Abstract
Defects in microtubule-based transport are implicated in many neuropathologies. The filamentous fungi Aspergillus nidulans and Ustilago maydis are valuable models for studying transport due to their yeast-like genetic and biochemical tractability and metazoan-like dependence on microtubule-based transport for cellular trafficking. In these organisms the role of microtubules in nuclear positioning is well studied, but recent work has expanded the range of cargos to include endosomes, messenger RNA, secretory vesicles, peroxisomes, and nuclear pore complexes, reflecting the diversity of metazoan systems. Furthermore, similarities in transport mechanisms exist between filamentous fungi and metazoan neurons, demonstrating the suitability of A. nidulans and U. maydis for studying the molecular basis of transport-related neuropathologies such as lissencephaly, motor neuron disease, and Perry syndrome.
Introduction
The size and polarization of many eukaryotic cells demands a system of fast, directed, and long-range transport of cellular cargos that typically occurs along microtubules. Defects in microtubule-based transport are pervasive in neurodegenerative and neurodevelopmental disorders [1], necessitating the development of tractable model systems to study transport processes. Several species of filamentous fungi have emerged as powerful model organisms for such purposes, due to their long hyphal compartments that grow via microtubule-dependent transport of cellular cargos that include endosomes, peroxisomes, nuclei, and mRNA. In this review we focus in on the fungal species Ustilago maydis and Aspergillus nidulans. U. maydis is a plant pathogen that exhibits both yeast-like cell division by budding and dikaryotic hyphal growth. In contrast, A. nidulans grows only as long polarized hyphae that can be maintained in either haploid or diploid states. Importantly, both systems have genetic tractability on par with that of S. cerevisiae [2,3] and fully sequenced and annotated genomes [4,5]. Exploitation of these traits in recent years has yielded significant advances in our understanding of transport regulation, demonstrating the broad utility of filamentous fungi in understanding microtubule-based transport.
Microtubule-based motors and their cargos
Like the dendrites and axons of metazoan neurons, both U. maydis and A. nidulans have discrete regions in which polarized microtubules are oriented in either anti-parallel or uniform arrays (Figure 1). Kinesin motors carry out plus-end-directed (anterograde) transport, while minus-end-directed (retrograde) transport is mediated by dynein. Humans encode 45 kinesins, of which 15 are members of the kinesin-1, -2, or -3 families used for transport of organelles and other cargo [6,7]. The genomes of filamentous fungi typically contain between 10 and 12 kinesins with 2–3 members of the kinesin-1 and -3 families participating in transport [8]. While multiple kinesin motors are used for anterograde motility, nearly all retrograde cytoplasmic movement in both metazoans and filamentous fungi is driven by a single cytoplasmic dynein motor (referred to here as dynein). Dynein activity is regulated by an array of associated proteins and protein complexes, including dynactin, Lis1, Nudel, and dynein-associated subunits, perhaps in order to achieve functional diversity [9]. Although S. cerevisiae encodes the basic dynein machinery (used exclusively for nuclear migration), it uses actin-based transport for most cellular trafficking [10] and lacks cargo-transporting kinesins along with several components of the dynactin complex and some dynein light chains that are shared between filamentous fungi and metazoans (Figure 2). Thus, filamentous fungi are ideal model systems for investigating the conserved microtubule-based transport processes of complex multicellular eukaryotes.
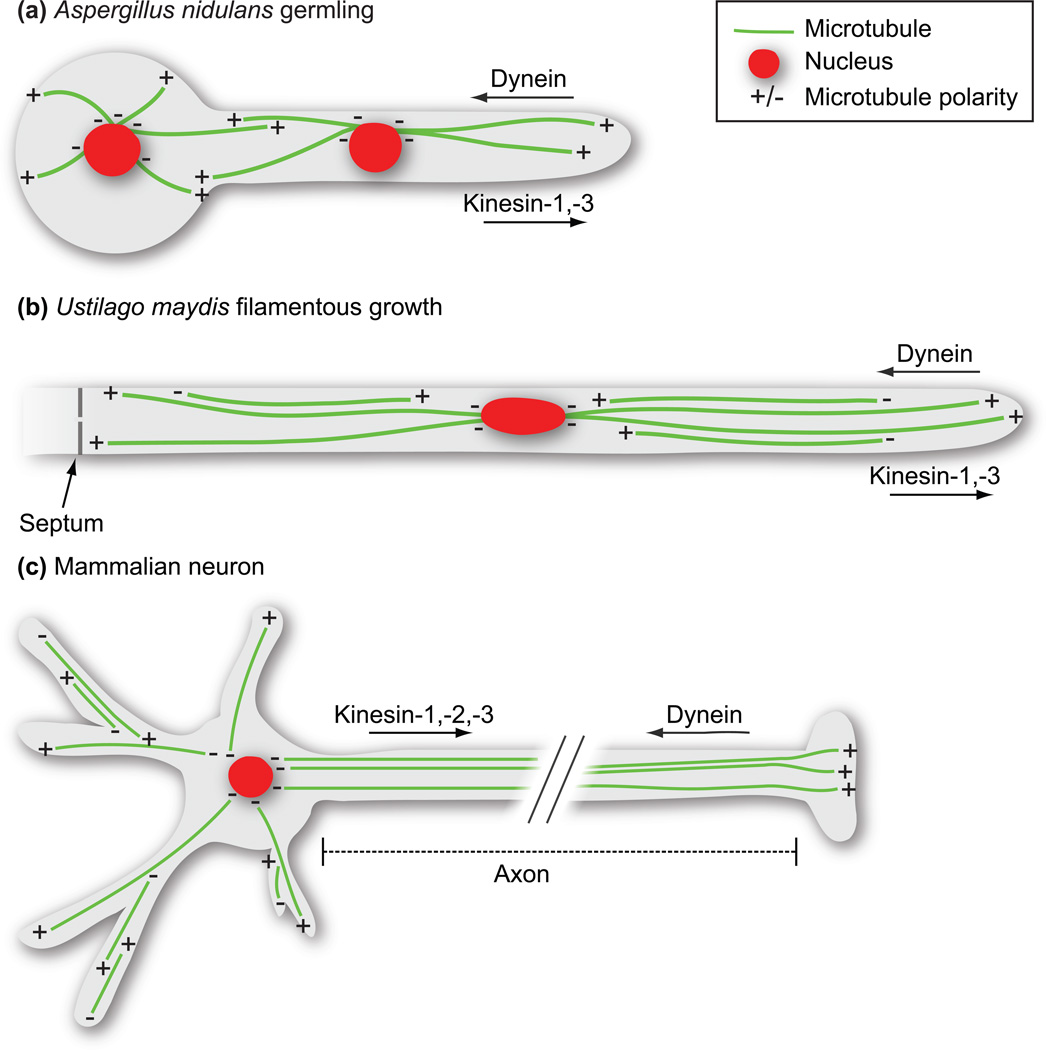
Figure 1. Microtubule organization and motor composition in filamentous fungal hyphae and mammalian neurons.
