Abstract
Objective
To estimate the prevalence and incidence of chronic fatigue syndrome in Olmsted County, Minnesota, using the 1994 case definition and describe exclusionary and comorbid conditions observed in patients who presented for evaluation of long-standing fatigue.
Patients and Methods
We conducted a retrospective medical record review of potential cases of chronic fatigue syndrome identified from January 1, 1998, through December 31, 2002, using the Rochester Epidemiology Project, a population-based database. Patients were classified as having chronic fatigue syndrome if the medical record review documented fatigue of 6 months' duration, at least 4 of 8 chronic fatigue syndrome–defining symptoms, and symptoms that interfered with daily work or activities. Patients not meeting all of the criteria were classified as having insufficient/idiopathic fatigue.
Results
We identified 686 potential patients with chronic fatigue, 2 of whom declined consent for medical record review. Of the remaining 684 patients, 151 (22%) met criteria for chronic fatigue syndrome or insufficient/idiopathic fatigue. The overall prevalence and incidence of chronic fatigue syndrome and insufficient/idiopathic fatigue were 71.34 per 100,000 persons and 13.16 per 100,000 person-years vs 73.70 per 100,000 persons and 13.58 per 100,000 person-years, respectively. The potential cases included 482 patients (70%) who had an exclusionary condition, and almost half the patients who met either criterion had at least one nonexclusionary comorbid condition.
Conclusion
The incidence and prevalence of chronic fatigue syndrome and insufficient/idiopathic fatigue are relatively low in Olmsted County. Careful clinical evaluation to identify whether fatigue could be attributed to exclusionary or comorbid conditions rather than chronic fatigue syndrome itself will ensure appropriate assessment for patients without chronic fatigue syndrome.
Abbreviations and Acronyms: CFS, chronic fatigue syndrome; H-ICDA, H-ICDA: Hospital Adaptation of ICDA; ICD-9, International Classification of Diseases, Ninth Revision; ISF, insufficient/idiopathic fatigue; REP, Rochester Epidemiology Project
The etiology and pathophysiology of chronic fatigue syndrome (CFS) remain poorly understood, and there are no tests, clinical signs, or physiologic markers that are diagnostic for this condition. Thus, the illness is diagnosed clinically, based on self-reported symptoms and clinical evaluation for medical and psychiatric conditions that present similarly.1 Research has identified some aspects of the CFS clinical course that may help clinicians manage the syndrome. For example, greater fatigue severity is associated with worse prognosis.2,3 The 1994 case definition1 includes a lengthy list of medical and psychiatric exclusions and comorbid conditions that may be confusing to health care professionals who lack familiarity with the case definition. This raises the potential for overdiagnosis or underdiagnosis. For example, in one study, 40% of American physicians reported making a diagnosis of CFS,4 and in another study, less than 20% of persons with the illness had been diagnosed as having it.5
A consistent standard to identify CFS cases is also important for estimating CFS incidence and prevalence. In a review of CFS, Afari and Buchwald6 noted that prevalence rates in the United States range from 0.007% to 2.8% in general populations and similarly ranged from 0.006% to 3.0% in primary care or general practices. Estimates of CFS occurrence and risk factors from population surveys reflect study design, survey population, and rigor of case definition. Studies from 1993 through 1999 reported prevalences of 0.004% to 0.56%,7-11 whereas more recent studies have reported prevalence rates of 0.24% to 2.6%.12-17 Estimates of CFS occurrence from clinical records also reflect study design, the nature of the clinical population, and rigor of case definition. The incidence of CFS has not been as well studied as prevalence. To date, the sole study of CFS incidence in the United States reported a rate of 180 per 100,000 persons.18 The incidence in that study was estimated by follow-up telephone interviews and clinical examinations of a preestablished cohort of persons who were not fatigued or were fatigued for less than 6 months at baseline.
An understanding of CFS incidence and prevalence is important for health care professionals and for those responsible for allocating clinical resources, who currently lack systematic and pragmatic guidelines concerning CFS diagnosis and management. Establishing an evidence base for prevalence, incidence, clinical course, and associated risk factors will provide the foundation for research involving CFS management, risk factors, and pathophysiology.
The Rochester Epidemiology Project (REP) represents a rigorous, longitudinal, population-based, clinical database suitable for identifying information on the prevalence and incidence of CFS and related conditions.19,20 The REP has collected comprehensive sociodemographic information and clinical records of the Olmsted County, Minnesota, population since 1907. The REP is a paper and electronic database that includes diagnostic codes for conditions identified by physicians at office visits or hospital stays and also includes lifetime records for a large proportion of patients. As with most clinical registries, the REP allows for identification of CFS by International Classification of Diseases, Ninth Revision (ICD-9) codes assigned at clinical visits. The REP is unique because information in physicians' clinical notes permits retrospective identification of persons with CFS based on whether symptoms of their illness meet criteria for CFS, independent of assigned ICD-9 code. Analysis of the REP further permits identification of incident and prevalent CFS cases, establishes the date of onset of CFS, and ascertains comorbid conditions.
