Abstract
Nitrogen-rich heterocyclic compounds have had a profound impact on human health, as these chemical motifs are found in a large number of drugs used to combat a broad range of diseases and pathophysiological conditions. Advances in transition metal-mediated cross-coupling have simplified the synthesis of such molecules; however, the development of practical and selective C–H functionalization methods that do not rely upon prefunctionalized starting materials is an underdeveloped area.1–9 Paradoxically, the innate properties of heterocycles that make them so desirable for biological applications render them challenging substrates for direct chemical functionalization, such as limited solubility, functional group incompatibilities, and reagent/catalyst deactivation. Herein we report that zinc sulfinate salts9 can be used to transfer alkyl radicals to heterocycles, allowing for a mild, direct and operationally simple formation of medicinally relevant C–C bonds while reacting in an orthogonal fashion to other innate C–H functionalization methods (Minisci, borono-Minisci, electrophilic aromatic substitution, transition metal-mediated C–H insertion, C–H deprotonation).2–7,9 A toolkit of these reagents was prepared and reacted across a wide range of heterocycles (natural products, drugs, building blocks) without recourse to protecting group chemistry, and can even be employed in a tandem fashion in a single pot in the presence of water and air.
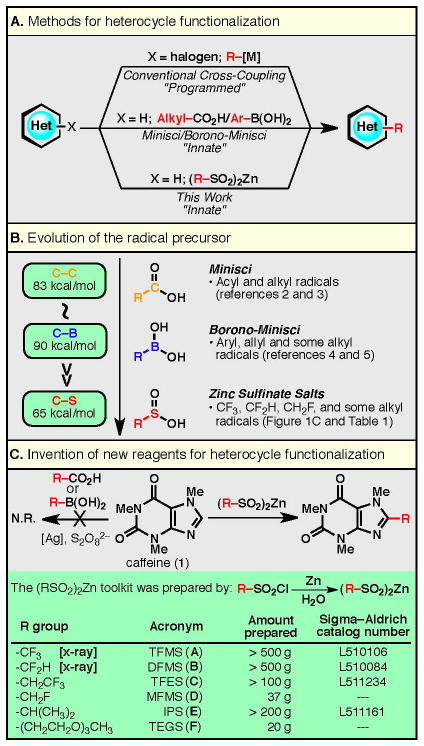
The strategy described herein was conceived with a strong desire to embrace the native chemical reactivity1 of nitrogen-rich heterocycles rather than attempting to override it with prefunctionalization (Fig. 1A). Minisci’s pioneering work clearly demonstrated the ability of heterocycles to react with certain alkyl and acyl radicals (derived from carboxylic acids and halides, see Fig. 1B).2 This form of innate C–H functionalization, however, is limited to a subset of alkyl and acyl donors, and often requires elevated temperatures, transition metal additives, and strongly oxidizing conditions.3 In 2010, our laboratory reported that aryl boronic acids can be used as aryl radical precursors for heterocycle functionalization.4 Later, we5 and others6 showed that alkyl boronic acids and trifluoroborate salts can serve as radical precursors. In 2011, we demonstrated7 that sodium trifluoromethanesulfinate, a reagent extensively studied by Langlois,8 was capable of trifluoromethylating heterocycles via a radical mechanism. Shortly thereafter, we described a procedure for difluoromethylation that relied upon the use of a zinc-derived sulfinate salt.9 Zinc sulfinate salts are easily prepared, bench-stable, free-flowing solids [with the exception of zinc triethyleneglycolsulfinate (TEGS; F), see Fig. 1C] that exhibit remarkably enhanced reactivity compared to sodium-derived analogs in radical additions to heterocycles (vide infra). Here we describe a modular, innate C–H functionalization of heterocycles using zinc sulfinate salts following a mild and practical reaction protocol. The invention of a zinc bis(alkanesulfinate) salt toolkit, much like available toolkits for cross-coupling chemistry, is used for the direct functionalization of a variety of heterocycles, all of which result in products that cannot be efficiently generated by standard Minisci protocols and would have otherwise required programmed, multi-step processes. These salts represent examples of what we anticipate to be a general reagent class that will permit the rapid diversification of heterocycles using an operationally simple protocol.10 It is of note that this work represents a significant improvement over sodium-based sulfinate salts7 and that it is a generalization of the zinc difluoromethanesulfinate chemistry developed previously in our laboratory.9
Figure 1. Development of a reagent toolkit for an innate C–H functionalization of heterocycles.
