Alzheimer’s disease
A physiological link between abnormal and phosphorylated tau, and mitochondrial dysfunction has been proposed in AD [99], suggesting that VADC1 may be associated with mitochondrial dysfunction found in AD. Researchers reported N-terminal tau associated with mitochondria in brain tissues from AD patients and AD mouse models. To determine whether phosphorylated tau interacts with VDAC1, we conducted immunoprecipitation analysis, using cortical protein lysates from the brains of AD patients and of control subjects, and APP/PS1, 3xTg.AD in brain tissues from transgenic mice and age-matched wild-type mice. We found a 32-kDa band of VDAC1 in the phosphorylated tau immunoprecipitation elutes in the brains from AD patients and from APP/PS1, 3xTg.AD transgenic mice. These results were cross-checked using a VDAC1 antibody for immunoprecipitation and a phosphorylated tau antibody for immunoblotting. We found a 60 kDa band of phosphorylated tau protein in the VDAC1-immunoprecipitation elutes, in the brain tissue from AD patients and from APP/PS1, 3xTg.AD transgenic mice. These findings suggest that phosphorylated tau interacts with VDAC1. These observations were further confirmed by double-labeling immunofluorescence analyses of VDAC1 and phosphorylated tau in brain tissues from AD patients and 3XTg.AD mice.
Overall, the results from our study suggest that VDAC1 interacts with phosphorylated tau in AD neurons and that these interactions increase with disease progression. The VDAC1-phosphortylated tau complexes blocks mitochondrial pores, interrupt the flux of metabolites between mitochondrial membranes and cytoplasm, impairs the gating of VDAC channel, leading to mitochondrial dysfunction and neuronal damage in AD neurons.
Conclusions and future directions
A large body evidence suggests that mitochondrial dysfunction and oxidative stress are involved in AD progression and pathogenesis. Further, recent research on AD postmortem brains, brain tissues from AD mouse models, and cells that express Aβ and phosphorylated tau revealed that Aβ and phosphorylated tau are associated with mitochondria, resulting in mitochondrial dysfunction and synaptic damage in AD-affected neurons. More specifically, several recent studies found Aβ interacting with the mitochondrial matrix proteins CypD and ABAD, and that this interaction leads to increased ROS production and mitochondrial dysfunction. In addition, recent research from the Reddy lab, that used postmortem brain tissues from AD patients and tissues from AD mouse models, revealed that in the mitochondrial outer membrane protein, VDAC1 is interacts with both Aβ and phosphorylated tau, resulting in the blockage of MPT pores and a disruption in the transport of proteins and metabolites between mitochondria and the rest of cell. Results from this study also showed that VDAC1 likely causes defects in oxidative phosphorylation and mitochondrial dysfunction in AD neurons. It is well-established that MPT pores are formed by VDAC1, ANT and CypD. Proper maintenance of pore opening and pore closure in mitochondria is critical for the transport of metabolites – including ADP, inorganic phosphorous, ATP, and proteins – from the cytoplasm to the mitochondria and vice versa. However, as described earlier, in the mitochondrial matrix, CypD is elevated, it interacts with Aβ in AD neurons, and may cause mitochondrial structural and functional abnormalities. Further, hippocampal neurons treated with Aβ exhibited elevated levels of the pore forming proteins VDAC1, CypD and ANT, and they revealed altered mitochondrial membrane potential and altered intracellular Ca2+ levels.