Our study had 3 objectives: (1) to identify patients with CFS defined by the 1994 case definition1 from their diagnostic codes in medical records, (2) to estimate the prevalence and incidence of CFS in the Olmsted County population from 1998 to 2002, and (3) to describe comorbid conditions, CFS clinical characteristics, and CFS exclusions.
Patients and Methods
This study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center. We reviewed the records of Olmsted County residents seen between 1998 and 2002 who participated in the REP and granted permission for review of their medical records.
REP Database
Most health care for residents of Olmsted County is delivered by Mayo Clinic, Olmsted Medical Center, and the Rochester Family Medicine Clinic. These institutions use a unit (or dossier) medical record system, whereby all data from an individual are linked and assembled in one place. Dossiers contain details from every outpatient visit to the offices, clinics, and emergency departments in the county; every physician visit to nursing homes or private homes; and every laboratory visit, every inpatient hospitalization, and all correspondence concerning each patient. These medical records are made available for approved research studies under the umbrella of the REP.19 The result is a linkage of medical records from essentially all sources of medical care available to and used by the Olmsted County population. The REP captures slightly more of the Olmsted County population than is predicted to reside in the county by the US Census.20 This complete capture of the population allows for assessment of the incidence and prevalence of diseases that come to medical attention in Olmsted County, Minnesota.
Data Collection and Case Ascertainment
Medical records in the REP are coded using the ICD-9 and the H-ICDA: Hospital Adaptation of ICDA (Table 1). We retrieved 2 lists of all ICD-9 and H-ICDA codes recorded for Olmsted County residents between January 1, 1998, and December 31, 2002. The first list included patients aged 18 years and older with H-ICDA codes 03106126 (syndrome, fatigue [chronic]) and 03106127 (fatigue, chronic) and ICD-9 code 780.71 (chronic fatigue syndrome). The second list used H-ICDA codes 07893110, 07893210, 07883110, and 07707150 and ICD-9 codes 307.4, 780.5, and 719.4, which identified CFS-defining symptoms of fatigue, sleep problems, muscle or joint pain, and memory problems.1 Only patients who had all 4 of these latter symptoms were included in the second list. Because this coding system is nonspecific, we did not expect all patients included in these lists to meet the CFS 1994 case definition criteria,1 nor did we expect that all patients with CFS seen during this time period would have codes for the disorder.
TABLE 1.
Diagnostic Codes for Case Identification
| H-ICDA codes | |
| 03106126 | Syndrome, fatigue (chronic) |
| 03106127 | Fatigue, chronic |
| 03164110 | Hypersomnia, psychophysiological |
| 07735110 | Insomnia, not otherwise specified |
| 07893110 | Myalgia, back |
| 07893210 | Myalgia, not otherwise specified (code also pain by site specified) |
| 07883110 | Pain, hip |
| 07707150 | Loss, memory |
| ICD-9 codes | |
| 780.71 | Chronic fatigue syndrome |
| 307.4 | Specific disorders of sleep of nonorganic origin |
| 780.5 | Sleep disturbances |
| 719.4 | Pain in joint |
H-ICDA = H-ICDA: Hospital Adaptation of ICDA; ICD-9 = International Classification of Diseases, Ninth Revision.
We conducted a detailed medical record review of all patients identified from both lists and documented whether criteria constituting the 1994 case definition for both CFS and insufficient/idiopathic fatigue (ISF) were present.1,18 Patients were classified as having CFS if the medical record documented fatigue of 6 months' duration or more; at least 4 of 8 CFS-defining symptoms (postexertional malaise, impaired memory/concentration, unrefreshing sleep, muscle pain, multijoint pain without redness or swelling, tender cervical or axillary lymph nodes, sore throat, and headache); and fatigue that interfered with daily work or activities.1 Patients not meeting all of the CFS criteria (ie, fatigue of less than 6 months' duration or fewer than 4 of 8 defining symptoms) were classified as having ISF.1 All CFS and ISF cases were reviewed and confirmed by 2 study investigators (A.V. and J.F.J.).