A modular set of zinc sulfinate salts were identified as being highly desirable for the installation of medicinally relevant moieties (Fig. 1C): zinc trifluoromethanesulfinate (TFMS; A), zinc difluoromethanesulfinate (DFMS; B), zinc trifluoroethanesulfinate (TFES; C), zinc monofluoromethanesulfinate (MFMS; D), zinc isopropylsulfinate (IPS; E), and zinc triethyleneglycolsulfinate (TEGS; F). The fluoroalkyl and alkyl groups that we wish to introduce hold privileged positions in drug discovery and are described in detail below.
The CF3 group is an excellent bioisostere of a methyl group, since the former is similar in size to the latter but does not suffer metabolic oxidation.11 It can also be used to tune the steric and electronic properties of a known scaffold, and grant favorable physicochemical attributes to a lead target.12,13 For these reasons, trifluoromethylation methodology has received much attention, with nucleophilic CF3 reagents (e.g., carbonyl functionalization),14 electrophilic CF3 reagents (e.g., enolate functionalization),14 and cross-coupling procedures dominating arene functionalization15,16 (metal-catalyzed C–H activation for trifluoromethylation is also known).17 However, the trifluoromethylation of heterocycles is less common due to substrate-mediated reagent or catalyst deactivation in these strongly basic, acidic or transition metal-catalyzed reactions. To fill this gap in methodology, we have begun to study a radical-based approach to trifluoromethylation.7 Although the introduction of the CF3 group by a radical pathway is known, reagents that achieve this radical transformation are difficult to handle and are toxic (CF3I, a gas),18 ozone-depleting (CF3Br, a gas),19 or corrosive (CF3SO2Cl, a low-boiling liquid).20,21 Furthermore, CF3CO2H does not generate radicals under Minisci conditions, and CF3B(OH)2, as an analogy to borono-Minisci chemistry, is an unknown chemical species (other CF3–boron sources such as CF3–B(OMe)3K and CF3–BF3K are not viable radical precursors7). While sodium trifluoromethanesulfinate (CF3SO2Na, a stable solid) addressed many of the above difficulties for heterocycle trifluoromethylation, TFMS (A) was found to be superior in both stability and reactivity [47% yield was obtained for a reaction of pentoxifylline (2) with CF3SO2Na in 2.5:1 CH2Cl2/H2O, rt, 48 h;7 79% yield was obtained for a reaction of pentoxifylline (2) with TFMS (A) in 2.5:1 CH2Cl2/H2O, rt, 3 h (see Table 1); 99% yield was obtained for a reaction of 2 with TFMS (A) in 2.5:1 CH2Cl2/H2O, 50 °C, 3 h; also see comparisons of reaction conversions for various substrates in the Supplementary Information].
Table 1. Substrate scope of the zinc sulfinate salt toolkit.