Elevated levels of VDAC1 were found in postmortem brain tissues from AD brains and AD transgenic mice, and evidence indicating an interaction between VDAC1 and Aβ and between VDAC1 and phosphorylated tau were found in both brain tissues [14]. Complexes of VDAC1-Aβ and VDAC1-phosphorylated tau may be involved in the blockage of mitochondrial pores, leading to the interruption of mitochondrial protein and metabolite transport, and to the impairment of VDAC channel conductance. In turn, these changes may lead to mitochondrial dysfunction in AD neurons. Based on these observations, reduced levels of VDAC1, Aβ, and phosphorylated tau may reduce the interactions between VDAC1 and Aβ, and between VDAC1 and phosphorylated tau in AD neurons–, resulting in the maintenance of normal mitochondrial pore opening and pore closure, ultimately leading to mitochondria supplying ATP to nerve terminals (as is the case in healthy neurons). If these hypotheses hold, then reduced interaction between VDAC1 and Aβ and phosphorylated tau may prove useful in boosting synaptic and cognitive functions in AD.
Research Highlights.
Mitochondrial dysfunction is an early event in Alzheimer’s disease.
VDAC1 protein levels are elevated in AD brains and cortical tissues from APP transgenic mice.
VDAC1 is interacted with Aβ and phospho tau, disrupts the transport of metabolites.
VDAC1+Aβ and Aβ+phospho tau complexes cause defects in OXPHOS in AD neurons.
Acknowledgements
This research was supported by NIH grants AG028072, RR000163, and Alzheimer Association grant IIRG-09-92429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy PH, Beal MF MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends. Mol. Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer Association, Alzheimer’s disease: Facts and Figures. 2012. [Google Scholar]
- 5.Mao P, Reddy PH PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim. Biophys. Acta. 2011;1812:1359–1370. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 7.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol. Exp Neurol. 2001:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 8.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20:S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl. Acad. Sci. U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X, Perry G, Smith MA, Wang X. Abnormal Mitochondrial Dynamics in the Pathogenesis of Alzheimer’s Disease. J. Alzheimers. Dis. 2012 Apr 24; doi: 10.3233/JAD-2012-129005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manczak M, Reddy PH. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau in Alzheimer’s disease neurons cause mitochondrial dysfunction. Hum. Mol. Genet. doi: 10.1093/hmg/dds360. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res. Brain. Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid. Redox. Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 17.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 18.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr. Pharm. Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 19.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects. Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Shoshan-Barmatz V, Golan M. Mitochondrial VDAC1: function in cell life and death and a target for cancer therapy. Curr. Med. Chem. 2012;19:714–735. doi: 10.2174/092986712798992110. [DOI] [PubMed] [Google Scholar]
- 21.Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12:24–34. doi: 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J. Membr. Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- 23.Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, Colombini M, Forte M. Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J. Biol. Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- 24.Blachly-Dyson E, Baldini A, Litt M, McCabe ER, Forte M. Human genes encoding the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane: mapping and identification of two new isoforms. Genomics. 1994;20:62–67. doi: 10.1006/geno.1994.1127. [DOI] [PubMed] [Google Scholar]
- 25.Hodge T, Colombini M M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 26.Rostovtseva T, Colombini M M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blachly-Dyson E, Forte M. VDAC channels. IUBMB Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 28.Colombini M. VDAC structure, selectivity, and dynamics. Biochim Biophys Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson MJ, Lovell RS, Davison DB, Craigen WJ. A novel mouse mitochondrial voltage-dependent anion channel gene localizes to chromosome 8. Genomics. 1996;36:192–196. doi: 10.1006/geno.1996.0445. [DOI] [PubMed] [Google Scholar]
- 30.Sampson MJ, Lovell RS, Craigen WJ. Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. Genomics. 1996;33:283–288. doi: 10.1006/geno.1996.0193. [DOI] [PubMed] [Google Scholar]