(A) Aspergillus nidulans grows by rapid tip extension forming polarized multinucleate hyphae. The region from the last nucleus to the hyphal tip contains uniformly orientated microtubule arrays, with their plus-ends pointing towards the direction of growth and their minus ends anchored at the spindle pole body/ microtubule organizing center. Kinesin-1 and -3 motors drive plus-end-directed movement, while dynein drives movement towards the microtubule minus end. Regions between nuclei contain microtubules of mixed polarity. (B) The dimorphic plant pathogenic fungus Ustilago maydis undergoes conditional filamentous growth resulting in highly polarized uninucleate infectious hyphae. Microtubules in U. maydis span the length of the infectious hyphae, but only regions ~12 µm from either the septum or the hyphal tip contain uniformly oriented microtubules. As in A. nidulans, kinesins-1 and -3 support plus-end-directed cargo transport, while dynein moves cargo towards the minus ends of microtubules. (C) Mammalian neurons contain unipolar microtubules within axons, with their plus ends oriented towards the synaptic terminal. Transport towards the synapse is mediated by kinesins-1, -2, and -3, while transport towards the cell body is largely dynein-driven. Microtubules are anti-parallel in dendrites.
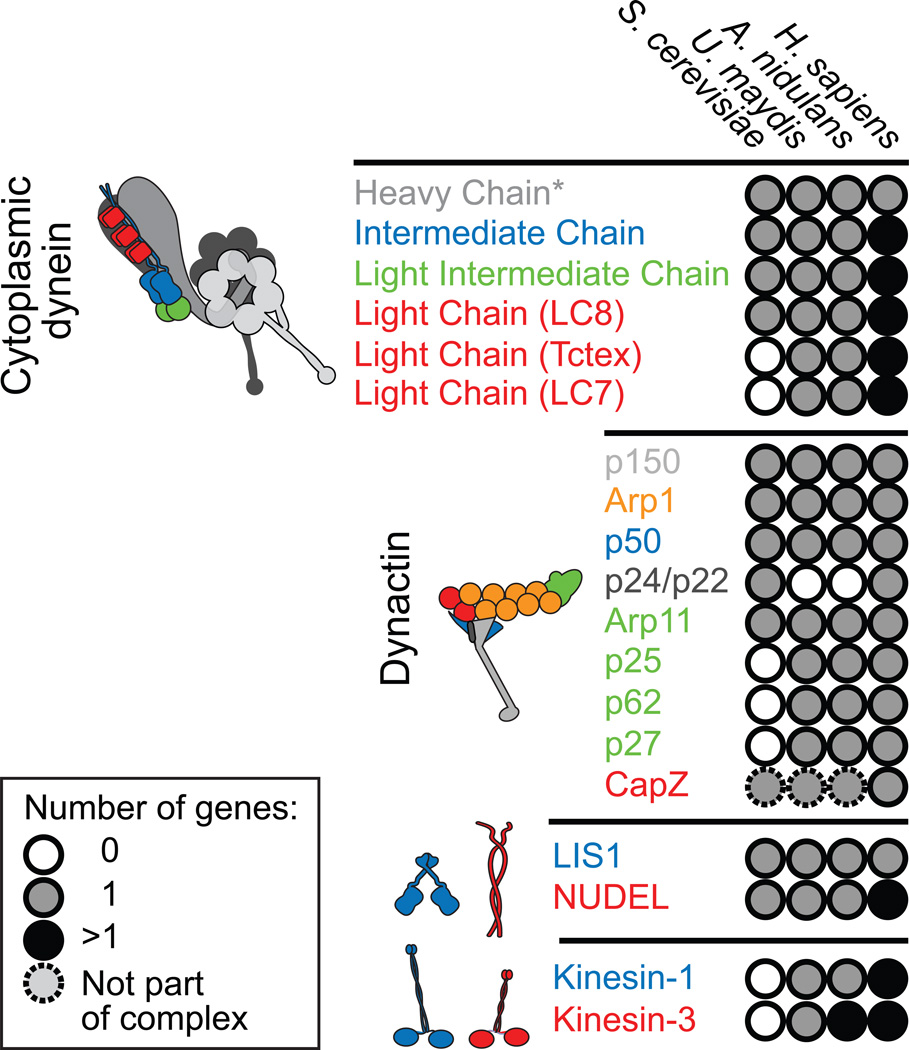
Figure 2. Genes and subunit composition of microtubule motors and their accessory factors in S. cerevisiae, U. Maydis, A. nidulans, and humans.
The number of genes found in each organism is color-coded as shown in the legend. S. cerevisiae lacks orthologues of 2 of the 3 dynein light chains, and 3 of the 4 dynactin subunits that form the pointed end complex of the Arp1 filament (shown in green), likely reflecting the fact that dynein does not participate in vesicular transport in this system. S. cerevisae also lacks kinesin-1 and -3 motors, instead relying on actin-based myosins for intracellular transport. In contrast, U. maydis and A. nidulans contain orthologues of most or all human dynein and dynactin subunits with the exception of p24/p22, and use kinesin-1 and kinesin-3s for cargo transport, making them excellent systems for dissecting the regulation of microtubule-based transport. *The single complete dynein heavy chain in U. maydis is encoded by two genes [59].