Isolated yields are displayed, % conversions by GC-MS are indicated between parentheses, and regioisomeric ratios are shown between square brackets. Compounds 1A, 2A, 3A, 4A and 5A have been previously synthesized with Langlois’ reagent (ref. 7); 1B, 2B, 3B, 4B, 5B and 8B have been previously prepared using DFMS (ref. 9) and are included herein for completeness; all other compounds in this table are new.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Zn salt; R =
|
CF3 (A) | CF2H (B) | CF2CF3 (C) | CF2F (D) | CH(CH3)2 (E) | (CH2CH2O)3CH3 (F) |
| heterocycle | ||||||
|
| ||||||
1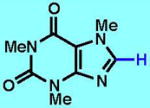
|
89 (100)b | 73 (57)bk | 51g | 80g | 41h | 40i |
| 1A | 1B | 1C | 1D | 1E | 1F | |
|
| ||||||
2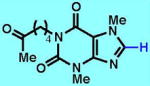
|
79 (100)b | 72 (41)bk | 44g | 75g | 37h | 49i |
| 2A | 2B | 2C | 2D | 2E | 2F | |
|
| ||||||
3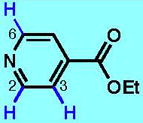
|
35 (77)b [4:1 C2:C3] |
66 (100)b | 18 (85)g [4:1 C2:C3] |
73fj [17:1 C2:C2&C6] |
47d | 41i |
| 3A | 3B | 3C | 3D | 3E | 3F | |
|
| ||||||
4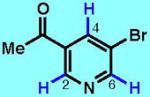
|
66 (65)bk [2.3:1 C6:C2] |
60 (96)b | 33g [1.4:1 C6:C4] |
N.R. | 41i | N.R. |
| 4A | 4B | 4C | 4D | 4E | 4F | |
|
| ||||||
5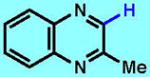
|
75 (100)b [5 products] |
50 (67)b | 31 (77)c | 56g | 43h | 32h |
| 5A | 5B | 5C | 5D | 5E | 5F | |
|
| ||||||
6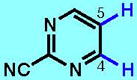
|
42 (44)e [2.7:1 C4:C5] |
21 (44)e [1.6:1 C4:C5] |
21h | N.R. | 46h [2.1:1 C4:C5] |
16i [3.4:1 C4:C5] |
| 6A | 6B | 6C | 6D | 6E | 6F | |
|
| ||||||
7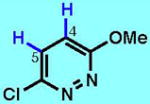
|
45 (90)e | 57 (71)e [6:1 C4:C5] | N.R. | N.R. | 49h [10:1 C4:C5] |
32 (38)i |
| 7A | 7B | 7C | 7D | 7E | 7F | |
|
| ||||||
8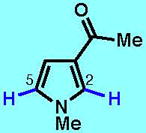
|
76 (91)b [7.4:1 C2:C5] |
65 (100)b | 58h [1.4:1 C2:C5] |
40d | 17h | 10 (43)i |
| 8A | 8B | 8C | 8D | 8E | 8F | |
Standard conditions involve heterocycle (1.0 equiv), zinc salt (2.0–3.0 equiv), TBHP (3.0–5.0 equiv) and solvent:H2O (2.5:1) at a specified temperature for a period of 3–12 h. Solvent/temperature:
CH2Cl2, RT;
ClCH2CH2Cl, RT;
ClCH2CH2Cl, 50 °C;
perfluorohexane, RT;
perfluorotoluene, RT;
perfluorotoluene, 50 °C;
DMSO, 50 °C;
anisole, 50 °C (note: the reaction time for anisole is 0.5–96 h, see Supplementary Information).
TFA was used as an additive.
When the GC % conversion is lower than the isolated yield, it signifies that only one addition of Zn salt was made for the “GC yield reaction”, but that a second addition of Zn salt and TBHP was performed after 12 h for the “isolated yield reaction”.
TBHP = tert-butyl hydroperoxide; TFA = trifluoroacetic acid; RT = room temperature; N.R. = no reaction.
The CF2H group can act as a lipophilic hydrogen bond donor and has been utilized in bioisostere design as a thiol, hydroxamic acid, hydroxyl or amide mimic.11 While the CF2H group can be formed using (diethylamino)sulfur trifluoride (DAST) on an aldehyde, and can be introduced using (trimethylsilyl)difluoromethane22 or other difluoromethyl precursors,23 direct difluoromethylation strategies on heterocycles had not been explored prior to the invention of DFMS (B).9
The CH2CF3 group is an excellent bioisostere of an ethyl group and acts as a mild electron-withdrawing group (trifluoroethanol is ca. 3.5 orders of magnitude more acidic than ethanol). Appendage of the CH2CF3 group is typically achieved by multistep strategies (such as nucleophilic addition of CF3 into an aldehyde followed by deoxygenation), and a catalytic cross-coupling method for this purpose was only reported recently.24 A direct, free radical-based approach for the introduction of the CH2CF3 group was unknown, and therefore TFES (C) was developed to fill this gap in methodology.