Several conditions are considered exclusionary for CFS.1,21 Patients identified as having CFS through the code lists and medical record review were excluded if they (1) were older than 65 years at the time of fatigue onset, (2) had cancer (except basal or squamous cell carcinoma) within 5 years before fatigue diagnosis, (3) were currently receiving chemotherapy, (4) had had an organ transplant, (5) were pregnant within 12 months before fatigue diagnosis, (6) had a body mass index (calculated as the weight in kilograms divided by the height in meters squared) greater than 40 kg/m2, or (7) had exclusionary medical conditions. The exclusionary medical conditions included chronic obstructive pulmonary disease, congestive heart failure, cirrhosis, hepatitis B or C infection, insulin-dependent diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, sickle cell anemia, stroke without full recovery, multiple sclerosis, Parkinson disease, dementia, epilepsy or seizure disorder, schizophrenia, bipolar disorder, depression with psychotic or melancholic features, anorexia nervosa or bulimia within 5 years before fatigue diagnosis, drug/alcohol/narcotic abuse within 2 years before fatigue diagnosis, narcolepsy, obstructive sleep apnea, sleep-disordered breathing, restless legs syndrome, and periodic limb movement disorder.
Data Management and Statistical Analyses
Study data were collected and managed using REDCap (Research Electronic Data Capture).22 Descriptive statistics are reported as mean (SD) and range for continuous variables or number and percentage for categorical variables. We used a 2-sample t test, χ2 test, or Fisher exact test, as appropriate, for comparison of demographic, comorbid, and clinical characteristics between CFS and ISF cases. P<.05 was considered statistically significant.
Prevalent cases were residents of Olmsted County, Minnesota, on December 31, 2002, with CFS or ISF based on medical record review, and incident cases were residents who first met criteria for CFS or ISF during the period 1998-2002. We calculated prevalence rates as number of subjects per 100,000 persons, assuming the entire adult population of Olmsted County was at risk. In contrast, incidence was restricted to adults 18 to 70 years of age, consistent with the case definition, and expressed per 100,000 person-years. Both incidence and prevalence rates were directly adjusted to the total US population in 2000.23 The denominators of age- and sex-specific person-years were derived from decennial US Census figures by linear interpolation. We estimated 95% CIs for incidence and prevalence assuming that the incidence cases followed a Poisson distribution and that the prevalence rates followed a binomial distribution. All analyses were carried out using SAS statistical software version 9.2 (SAS Institute).
Results
Using the medical indexing of the REP, we identified 686 possible cases of CFS based on ICD-9/H-ICDA codes (Figure). Two patients declined consent for medical record review; review of the medical records of the remaining 684 patients identified 482 (70%) with an exclusionary condition (Table 2). Of the remaining 202 patients, we classified 76 as meeting criteria for CFS, 75 as meeting criteria for ISF, and 51 as having neither (Figure). Of the 76 cases that met CFS criteria, 50 (66%) were identified by means of ICD-9/H-ICDA codes, as compared with 46 (61%) of 75 ISF cases (data not shown).
FIGURE.
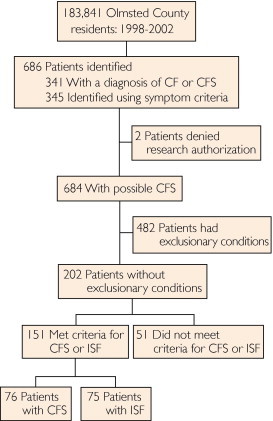
Identification and classification of patients with chronic fatigue (CF) syndrome (CFS) and insufficient/idiopathic fatigue (ISF).
TABLE 2.
Reasons for CFS Exclusion (N=482)a
| Exclusionary condition | No. (%) of patientsb |
|---|---|
| Obstructive sleep apnea | 158 (33) |
| Advanced age (>65 y at fatigue onset) | 97 (20) |
| Restless legs syndrome/periodic limb movement disorder | 58 (12) |
| Substance abuse (drugs/alcohol/narcotics) within 2 y before CFS diagnosis | 49 (10) |
| Chronic obstructive pulmonary disease | 42 (9) |
| Congestive heart failure | 35 (7) |
| Bipolar I/II disorder | 29 (6) |
| Pregnancy within 12 mo before CFS diagnosis | 29 (6) |
| Cancer within 5 y before CFS diagnosis (except basal or squamous cell carcinoma) | 23 (5) |
| Dementia | 22 (5) |
| Rheumatoid arthritis | 16 (3) |
| Hepatitis B or C | 13 (3) |
| Stroke without full recovery | 13 (3) |
| Epilepsy or seizure disorder | 12 (2) |
| Schizophrenia | 9 (2) |
| Parkinson disease | 8 (2) |
| Insulin-dependent diabetes mellitus | 8 (2) |
| Narcolepsy | 6 (1) |
| Depression with psychotic features | 6 (1) |
| Systemic lupus erythematosus | 5 (1) |
| Melancholic depression | 5 (1) |
| Eating disorder (anorexia nervosa or bulimia) within 5 y before CFS diagnosis | 3 (1) |
| Multiple sclerosis | 3 (1) |
| Cirrhosis | 3 (1) |
| Current chemotherapy | 1 (<1) |
| Organ transplant | 1 (<1) |
| Other (eg, morbid obesity, polymyalgia rheumatica) | 64 (13) |
CFS = chronic fatigue syndrome.