Nuclei were the first microtubule-dependent cargo described in filamentous fungi [11] and studies in these organisms continue to provide important insight into the mechanism of nuclear distribution. The list of cellular cargos transported by microtubule-based motors in filamentous fungi has since grown considerably, and it is likely that additional fungal cargos remain to be discovered. Here, we highlight the expanding diversity of known cargos for dynein and kinesin in A. nidulans and U. maydis.
Endosomes
It is well established that dynein and kinesin-3 support the motility of early endosomes in filamentous fungi, which is thought to facilitate long-range communication between the nucleus and the growing hyphal tip [12–16]. This makes endosome trafficking a valuable tool in dissecting bi-directional transport and its regulation. In U. maydis hyphae, which contain both unipolar and anti-parallel microtubules arrays (Figure 1), recent work shows that dynein moves endosomes through unipolar regions, while kinesin-3 is used in anti-parallel regions for further transport toward the nucleus [17**]. Thus, cooperation between opposite-polarity motors supports the transport of endosomes across the entire length of the cell. In A. nidulans, which also uses dynein and kinesin-3 for endosome transport [13**–16], the p25 subunit of the dynactin complex is required for the physical interaction of dynein with early endosomes [15**], demonstrating a cargo adaptor role for dynactin in filamentous fungi that is consistent with reported roles for dynactin as a cargo adaptor in metazoans [18,19]. Post-translational tubulin modifications may provide another layer of regulation for endosome traffic. In A. nidulans, endosome-transporting kinesin-3/UncA was found to prefer a subpopulation of detyrosinated microtubules [20,21]. Intriguingly, a similar preference for detyrosinated microtubules allows kinesin-1, but not kinesin-3, to discriminate between axons and dendrites in mammalian neurons [22,23].
Messenger RNA
Microtubule motor-driven transport and subsequent localized translation of mRNA is an important feature of eukaryotic biology. Recently, microtubule-based mRNA transport was also reported in U. maydis, where the RNA binding protein Rrm4 mediates the recognition and shuttling of target mRNAs [24]. In this study, Rrm4 recognized target mRNAs by a ‘zipcode’ sequence in the 3’-UTR of the transcript, promoting the packaging of the mRNA into ribonucleoprotein complexes (mRNPs) for subsequent delivery [24]. This is reminiscent of the mechanism by which β-actin mRNA is transported to the cell periphery via microtubules in mammalian systems, reflecting the likely conservation of microtubule-based mRNA transport mechanisms between filamentous fungi and metazoans [25]. A subsequent study showed that bi-directional Rrm4-mediated mRNA transport is driven by dynein and kinesin-3 [26**]. Interestingly, Rrm4-containing mRNPs also required functional endosomes for microtubule-based movement, suggesting that these mRNPs may hitch a ride on dynein and kinesin-3-driven early endosomes [26**]. A similar relationship between membrane trafficking and localized mRNA has been proposed in S. cerevisiae [27]. The significance of this mRNA transport in U. maydis was highlighted in another recent study demonstrating that efficient secretion of an endochitinase, Cts1, is dependent on Rrm4-mediated mRNA shuttling [28].
Secretory vesicles
In mammalian systems, a large and diverse array of cellular cargos have been identified for kinesin-1 [7], while in filamentous fungi organellar kinesin-1 cargos have remained elusive. However, a recent study in U. maydis supports a direct role for kinesin-1 in polarized secretion [29**]. Kinesin-1, along with the actin-based motor myosin-5, is needed for the delivery of chitin-synthase-containing vesicles (CSVs) to the polarized growth region for exocytic secretion [29**]. In this pathway, retrograde dynein activity likely functions in the removal of excess CSVs from the region of polarized growth, therein generating a gradient of chitin synthase for secretion [29**].
Peroxisomes
Peroxisomes are ubiquitous single membrane-bound organelles that perform important metabolic functions. In mammalian cells, a small population of peroxisomes undergo long-range dynein- and dynactin-dependent saltatory movements [30]. In A. nidulans, peroxisome behavior is strikingly similar, with small populations undergoing dynein and kinesin-3 dependent bi-directional movements, while the majority remain immotile [13**]. Recent work in A. nidulans suggests that a subpopulation of peroxisomes may act as vehicles for transporting proteins associated with microtubule-organizing centers (MTOCs) [31*], therefore the idea of some cargos ‘piggy-backing’ on others for transport may not be limited to the relationship between mRNPs and endosomes. In yeast, actin-based transport of peroxisomes to the bud neck during cell division is thought to facilitate equal inheritance of the organelle between mother and daughter cells [10]. In filamentous fungi, peroxisome transport likely serves a similar function, as the distribution of the organelle must keep pace with the growing hyphal tip.
Nuclear pore complexes
Nuclear pore complexes (NPCs) were also recently identified as microtubule-based cargos in U. maydis, which, along with other fungi, lacks nuclear lamina [32*]. The NPCs displayed dynein- and kinesin-1-dependent movements within the nuclear envelope that were required for both NPC and chromatin distribution [32*]. These results raise the interesting possibility that microtubule-dependent NPC positioning may play a role in transcriptional regulation.
Transport initiation at microtubule plus ends
In filamentous fungi, as well as in metazoans, dynamic microtubule plus ends are sites for cargo loading and transport initiation [12,13**,33,34]. Consistent with this idea, dynein and/ or its regulators accumulate at microtubule plus ends in many cell types [9]. Here we highlight recent progress made in filamentous fungi towards understanding how dynein gets to and is retained at microtubule plus ends, and how it is subsequently loaded onto cargo.
In both U. maydis and A. nidulans, dynein is recruited to the dynamic plus ends of cytoplasmic microtubules in a kinesin-1-dependent manner [12,13**,35]. In U. maydis an interaction between the end-binding protein, EB1, and dynactin is important for retention of a sub-population of dynein [36**], though other mechanisms are likely to be involved (see below). Ultimately, this plus end recruitment generates spatially confined dynein pools that are proposed to function as ‘loading zones’ for the capture of incoming kinesin-3-driven early endosomes [12,36**]. Evidence suggests similar spatial loading of dynein onto endosomes at microtubule plus ends in A. nidulans [13**], though the situation in U. maydis may be more complex, as dynein can also be loaded onto moving endosomes before they reach the established dynein ‘loading zone’ at the hyphal tip [37**].