Much like the CF3 group, the CH2F group can act as a useful bioisostere of a methyl group as the fluorine atom can also prevent metabolic oxidation due to its electron-withdrawing effect. Furthermore, the CH2F group has also been considered bioisosteric with hydroxymethyl (CH2OH) and methoxymethyl (CH2OCH3) groups.25 However, monofluoromethylation methods are limited and less explored compared to trifluoromethylation and are typically introduced using “auxiliary” approaches,23 for example, appending a fluoro(phenylsulfonyl)methyl group and then removing the phenylsulfonyl group.26 The generation of a monofluoromethyl radical is practically unknown, with only one report made on its characterization by a matrix reaction of bromofluoromethane with alkali metals, without demonstration of synthetic applicability.27 As fluoroacetic acid (CH2F-CO2H) cannot generate radicals under standard Minisci conditions, a practical CH2F radical precursor and its application to synthetic chemistry is required; MFMS (D) is a new reagent devised for this purpose.
The ability to add steric bulk and lipophilicity where needed may prove valuable when probing for structure-activity relationships (SARs) or when blocking metabolically labile positions. Methods for the direct introduction of this hindered group include the use of toxic isopropylmercurial reagent [(CH3)2CH-HgCl],28 or the use of air- and water-sensitive isopropyllithium or isopropylmagnesium chloride. Furthermore, the use of (CH3)2CH-CO2H or (CH3)2CH-I with the Minisci protocol on caffeine (1) did not afford the desired product at room temperature or elevated temperatures. A “programmed” approach to installing an isopropyl group is often low-yielding and problematic using Suzuki- or Negishi-type couplings (with a few notable exceptions29) and therefore an “innate” approach using IPS (E) was conceived.
Ethylene glycol units are excellent solubilizing units and the utility of poly(ethylene glycol) (PEG) chains in drug delivery has been extensively investigated.30 As a proof-of-concept for the appendage of PEG units onto heterocycles, we planned to introduce a shorter triethylene glycol (TEG) chain that might still retain the ability to increase the hydrophilicity of a lead target. Methods to directly append an oligo(ethylene glycol) unit onto heterocyclic frameworks are unknown, and therefore TEGS (F) was devised.
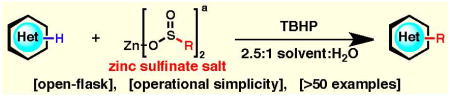
The ability to incorporate fluoroalkyl, alkyl, and alkoxyalkyl groups into heterocyclic frameworks is highly desirable, however, until now the chemistry to rapidly access substituted heterocycles has been underdeveloped and of limited scope. The power of the zinc sulfinate toolkit is demonstrated herein with a synthesis of an exemplary set of medicinally relevant heterocycles (> 50 examples), most of which represent new chemical entities.
As depicted in Table 1, an operationally simple procedure was used to functionalize xanthines (1 and 2), pyridines (3 and 4), quinoxalines (5), pyrimidines (6), pyridazines (7), and pyrroles (8): the heterocycle substrate and the zinc sulfinate salt were combined with a organic solvent/water mixture, cooled to 0 °C, and tert-butyl hydroperoxide (TBHP) was added, followed by warming to room temperature or 50 °C. Exclusion of air or purification of solvents is unnecessary, and the reaction flask is only sealed with a plastic cap to prevent solvent evaporation. Fluoroalkylated zinc sulfinate reagents (A–D) performed best in halogenated solvents such as CH2Cl2, ClCH2CH2Cl, perfluorotoluene or perfluorohexane, whereas alkylated zinc salts (E and F) reacted more favorably in DMSO or in an electron-rich aromatic solvent such as anisole. For substrates that showed low conversion, a second addition of the zinc sulfinate salt and/or the addition of trifluoroacetic acid (TFA) was performed.