- 31.Messina A, Reina S, Guarino F, De Pinto V. VDAC isoforms in mammals. Biochim. Biophys. Acta. 2012;1818:1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Young MJ, Bay DC, Hausner G, Court DA. The evolutionary history of mitochondrial porins. BMC Evol Biol. 2007;7:31. doi: 10.1186/1471-2148-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saccone C, Caggese C, D'Erchia AM, Lanave C, Oliva M, Pesole G. Molecular clock and gene function. J. Mol. Evol. 2003;57:S277–S285. doi: 10.1007/s00239-003-0037-9. [DOI] [PubMed] [Google Scholar]
- 34.Craigen WJ, Graham BH BH. Genetic strategies for dissecting mammalian and Drosophila voltage-dependent anion channel functions. J. Bioenerg. Biomembr. 2008;40:207–212. doi: 10.1007/s10863-008-9146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yoshimura Y, Yamauchi T, Kataoka M, Nagata T, Terada H, Shinohara Y. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J. Proteome. Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- 36.Sampson MJ, Ross L, Decker WK, Craigen WJ WJ. A novel isoform of the mitochondrial outer membrane protein VDAC3 via alternative splicing of a 3-base exon. Functional characteristics and subcellular localization. J. Biol. Chem. 1998;273:30482–30486. doi: 10.1074/jbc.273.46.30482. [DOI] [PubMed] [Google Scholar]
- 37.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Raghavan A, Sheiko T, Graham BH, Craigen WJ WJ. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim Biophys Acta. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogeniccytochrome c by the mitochondrial channel VDAC. Nature. 1993;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr. Biol. 1999;170:89–102. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- 41.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 42.Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane Ion. Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- 43.Duan S, Hajek P, Lin C, Shin SK, Attardi G, Chomyn A. Mitochondrial outer membrane permeability change and hypersensitivity to digitonin early in staurosporine-induced apoptosis. J. Biol. Chem. 2003;278:1346–1353. doi: 10.1074/jbc.M209269200. [DOI] [PubMed] [Google Scholar]
- 44.Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, Bezrukov SM. Bid, but not Bax, regulates VDAC channels, S.M. Bezrukov, Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- 46.Rostovtseva TK, Bezrukov SM. VDAC inhibition by tubulin and its physiological implications. Biochim Biophys Acta. 2012;1818:1526–1535. doi: 10.1016/j.bbamem.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerner J, Lee K, Tandler B, Hoppel CL. VDAC proteomics: Post-translation modifications. Biochim Biophys Acta. 2012;1818:1520–1525. doi: 10.1016/j.bbamem.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemasters JJ, Holmuhamedov EL, Czerny C, Zhong Z, Maldonado EN EN. Regulation of mitochondrialfunction by voltage dependent anion channels in ethanol metabolism and the Warburg effect. Biochim Biophys Acta. 2012;1818:1536–1544. doi: 10.1016/j.bbamem.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baines CP, Son CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer. Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim. Biophys. Acta. 1999;1452:68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 52.Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, Sweatt JD, Craigen WJ. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J. Biol. Chem. 2002;277:18891–18897. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- 53.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 54.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 55.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 57.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA MA. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 59.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 60.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum. Mol. Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 62.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 63.Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J. Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. (2004) [DOI] [PubMed] [Google Scholar]
- 64.Eckert A, Hauptmann S, Scherping I, Rhein V, Müller-Spahn F, Götz J, Müller WE. Soluble beta amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener. Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 65.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 66.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SS. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 67.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manczak M, Mao P, Calkins CJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J. Alzheimers. Dis. 2010;20:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2010;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calkins MJ MJ, Reddy PH. Assessment of newly synthesized mitochondrial DNA using BrdU labeling in primary neurons from Alzheimer’s disease mice: Implications for impaired mitochondrial biogenesis and synaptic damage. Biochim. Biophys. Acta. 2011;1812:1182–1189. doi: 10.1016/j.bbadis.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diana A, Simić G, Sinforiani E, Orrù N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to beta-amyloid(1–42) peptide. Coll. Antropol. 2008;32:51–58. [PubMed] [Google Scholar]