All of the known cellular functions of dynein, including its accumulation at the microtubule plus end, are dependent on the largest subunit of the dynactin complex, p150Glued (referred to here as p150). The N-terminus of p150 contains a conserved microtubule-binding region consisting of a cytoskeleton-associated protein glycine rich (CAP-Gly) domain, immediately followed by a region rich in basic amino acids. CAP-Gly domains have been shown to interact directly with EEY/F-COO− motifs, which are sequence elements found in α-tubulin and microtubule end-binding proteins, such as EB1 [38]. A recent functional analysis of A. nidulans p150 demonstrated that the CAP-Gly domain is not required for the microtubule plus end localization of p150 [39**]. Instead, the basic domain was necessary for the plus end localization of both p150 and dynein, and therefore essential for normal early endosome motility [39**]. Furthermore, the basic domain was required for the interaction of p150 with microtubules in vivo, which may be important for the kinesin-1-dependent recruitment of dynein and dynactin to microtubule plus ends in A. nidulans [39**].
An additional layer of regulation may be to inhibit dynein activity at microtubule plus ends prior to cargo loading. For example, in both A. nidulans and U. maydis the orthologue of Lis1 appears to play an important role in initiating dynein-mediated transport from the microtubule plus end and may function in loading dynein onto cargos to initiate retrograde motility [12,13**]. In A. nidulans, in the absence of Lis1 both dynein and its cargos leave plus ends with a dramatically reduced frequency, but moving molecules or cargos do so at normal speeds [13**] (Figure 3). Further support for a transport initiation role for Lis1 comes from experiments in S. cerevisiae, where dynein positions daughter nuclei by pulling on nucleus-attached astral microtubules while anchored to the cell cortex [40]. Here, Lis1/Pac1 is required for dynein plus end localization and subsequent ‘off-loading’ to the cell cortex [40], a scenario that is analogous to loading dynein onto organellar cargo. Recent work suggests a molecular mechanism for how Lis1 regulates dynein [41**]. In this work, single-molecule motility assays and single-particle electron microscopy suggested that Lis1 binds at the junction of the dynein ATPase ring and microtubule-binding stalk. When bound to dynein, Lis1 appeared to sever communication between these domains, causing dynein to become anchored to the microtubule [41**]. Thus, by binding dynein, Lis1 may increase the residence time of dynein at the microtubule plus end, and assist in the kinetics of cargo loading [41**].
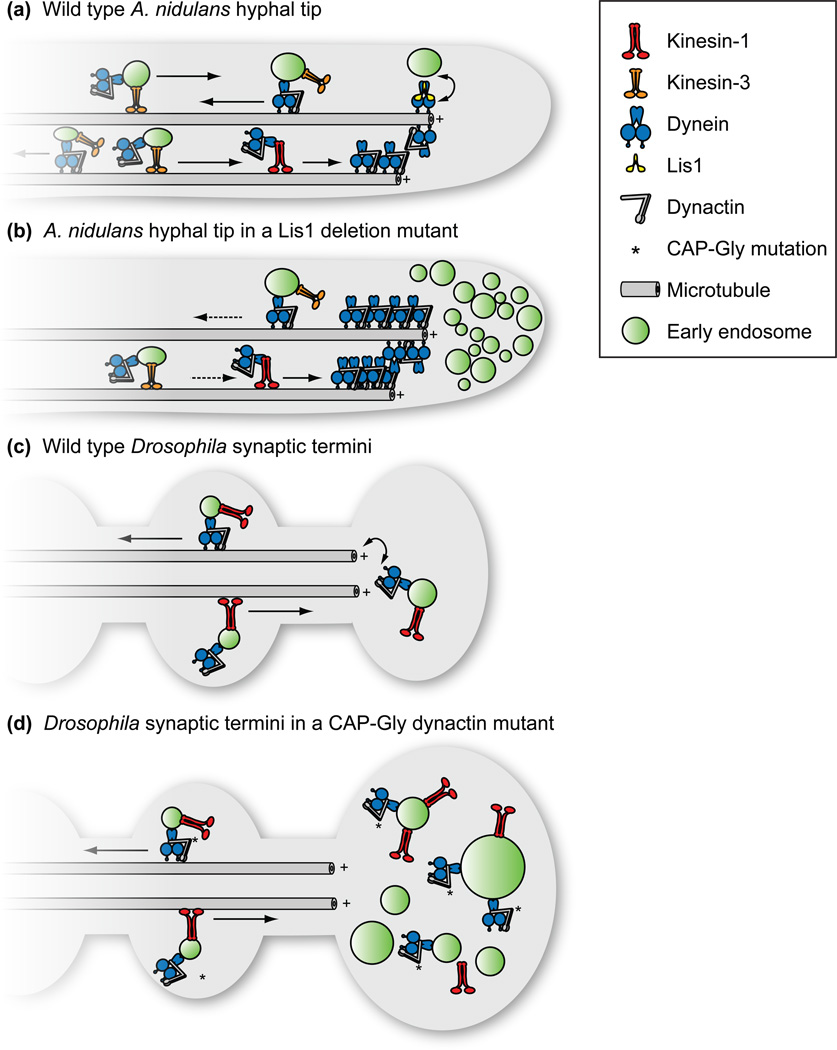
Figure 3. Models for transport initiation in A. nidulans and Drosophila.