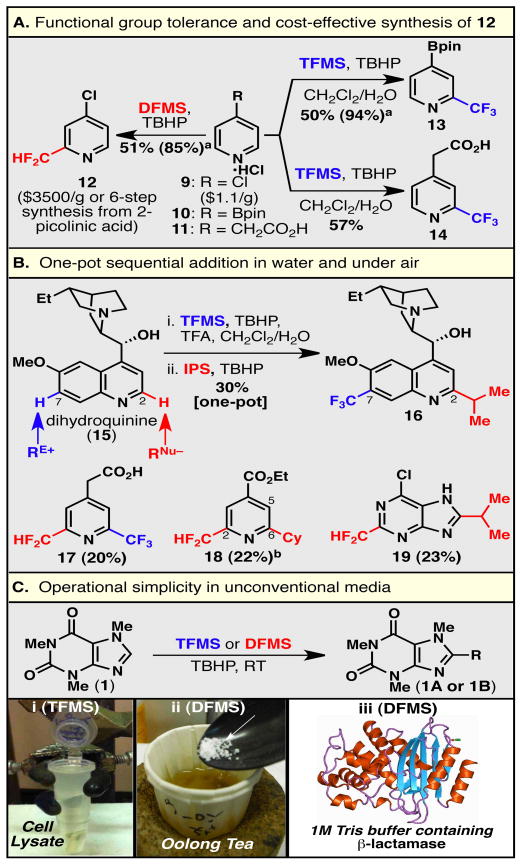
Perhaps the most notable aspect of C–C bond formation using zinc sulfinate chemistry is the mildness of the reaction conditions. As shown above, reactive groups such as nitriles, ketones, and esters are tolerated. The level of functional group compatibility can even be extended to reactive aryl halides, free carboxylic acids, and boronate esters, three groups of particular utility in pharmaceutical chemistry due to the ease of further functionalization via cross-coupling methods or amide bond formation (Fig. 2A). These groups are typically incompatible with most organic reactions, let alone C–H functionalization strategies; however, under our reaction conditions, simple pyridine building blocks bearing a reactive chloro (9), pinacolatoboron (Bpin; 10), or carboxylic acid (11) moiety can be functionalized with CF3 or CF2H groups. In particular, carboxylic acid 11 is a quintessential example of the unique chemoselectivity and mildness of the reaction conditions, as Minisci or borono-Minisci conditions would not leave the carboxylic acid unit intact. It is of note that the modest yields observed in these reactions are due to difficulties in product isolation, for example, due to the high volatility of difluoromethylated product 12. It is of further note that 12 is commercially available but is prohibitively expensive ($3500/gram from Accel Pharmtech), and was previously prepared from 4-chloro-2-pyridinecarbaldehyde ($432/gram from Matrix Scientific) using DAST or from 2-picolinic acid using a laborious six-step protocol; the zinc sulfinate procedure allowed the synthesis of 12 from a cheap precursor ($1.1/gram from Oakwood Products), 4-chloropyridinium hydrochloride (9).
Figure 2. Chemoselectivity, rapid diversity and complexity generation, and practical utility.
Cy = cyclohexyl. a % conversion as observed by GC-MS analysis. b 6% yield of the C5-cyclohexyl isomer was also obtained.
The specificity of this transformation to react at the position defined by the innate reactivity of the given heterocycle was further tested by a one-pot sequential C–H functionalization of the anti-malarial drug dihydroquinine (15; Fig. 2B). Since our previous studies suggested that electrophilic radicals add to the C7 position of 157 and nucleophilic radicals add to the C2 position of 15,9 we expected 15 to react sequentially when an electrophilic and a nucleophilic radical are added in one pot. To this end, TFMS (A) then IPS (E) were added sequentially to a reaction vessel containing dihydroquinine (15), after which difunctionalized dihydroquinine derivative 16 was isolated in 30% yield. The scope of this sequential functionalization was further extended to examples including pyridines (17 and 18) and purines (19). This strategy is particularly useful for the construction of lipophilic derivatives of hydrophilic molecules such as dihydroquinine (15) and 6-chloropurine, since the isolation of water-soluble intermediates can be avoided and only the lipophilic bis-substituted product is isolated.