- 74.Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol. Psychiatry. 2008;13:953–969. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J. Neural. Transm. 2006;113:125–135. doi: 10.1007/s00702-005-0318-0. [DOI] [PubMed] [Google Scholar]
- 76.Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. Gradual alteration of mitochondrial structure and function by beta amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 2005;37:207–225. doi: 10.1007/s10863-005-6631-3. [DOI] [PubMed] [Google Scholar]
- 77.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 78.Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994;24:336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 79.Chandrasekaran K, Hatanpää K, Rapoport SI, Brady BR. Decreased expression of nuclear and mitochondrial DNA-encoded genes of oxidative phosphorylation in association neocortex in Alzheimer disease. Brain. Res. Mol. Brain. Res. 1997;44:99–104. doi: 10.1016/s0169-328x(96)00191-x. [DOI] [PubMed] [Google Scholar]
- 80.Simonian NA, Hyman BT. Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J. Neuropathol. Exp. Neurol. 1994;53:508–512. doi: 10.1097/00005072-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J. Neural. Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, Xiong S, Xie C, MarkesberY WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 83.Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J. Alzheimers. Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- 84.Du HL, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice. Neurobiol Dis. 2012;45:417–424. doi: 10.1016/j.nbd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J. Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao J, Du H, Yan S, Fang F, Wang C, Lue LF, Guo L, Chen D, Stern DM, Gunn Moore FJ, Xi Chen J, Arancio O, Yan SS. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J. Neurosci. 2011;31:2313–2320. doi: 10.1523/JNEUROSCI.4717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell. Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keil U, Bonert A, Marques CA, Strosznajder JB, Müller-Spahn F, Müller WE, Eckert A. Elevated nitric oxide production mediates beta-amyloid-induced mitochondria failure. Pol. J. Pharmacol. 2004;56:631–634. [PubMed] [Google Scholar]
- 91.Park HJ, Kim SS, Seong YM, Kim KH, Goo HG, Yoon EJ, Min do S, Kang S, Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J. Biol. Chem. 2006;281:34277–34287. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- 92.Amadoro G, Corsetti V, Ciotti MT, Florenzano F, Capsoni S, Amato G, Calissano P. Endogeno Aβ causes cell death via early tau hyperphosphorylation. Neurobiol Aging. 2011;32:969–990. doi: 10.1016/j.neurobiolaging.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 93.Atlante A, Amadoro G, Bobba A, de Bari L, Corsetti V, Pappalardo G, Marra E, Calissano P, Passarella S. A peptide containing residues 26–44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim Biophys Acta. 2008;1777:1289–1300. doi: 10.1016/j.bbabio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren R, Zhang Y, Li B, Wu Y, Li B. Effect of β-amyloid (25–35) on mitochondrial function and expression of mitochondrial permeability transition pore proteins in rat hippocampal neurons. J. Cell. Biochem. 2011;112:1450–1457. doi: 10.1002/jcb.23062. [DOI] [PubMed] [Google Scholar]
- 96.Cuadrado-Tejedor M, Vilariño M, Cabodevilla F, Del Río J, Frechilla D, Pérez-Mediavilla A. Enhanced expression of the voltage-dependent anion channel 1 (VDAC1) in Alzheimer’s disease transgenic mice: an insight into the pathogenic effects of amyloid-β. J. Alzheimers. Dis. 2011;23:195–206. doi: 10.3233/JAD-2010-100966. [DOI] [PubMed] [Google Scholar]
- 97.Thinnes FP. Apoptogenic interactions of plasmalemmal type-1 VDAC and Aβ peptides via GxxxG motifs induce Alzheimer’s disease - a basic model of apoptosis? Wien Med Wochenschr. 2011;161:274–276. doi: 10.1007/s10354-011-0887-5. [DOI] [PubMed] [Google Scholar]
- 98.Ramírez CM, González M, Díaz M, Alonso R, Ferrer I, Santpere G, Puig B, Meyer G, Marin R. VDAC and ERalpha interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol. Cell. Neurosci. 2009;42:172–183. doi: 10.1016/j.mcn.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain. Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]