(A) In wild type A. nidulans hyphae, dynein accumulates at dynamic microtubule plus ends in a process dependent on kinesin-1. Efficient loading of dynein onto early endosomes requires the accessory protein Lis1. (B) In the absence of Lis1, dynein and cargos accumulate aberrantly at the hyphal tip [12,13**,35]. Reflecting a role for Lis1 in loading dynein onto endosomes, the frequency but not velocity of endosome movements is dramatically affected by the deletion of Lis1. (C) In Drosophila neurons the CAP-Gly domain of the p150 subunit of the dynactin complex is required for the initiation of dynein-dependent vesicle transport from axonal termini. (D) Introduction of a loss-of-function and motor neuron disease-associated mutation within the CAP-Gly domain causes accumulation of cargo, yet axonal transport is unperturbed [43*].
Molecular motor recycling
Many eukaryotic cell types exhibit a mutual interdependence between opposite polarity motors for bi-directional motility (discussed recently in [13**]). How molecular motors coordinate with each other to achieve this interdependence is an area of active inquiry, but recent work suggests a mechanism of motor ‘recycling’ in which dynein and kinesin themselves function as cargo for an opposite polarity motor, facilitating their return to their respective starting points on the microtubule track. In both A. nidulans and U. maydis, as well as in mammalian neurons, the plus end localization of dynactin, and thus proper retrograde transport, is dependent on kinesin-1 [12,13**,35,42*]. Similarly, a Drosophila kinesin-1 orthologue functions synergistically with plus end localized dynactin at presynaptic axon terminals to initiate retrograde transport [43*]. Demonstrating the reciprocity of this relationship, the genetic absence of dynein in A. nidulans causes kinesin-3 accumulation at microtubule plus ends, while in Drosophila neurons, kinesin-1 accumulates at axon terminals as a consequence of dynactin dysfunction [13**,43*]. Collectively, these experiments provide compelling evidence for a kind of molecular conveyor belt on which dyneins and kinesins are continuously recycled between opposite microtubule ends.
Filamentous fungi as models for understanding disease
Defects in microtubule-based transport are associated with several neuropathologies [1]. One such pathology, lissencephaly, is caused by dominantly inherited loss of function mutations in the human LIS1 gene [44]. The connection between Lis1 and dynein was first discovered in A. nidulans, in which loss-of-function mutants of the LIS1 orthologue (nudF) phenocopies the severe nuclear distribution and growth defects of dynein loss-of-function mutants [45]. The identification of a point mutation (R3086C) in AAA4 of the dynein motor domain that suppressed the Lis1 null phenotype in A. nidulans provided a clue regarding the relationship between these proteins [46,47]. In addition to mitigating the nuclear migration defects of the Lis1 null strain, the suppressor mutation induced a dynein localization pattern of discrete, nearly immobile puncta along microtubules [46]. When mapped onto recent high-resolution dynein crystal structures [48,49], the mutated residue appeared to form an ‘arginine finger’ motif, which likely transmits conformational changes within the dynein ATPase motor ring [41**]. Interestingly, analogous mutations in yeast dynein mimicked regulation by Lis1 (see above), corroborating its proposed role in regulating allosteric communication within the dynein motor [41**].
In addition to lissencephaly, filamentous fungi are poised to broaden our understanding of microtubule-based transport in motor neuron diseases and the Parkinson’s-like Perry syndrome, in which mutations in both dynein and dynactin have been identified in human patients [1,50]. In particular, the functional integrity of the CAP-Gly domain of the dynactin subunit p150 appears to be an important mediator of disease. Causative mutations for these divergent disorders reside less than 15 residues apart in p150’s CAP-Gly domain, indicating that CAP-Gly dysfunction may promote pathology by multiple mechanisms. Despite this, the CAP-Gly domain appears to be dispensable for microtubule-based transport processes in several experimental systems. As described above, in A. nidulans deletion of the CAP-Gly domain yields normal growth and nuclear migration phenotypes and does not perturb the retrograde velocities of motile endosomes, or the frequency with which they leave the hyphal tip [39**]. Similarly, depletion of the CAP-Gly domain in non-neuronal mammalian and Drosophila cells is not deleterious for organelle transport or distribution [51,52]. In S. cerevisiae the CAP-Gly domain was found to be important for initiating dynein-mediated movement of dividing nuclei into the bud neck, but dispensable for dynactin-mediated increases in dynein processivity in vitro [53,54]. These studies suggest that the functional role of the CAP-Gly domain may be subtle or exclusive to high load functions, such as nuclear positioning. Consistent with its proposed role in yeast, recent studies in Drosophila and mouse neurons suggest that the p150 CAP-Gly domain is important for retrograde transport initiation, but not transport itself [42*,43*]. In these systems the CAP-Gly domain is dispensable for cargo flux, velocity, and run-length within the axon but required to enrich the dynactin complex and promote retrograde transport from synaptic terminals (Figure 3).
Thus, both dynactin and Lis1 are important for transport initiation (Figure 3). While the connection between dynactin and Lis1 in transport initiation is currently unclear, their mechanisms are likely different making this an exciting area for future study. One possibility is that Lis1 and dynactin may have cell-type or organism-specific functions. In filamentous fungi and in non-neuronal mammalian cells, both dynactin and dynein localize to microtubule plus ends [9,12,13**,35]. However, in mammalian neurons only dynactin plus end localization is observed, perhaps because dynein recruitment to axon terminals is rate limiting for transport in neurons, whereas other factors such as the availability of cargo, may be rate limiting in other cell types [42*]. In neurons, p150 may serve to concentrate dynactin, which is essential for dynein function in vivo, for efficient and immediate engagement in retrograde motility once motors become available.
Another possibility is that dynactin and Lis1 are not mutually exclusive in transport initiation, but rather cooperative. In both S. cerevisiae and A. nidulans, Lis1 is proposed to ‘prime’ dynein for cargo loading at microtubule plus ends [13**,41**]. Once loaded onto the cell cortex in yeast (which effectively functions as dynein cargo), the activity of the p150 CAP-Gly domain is important in initiating, but not sustaining, the dynein-driven microtubule sliding that positions the daughter nucleus [54], perhaps through the displacement of Lis1 from the dynein complex [55,56]. This is consistent with the observed absence of Lis1 on retrograde cargo leaving the microtubule plus ends in A. nidulans [13**]. The loading and transport of diverse cellular cargos by only a single retrograde motor are likely to be highly regulated processes mediated by the sequential or combinatorial activities of many factors, including Lis1 and dynactin.