Finally, the versatile nature of this transformation is demonstrated by the ability to carry out difluoromethylation and trifluoromethylation in unconventional media (conversion to product confirmed by HPLC), including cell lysate (Fig. 2Ci), tea (Fig. 2Cii), or a Tris buffer solution in the presence of serine based β-lactamase (Fig. 2Ciii), which retained its functional activity after reisolation. These findings may have important implications in the area of bioconjugation.10
Although zinc sulfinate chemistry can provide a wellspring of new chemical entities that are difficult or impossible to access in other ways while exhibiting high levels of chemoselectivity, much like other methods, this procedure is not without limitations. For instance, some nucleophilic radicals gave markedly lower yields when reacting with certain electron-rich substrates, for example, a 17% yield in the reaction of IPS (E) with pyrrole 8 (see Table 1). At present, multiple solvents sometimes had to be screened to achieve the desired reaction [e.g., for MFMS (D)]. Nevertheless, we believe that the practicality, versatility and functional group tolerance of this innate functionalization of heterocycles makes it a valuable addition to the range of C–H functionalization methods that can be used to construct pharmaceutically important targets. In addition, if one were to compare traditional programmed approaches (of which none exist for three of the six salts described in Table 1) to the synthesis of compounds in Table 1 and especially to those in Fig. 2, it would be extremely difficult to derive a faster, simpler, or cheaper way to make such molecules. In most contexts of discovery chemistry, reactions that can be conducted open to the air on unprotected late-stage molecules proceeding in 10–50% yield are preferable to time-intensive multistep routes, especially when the overall yield of such routes (for instance, a typical sequence of protection, lithiation, iodination, cross-coupling, deprotection) is comparable to this direct approach. It is notable that 50 among the 52 compounds reported herein are prepared for the first time or have previously been described by our laboratory7,9 (several other methods show up in compound databases but no preparations are reported). Zinc sulfinate chemistry is not limited to the six salts presented above as salts have also been prepared for the transfer of CH2Cl, CH2CO2Me, cyclohexyl (e.g., see 18 in Fig. 2B) and perfluoroalkyl (e.g., C6F13) groups, and the extent of their scope is currently being examined (see Supplementary Information for details).
In this article, the invention of a zinc sulfinate toolkit containing ten different groups (CF3, CF2H, CH2CF3, CH2F, CH(CH3)2, (CH2CH2O)3CH3, CH2Cl, CH2CO2CH3, cyclohexyl, and C6F13) for the simple and rapid diversification of heterocycles has been described. Four of these reagents are currently commercially available from Sigma-Aldrich (TFMS catalog no. L510106; DFMS catalog no. L510084; TFES catalog no. L511234; IPS catalog no. L511161). Highlights of this chemistry include: 1) the superiority of zinc over sodium alkylsulfinates, which led to the identification of a highly active counter cation and which allowed for an efficient radical generation from alkylsulfinates; 2) the mildness of the reaction conditions, which tolerated highly sensitive functional groups such as chloro and boryl groups; 3) a different, transition-metal-free mode of radical generation compared to Minisci chemistry, since benzylic carboxylic acids were tolerated; 4) the feasibility of a sequential, one-pot operation that allowed for a site-selective bis-alkylation of heterocycles using two different alkylating agents; and 5) the ability to conduct these reactions under open air, without organic co-solvent and in the presence of extensive impurities in the reaction medium, which may have potential applications in bioconjugation. This chemistry has already been “field-tested” and is now widely adopted within medicinal chemistry at Pfizer; several of the building blocks reported in this work are currently in use there (3–5, 7, 13, 14). In many cases, building blocks that would be regarded as “low priority” at Pfizer due to prohibitively high cost or lengthy routes of synthesis are now easily accessible. The extreme operational ease, functional group tolerance, and ability to run reactions in unconventional media open to air bode well for the widespread application of zinc sulfinate salts in chemical synthesis. As a further outlook for the utility of innate heterocycle functionalization, zinc sulfinate reagents could potentially be used to cap metabolically susceptible positions in bioactive molecules and to identify the most reactive sites of a given (hetero)arene. The development of such a “profiling system” that provides a set of empirically determined reactivity rules to organic chemists is a topic of ongoing research in our laboratory.