Conclusion
Many questions remain regarding the regulation of microtubule-based transport and how defects in this regulation lead to disease in humans. From the expansion of known cargos to the elucidation of retrograde transport initiation mechanisms, recent work in both U. maydis and A. nidulans demonstrates the utility of these organisms in understanding evolutionarily conserved microtubule-based transport processes. Moving forward, the biochemical and genetic tractability of filamentous fungi give them tremendous potential in understanding motor mechanisms as well as for high-throughput screening to identify novel drug targets and therapeutics. Recent descriptions of tetracycline-inducible expression systems for a related Aspergillus species, A. niger, as well as protocols for the purification of enzymatically active proteins from A. nidulans emphasize the suitability of filamentous fungi for heterologous protein expression and in vitro characterization [57,58]. Furthermore, the improving annotation of fungal genomes and feasibility of whole-genome sequencing makes these organisms amenable to the identification of novel genes involved in microtubule-based transport through genetic screens. With these strengths, future work, both in vivo and in vitro, is likely to lean heavily on conclusions drawn from filamentous fungi.
Highlights.
Dynein- and kinesin-driven microtubule-based transport is linked to neuropathology.
Filamentous fungi are valuable models to study transport by dynein and kinesin.
The diversity of fungal cargos that use microtubule-based transport is expanding.
Fungal models provide insight into transport initiation at microtubule terminals.
Knowledge of transport regulation in fungi informs efforts to understand neurological disease.
Acknowledgements
SRP is funded by the Rita Allen Foundation, the Harvard Armenise Foundation, and a NIH New Innovator award (1 DP2 OD004268-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Larrondo LF, Colot HV, Baker CL, Loros JJ, Dunlap JC. Fungal functional genomics: tunable knockout-knock-in expression and tagging strategies. Eukaryotic Cell. 2009;8:800–804. doi: 10.1128/EC.00072-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todd RB, Davis MA, Hynes MJ. Genetic manipulation of Aspergillus nidulans: meiotic progeny for genetic analysis and strain construction. Nat. Protoc. 2007;2:811–821. doi: 10.1038/nprot.2007.112. [DOI] [PubMed] [Google Scholar]
- 4.Galagan JE, Calvo SE, Cuomo C, Ma L-J, Wortman JR, Batzoglou S, Lee S-I, Baştürkmen M, Spevak CC, Clutterbuck J, et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 5.Kämper J, Kahmann R, Bölker M, Ma L-J, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Müller O, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- 6.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg G. Motors in fungal morphogenesis: cooperation versus competition. Curr. Opin. Microbiol. 2011;14:660–667. doi: 10.1016/j.mib.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammer JA, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 2012;13:13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- 11.Xiang X, Fischer R. Nuclear migration and positioning in filamentous fungi. Fungal Genet. Biol. 2004;41:411–419. doi: 10.1016/j.fgb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Lenz JH, Schuchardt I, Straube A, Steinberg G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. The EMBO Journal. 2006;25:2275–2286. doi: 10.1038/sj.emboj.7601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egan MJ, Tan K, Reck-Peterson SL. Lis1 is an initiation factor for dynein-driven organelle transport. The Journal of Cell Biology. 2012;197:971–982. doi: 10.1083/jcb.201112101. In this study, the authors use live-cell fluorescent imaging to investigate the role of Lis1 in microtubule-based transport in A. nidulans. They show that Lis1 promotes dynein-driven organelle flux from microtubule plus-ends, but is not strictly required for organelle motility. Thus, Lis1 is an initiation factor in retrograde transport rather than a constitutive component of minus-end-directed motor complexes.
- 14.Zhang J, Zhuang L, Lee Y, Abenza JF, Peñalva MA, Xiang X. The microtubule plus-end localization of Aspergillus dynein is important for dynein-early-endosome interaction but not for dynein ATPase activation. Journal of Cell Science. 2010;123:3596–3604. doi: 10.1242/jcs.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Yao X, Fischer L, Abenza JF, Peñalva MA, Xiang X. The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. The Journal of Cell Biology. 2011;193:1245–1255. doi: 10.1083/jcb.201011022. Though the dynactin complex is widely thought to facilitate interactions between dynein and its cargo, little is known about the specific proteins that participate in these interactions. This paper shows that the p25 subunit of dynactin is important in the specific recognition of endosomes as dynein cargo for retrograde motility in A. nidulans, providing valuable insight regarding how dynactin components identify unique cargos.
- 16.Abenza JF, Pantazopoulou A, Rodr guez JM, Galindo A, Peñalva MA. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic. 2009;10:57–75. doi: 10.1111/j.1600-0854.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 17. Schuster M, Kilaru S, Fink G, Collemare J, Roger Y, Steinberg G. Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a nonuniform microtubule array. Molecular Biology of the Cell. 2011;22:3645–3657. doi: 10.1091/mbc.E11-03-0217. How opposite polarity motors cooperate to achieve long-range transport is of great importance to the field. This study highlights how dynein and kinesin-3 can exploit microtubule polarity to maintain overall unidirectional transport by having dynein ‘hand off’ cargo to kinesin-3 at the interface between uniform and anti-parallel microtubule arrays.
- 18.Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. beta III spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 19.Yeh T-Y, Quintyne NJ, Scipioni BR, Eckley DM, Schroer TA. Dynactin's pointed-end complex is a cargo-targeting module. Molecular Biology of the Cell. 2012;23:3827–3837. doi: 10.1091/mbc.E12-07-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zekert N, Fischer R. The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Molecular Biology of the Cell. 2009;20:673–684. doi: 10.1091/mbc.E08-07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel C, Zekert N, Fischer R. The Aspergillus nidulans kinesin-3 tail is necessary and sufficient to recognize modified microtubules. PLoS ONE. 2012;7:e30976. doi: 10.1371/journal.pone.0030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 2009;10:765–777. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 23.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- 24.König J, Baumann S, Koepke J, Pohlmann T, Zarnack K, Feldbrügge M. The fungal RNA-binding protein Rrm4 mediates long-distance transport of ubi1 and rho3 mRNAs. The EMBO Journal. 2009;28:1855–1866. doi: 10.1038/emboj.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnelly CJ, Fainzilber M, Twiss JL. Subcellular Communication Through RNA Transport and Localized Protein Synthesis. Traffic. 2010;11:1498–1505. doi: 10.1111/j.1600-0854.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baumann S, Pohlmann T, Jungbluth M, Brachmann A, Feldbrügge M. Kinesin-3 dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. Journal of Cell Science. 2012;125:2740–2752. doi: 10.1242/jcs.101212. RNA metabolism processes, including the regulation of long-range mRNA transport in neurons, are emerging as important factors in neurodegenerative diseases. The demonstration in this paper that U. maydis uses dynein and kinesin-3 to transport mRNA within hyphae indicates that filamentous fungi are useful organisms for understanding the role of RNA transport in disease. Additionally, the authors propose a novel ‘hitchhiking’ mechanism of intracellular transport in which interactions between mRNPs and endosomes facilitate co-transport of these organelles.
- 27.Gerst JE. Message on the web: mRNA and ER co-trafficking. Trends Cell Biol. 2008;18:68–76. doi: 10.1016/j.tcb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Koepke J, Kaffarnik F, Haag C, Zarnack K, Luscombe NM, König J, Ule J, Kellner R, Begerow D, Feldbrügge M. The RNA-binding protein Rrm4 is essential for efficient secretion of endochitinase Cts1. Mol. Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.011213. M111.011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuster M, Treitschke S, Kilaru S, Molloy J, Harmer NJ, Steinberg G. Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. The EMBO Journal. 2012;31:214–227. doi: 10.1038/emboj.2011.361. Evidence suggests that kinesin-1 in filamentous fungi is important in several secretory pathways. The authors demonstrate that kinesin-1 and myosin-5 mediate the transport of vesicles containing chitin synthase to the growing hyphal tip for exocytosis, while dynein returns these vesicles to the cell center if unsuccessfully secreted. This provides valuable insight not only into the emerging role of kinesing-1, but also into how microtubule-based motors coordinate with actin-based transport mechanisms.
- 30.Schrader M, King SJ, Stroh TA, Schroer TA. Real time imaging reveals a peroxisomal reticulum in living cells. Journal of Cell Science. 2000;113(Pt 20):3663–3671. doi: 10.1242/jcs.113.20.3663. [DOI] [PubMed] [Google Scholar]
- 31. Zekert N, Veith D, Fischer R. Interaction of the Aspergillus nidulans microtubule-organizing center (MTOC) component ApsB with gamma-tubulin and evidence for a role of a subclass of peroxisomes in the formation of septal MTOCs. Eukaryotic Cell. 2010;9:795–805. doi: 10.1128/EC.00058-10. Relatively little is known about the role of peroxisome transport in filamentous fungi. The authors of this study identify a subpopulation of peroxisomes to which components of microtubule-organizing centers (MTOCs) localize, leading to the hypothesis that transport of these peroxisomes facilitates the generation and maintenance of MTOCs at septa within A. nidulans hyphae.
- 32. Steinberg G, Schuster M, Theisen U, Kilaru S, Forge A, Martin-Urdiroz M. Motor-driven motility of fungal nuclear pores organizes chromosomes and fosters nucleocytoplasmic transport. The Journal of Cell Biology. 2012;198:343–355. doi: 10.1083/jcb.201201087. This paper describes dynein- and kinesin-1-driven motility of nuclear pore complexes (NPCs) within the membranes of U. maydis nuclei. This motility was found to be important in maintaining a uniform distribution of NPCs in the nuclear membrane and for the proper trafficking of cellular contents between the nucleus and the cytoplasm. Additionally, the observation that NPC motility and distribution maintains chromosomal organization within the nucleus presents the exciting possibility of motor-based genetic regulation in filamentous fungi.
- 33.Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150Glued to microtubule plus ends in organelle transport. The Journal of Cell Biology. 2002;158:305–319. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lomakin AJ, Semenova I, Zaliapin I, Kraikivski P, Nadezhdina E, Slepchenko BM, Akhmanova A, Rodionov V. CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Developmental Cell. 2009;17:323–333. doi: 10.1016/j.devcel.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Li S, Fischer R, Xiang X. Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Molecular Biology of the Cell. 2003;14:1479–1488. doi: 10.1091/mbc.E02-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuster M, Kilaru S, Ashwin P, Lin C, Severs NJ, Steinberg G. Controlled and stochastic retention concentrates dynein at microtubule ends to keep endosomes on track. The EMBO Journal. 2011;30:652–664. doi: 10.1038/emboj.2010.360. The localization and subsequent activation of retrograde transport machinery at microtubule plus-ends has been an active topic of pursuit in several model systems during the last year. This paper quantitatively describes the active retention of approximately half of the dynein motors by EB1 at the hyphal tip of filamentous U. maydis, with the remaining half being retained stochastically by a motor crowding mechanism at microtubule plus-ends.
- 37. Schuster M, Lipowsky R, Assmann M-A, Lenz P, Steinberg G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3618–3623. doi: 10.1073/pnas.1015839108. How opposite polarity motors on the same cargo coordinate to achieve directed transport is not well understood. In this paper, the authors find that directionality of endosomes in U. maydis is dictated by the presence or absence of a single dynein motor on cargos to which multiple kinesin-3 motors are constitutively bound. Additionally, the observation that anterograde cargos change direction prior to reaching the dynein pool at the hyphal tip led the authors to hypothesize that dynein released from microtubule plus-ends can load cargo ‘on the run’.
- 38.Honnappa S, Okhrimenko O, Jaussi R, Jawhari H, Jelesarov I, Winkler FK, Steinmetz MO. Key interaction modes of dynamic +TIP networks. Mol. Cell. 2006;23:663–671. doi: 10.1016/j.molcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 39. Yao X, Zhang J, Zhou H, Wang E, Xiang X. In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function. Traffic. 2012;13:375–387. doi: 10.1111/j.1600-0854.2011.01312.x. The in vivo role of the CAP-Gly domain of p150 is emerging, but still mysterious. In this paper, the authors find the CAP-Gly domain to be dispensable for endosome transport, distribution, and plus-end tracking of dynein and dynactin in A. nidulans. Instead, the authors determined the basic domain that follows the CAP-Gly domain to be important for these functions, suggesting that the in vivo significance of the basic domain’s microtubule binding capacity may be underemphasized.