METHODS
Standard procedure for the functionalization of heterocycles
A solution of heterocycle (0.125–0.250 mmol, 1.0 equiv) and Zn salt (2.0–3.0 equiv) in solvent was cooled to 0 °C, followed by a slow addition of tert-butyl hydroperoxide (70% solution in water, 54–179 μL, 3.0–5.0 equiv) by an Eppendorf pipette (metal needles should not be used as they decompose the reagent) with vigorous stirring. The reaction mixture was warmed to room temperature or 50 °C and monitored by thin-layer chromatography until reaction completion. For substrates that do not go to completion in 12–24 h, a second addition of Zn salt (2.0–3.0 equiv) and tert-butyl hydroperoxide (3.0–5.0 equiv) may be added to drive the reaction further. Upon consumption of the starting material, the reaction was partitioned between EtOAc or CH2Cl2 (5.0 mL) and saturated NaHCO3 (5.0 mL). The organic layer was separated, and the aqueous layer was extracted with EtOAc or CH2Cl2 (3 × 5.0 mL). The organic layers were dried with Na2SO4, concentrated and purified by column chromatography on silica gel. NOTE: If the addition of tert-butyl hydroperoxide is performed too rapidly, the resulting exotherm can result in reduced yield and selectivity. This is especially important on larger scales, where a syringe pump may be used to add in tert-butyl hydroperoxide.
Supplementary Material
Acknowledgments
We thank D.-H. Huang and L. Pasternack for NMR spectroscopic assistance, A. Schuyler and W. Uritboonthai for mass spectrometry assistance, and D. Cayer for the analysis of the β-lactamase activity. Financial support for this work was provided by the NIH/NIGMS (GM-073949), Uehara Memorial Foundation (postdoctoral fellowship for Y.F.), US-UK Fulbright Commission (postdoctoral fellowship for F.O.), Aarhus University, OChem Graduate School, CDNA, CFIN and NABIIT (fellowship for E.D.F.), NIH (graduate fellowship for R.A.R.), and Pfizer Inc. (postdoctoral fellowship for R.D.B.).
Footnotes
Author contributions
Y.F., J.A.D., D.D.D., R.A.R. and P.S.B. conceived the work; Y.F., J.A.D., F.O., E.D.F., D.D.D., R.A.R., R.D.B., B.H., N.S. and M.R.C. performed the experiments; Y.F., J.A.D., F.O., E.D.F., D.D.D., R.A.R., R.D.B., B.H., N.S., M.R.C. and P.S.B. analysed the data; R.A.R., Y.I. and P.S.B. wrote the manuscript.
Additional information
The authors declare no competing financial interests. Supplementary Information and chemical compound information accompany this paper at http://www.nature.com/nature. Reprints and permission information is available online at http://www.nature.com/reprints/.
References
- 1.Brückl T, Baxter RD, Ishihara Y, Baran PS. Innate and guided C–H functionalization logic. Acc Chem Res. 2012;45:826–839. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minisci F, Vismara E, Fontana F. Recent developments of free-radical substitutions of heteroaromatic bases. Heterocycles. 1989;28:489–519. [Google Scholar]
- 3.Duncton MAJ. Minisci reactions: versatile CH-functionalizations for medicinal chemists. Med Chem Comm. 2011;2:1135–1161. [Google Scholar]
- 4.Seiple IB, et al. Direct C–H arylation of electron-deficient heterocycles with arylboronic acids. J Am Chem Soc. 2010;132:13194–13196. doi: 10.1021/ja1066459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara Y, et al. Practical C–H functionalization of quinones with boronic acids. J Am Chem Soc. 2011;133:3292–3295. doi: 10.1021/ja111152z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molander GA, Colombel V, Braz VA. Direct alkylation of heteroaryls using potassium alkyl- and alkoxymethyltrifluoroborates. Org Lett. 2011;13:1852–1855. doi: 10.1021/ol2003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y, et al. Innate C–H trifluoromethylation of heterocycles. Proc Natl Acad Sci. 2011;108:14411–14415. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langlois BR, Laurent E, Roidot N. Trifluoromethylation of aromatic compounds with sodium trifluoromethanesulfinate under oxidative conditions. Tetrahedron Lett. 1991;32:7525–7528. [Google Scholar]
- 9.Fujiwara Y, et al. A new reagent for direct difluoromethylation. J Am Chem Soc. 2012;134:1494–1497. doi: 10.1021/ja211422g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Meanwell NA. Synopsis of some recent tactical application of bioisosteres in drug design. J Med Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 12.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37:320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- 13.Kirk KL. Fluorination in medicinal chemistry: methods, strategies, and recent developments. Org Process Res Dev. 2008;12:305–321. [Google Scholar]
- 14.Ma JA, Cahard D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J Fluorine Chem. 2007;128:975–996. [Google Scholar]
- 15.Cho EJ, et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science. 2010;328:1679–1681. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto H, Tsubogo T, Litvinas ND, Hartwig JF. A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides. Angew Chem Int Ed. 2011;50:3793–3798. doi: 10.1002/anie.201100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Truesdale L, Yu JQ. Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter. J Am Chem Soc. 2010;132:3648–3649. doi: 10.1021/ja909522s. [DOI] [PubMed] [Google Scholar]
- 18.Kino T, et al. Trifluoromethylation of various aromatic compounds by CF3I in the presence of Fe(II) compound, H2O2 and dimethylsulfoxide. J Fluorine Chem. 2010;131:98–105. [Google Scholar]
- 19.Qi Q, Shen Q, Lu L. Polyfluoroalkylation of 2-aminothiazoles. J Fluorine Chem. 2012;133:115–119. [Google Scholar]
- 20.Kamigata N, Ohtsuka T, Fukushima T, Yoshida M, Shimizu T. Direct perfluoroalkylation of aromatic and heteroaromatic compounds with perfluoroalkanesulfonyl chlorides catalysed by a ruthenium(II) phosphine complex. J Chem Soc, Perkin Trans. 1994;1:1339–1346. [Google Scholar]
- 21.Nagib DA, MacMillan DWC. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature. 2011;480:224–228. doi: 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fier PS, Hartwig JF. Copper-mediated difluoromethylation of aryl and vinyl iodides. J Am Chem Soc. 2012;134:5524–5527. doi: 10.1021/ja301013h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J, Zhang W, Wang F. Selective difluoromethylation and monofluoromethylation reactions. Chem Commun. 2009:7465–7478. doi: 10.1039/b916463d. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Hu J. Palladium-catalyzed 2,2,2-trifluoroethylation of organoboronic acids and esters. Angew Chem Int Ed. 2012;51:1033–1036. doi: 10.1002/anie.201106742. [DOI] [PubMed] [Google Scholar]
- 25.Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. Stereoselective nucleophilic monofluoromethylation of N-(tert-butanesulfinyl)imines with fluoromethyl phenyl sulfone. Org Lett. 2006;8:1693–1696. doi: 10.1021/ol060322t. [DOI] [PubMed] [Google Scholar]
- 27.Raymond JI, Andrews L. Matrix reactions of fluorohalomethanes with alkali metals. Infrared spectrum and bonding in the monofluoromethyl radical. J Phys Chem. 1971;75:3235–3242. [Google Scholar]
- 28.Russell GA, Guo D, Khanna RK. Alkylation of pyridine in free radical chain reactions utilizing alkylmercurials. J Org Chem. 1985;50:3423–3425. [Google Scholar]
- 29.Han C, Buchwald SL. Negishi coupling of secondary alkylzinc halides with aryl bromides and chlorides. J Am Chem Soc. 2009;131:7532–7533. doi: 10.1021/ja902046m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.