- 40.Moore JK, Cooper JA. Coordinating mitosis with cell polarity: Molecular motors at the cell cortex. Semin. Cell Dev. Biol. 2010;21:283–289. doi: 10.1016/j.semcdb.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang J, Roberts AJ, Leschziner AE, Reck-Peterson SL. Lis1 Acts as a “Clutch” between the ATPase and Microtubule-Binding Domains of the Dynein Motor. Cell. 2012;150:975–986. doi: 10.1016/j.cell.2012.07.022. How Lis1 regulates dynein is of great interest as mutations in Lis1 cause lissencephaly in humans. This study combines structural, biochemical, and single-molecule experiments with purified yeast dynein to demonstrate that Lis1 binding between AAA3 and AAA4 of the dynein motor domain disrupts allosteric communication between the microtubule-binding domain and ATPase domain, effectively anchoring the motor in a high microtubule affinity state despite continued hydrolysis of ATP.
- 42. Moughamian AJ, Holzbaur ELF. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74:331–343. doi: 10.1016/j.neuron.2012.02.025. This paper supports a role for the CAP-Gly domain of p150 in retrograde transport initiation in mammalian neurons. Metrics for the integrity of axonal transport were largely normal in cells depleted of the CAP-Gly domain with the exception of a decrease in flux from the axon terminal. The CAP-Gly domain was found to enrich dynactin at microtubule plus-ends, facilitating efficient transport from the terminal. Additionally, the authors propose mechanisms by which mutations in different regions of the CAP-Gly domain cause motor neuron disease or Perry syndrome.
- 43. Lloyd TE, Machamer J, O'Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan ES, et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74:344–360. doi: 10.1016/j.neuron.2012.02.026. Using Drosophila neurons, this paper provides additional evidence for the regulation of transport initiation by the CAP-Gly domain of p150. With CAP-Gly utility impaired by a loss-of-function point mutation associated with motor neuron disease, the authors observe normal organelle trafficking within axons, but decreased retrograde flux from axon terminals. Coupled with the observed accumulation of cargo and dynein heavy chain at these axon terminals, this points to an inability of dynein to engage the microtubule track at plus-ends in the absence of a functional CAP-Gly domain.
- 44.Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 45.Xiang X, Osmani AH, Osmani SA, Xin M, Morris NR. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Molecular Biology of the Cell. 1995;6:297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhuang L, Zhang J, Xiang X. Point mutations in the stem region and the fourth AAA domain of cytoplasmic dynein heavy chain partially suppress the phenotype of NUDF/LIS1 loss in Aspergillus nidulans. Genetics. 2007;175:1185–1196. doi: 10.1534/genetics.106.069013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willins DA, Liu B, Xiang X, Morris NR. Mutations in the heavy chain of cytoplasmic dynein suppress the nudF nuclear migration mutation of Aspergillus nidulans. Mol. Gen. Genet. 1997;255:194–200. doi: 10.1007/s004380050489. [DOI] [PubMed] [Google Scholar]
- 48.Kon T, Oyama T, Shimo-Kon R, Imamula K, Shima T, Sutoh K, Kurisu G. The 2.8 Å crystal structure of the dynein motor domain. Nature. 2012;484:345–350. doi: 10.1038/nature10955. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H, Gleave ES, Carter AP. Insights into dynein motor domain function from a 3.3-Å crystal structure. Nat. Struct. Mol. Biol. 2012;19 doi: 10.1038/nsmb.2272. 492–7–S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harms MB, Ori-McKenney KM, Scoto M, Tuck EP, Bell S, Ma D, Masi S, Allred P, Al-Lozi M, Reilly MM, et al. Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology. 2012;78:1714–1720. doi: 10.1212/WNL.0b013e3182556c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur ELF. Regulation of dynactin through the differential expression of p150Glued isoforms. J. Biol. Chem. 2008;283:33611–33619. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim H, Ling SC, Rogers GC, Kural C, Selvin PR, Rogers SL, Gelfand VI. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. The Journal of Cell Biology. 2007;176:641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kardon JR, Reck-Peterson SL, Vale RD. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5669–5674. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore JK, Sept D, Cooper JA. Neurodegeneration mutations in dynactin impair dynein-dependent nuclear migration. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5147–5152. doi: 10.1073/pnas.0810828106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKenney RJ, Weil SJ, Scherer J, Vallee RB. Mutually exclusive cytoplasmic dynein regulation by NudE-Lis1 and dynactin. Journal of Biological Chemistry. 2011;286:39615–39622. doi: 10.1074/jbc.M111.289017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nyarko A, Song Y, Barbar E. Intrinsic Disorder in Dynein Intermediate Chain Modulates Its Interactions with NudE and Dynactin. Journal of Biological Chemistry. 2012;287:24884–24893. doi: 10.1074/jbc.M112.376038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer V, Wanka F, van Gent J, Arentshorst M, van den Hondel CAMJJ, Ram AFJ. Fungal gene expression on demand: an inducible, tunable, and metabolism-independent expression system for Aspergillus niger. Appl. Environ. Microbiol. 2011;77:2975–2983. doi: 10.1128/AEM.02740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H-L, Osmani AH, Ukil L, Son S, Markossian S, Shen K-F, Govindaraghavan M, Varadaraj A, Hashmi SB, De Souza CP, et al. Single-step affinity purification for fungal proteomics. Eukaryotic Cell. 2010;9:831–833. doi: 10.1128/EC.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straube A, Enard W, Berner A, Wedlich-Söldner R, Kahmann R, Steinberg G. A split motor domain in a cytoplasmic dynein. The EMBO Journal. 2001;20:5091–5100. doi: 10.1093/emboj/20.18.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]