Patients could have more than one exclusionary condition.
Prevalence and Incidence of CFS
Sex-specific and total age-adjusted prevalence and incidence estimates for CFS and ISF are shown in Table 3. The overall prevalence of CFS was 71.34 per 100,000 persons (95% CI, 54.33-88.35), and the overall prevalence of ISF was 73.70 per 100,000 persons (95% CI, 56.54-90.86). The overall incidence of CFS was 13.16 per 100,000 person-years (95% CI, 9.68-16.65), and the overall incidence of ISF was 13.58 per 100,000 person-years (95% CI, 10.02-17.15). Prevalence and incidence estimates differed significantly by sex and were higher in women than in men for both CFS and ISF.
TABLE 3.
| Prevalencec |
Incidenced |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFS |
ISF |
CFS |
ISF |
|||||||||
| Adjustment | No. | Rate per 100,000 persons (SE) | 95% CI | No. | Rate per 100,000 persons (SE) | 95% CI | No. | Rate per 100,000 person-years (SE) | 95% CI | No. | Rate per 100,000 person-years (SE) | 95% CI |
| Age-adjusted, women | 60 | 122.1 (15.8) | 91.23-153.15 | 59 | 120.4 (15.7) | 89.73-151.25 | 46 | 21.74 (3.21) | 15.45-28.03 | 47 | 22.48 (3.28) | 16.04-28.91 |
| Age-adjusted, men | 8 | 17.63 (6.25) | 5.38-29.88 | 12 | 26.58 (7.69) | 11.51-41.66 | 9 | 4.39 (1.47) | 1.52-7.27 | 9 | 4.62 (1.54) | 1.60-7.64 |
| Age-adjusted, total | 68 | 71.66 (8.71) | 54.58-88.74 | 71 | 74.33 (8.83) | 57.01-91.64 | 55 | 13.23 (1.79) | 9.73-16.74 | 56 | 13.70 (1.83) | 10.10-17.29 |
| Age- and sex-adjusted, total | 68 | 71.34 (8.68) | 54.33-88.35 | 71 | 73.70 (8.75) | 56.54-90.86 | 55 | 13.16 (1.78) | 9.68-16.65 | 56 | 13.58 (1.82) | 10.02-17.15 |
CFS = chronic fatigue syndrome; ISF = insufficient/idiopathic fatigue.
All rates are adjusted to the 2000 US population; 10-year age groups of 18-70 y for incidence and 18-110 y for prevalence.
Variability in rates is based on binomial distribution for prevalence.
Variability in rates is based on Poisson distribution for incidence.
Demographic and Clinical Characteristics and Comorbid Conditions
Demographic and clinical characteristics and comorbid conditions of patients with CFS and ISF are shown in Table 4. The 151 patients comprising the CFS and ISF groups were predominantly white (90%), female (83%), and well educated (mean [SD], 14.3 [2.3] years of education), with a mean (SD) age at fatigue onset of 38.2 (10.4) years. The 4 most common symptoms in the CFS group were muscle pain (74 patients [97%]), nonrestorative sleep (69 patients [91%]), multijoint pain (69 patients [91%]), and headaches (58 patients [76%]). Postexertional malaise was only documented in the medical records of 27 patients (36%). Overall, the ISF group had lower rates of the CFS symptom criteria than the CFS group. Fatigue and symptoms affected daily activities and work in both groups (CFS, 71 of 75 patients [95%]; ISF, 40 of 49 patients [82%]).
TABLE 4.
Demographic Characteristics, Comorbid Conditions, and Clinical Findings in Patients With CFS and ISFa,b
| Variable | CFS (N=76)c | ISF (N=75) | P valued |
|---|---|---|---|
| Age (y) at fatigue onset | 38.2 (10.3) [17.0-62.2] | 38.3 (10.6) [17.9-58.1] | .95e |
| Female | 64 (84) | 62 (83) | .80 |
| Race | .28f | ||
| White | 70 (92) | 66 (88) | |
| Black | 1 (1) | 0 | |
| American Indian/Asian/Pacific Islander | 2 (3) | 1 (1) | |
| Other | 0 | 3 (4) | |
| Unknown | 3 (4) | 5 (7) | |
| Hispanic ethnicity | 0 | 1 (1) | .48f |
| Education (y) | 14.1 (2.5) [3.0-17.0] | 14.4 (2.2) [8.0-20.0] | .58e |
| Comorbid conditionsg | |||
| Type 2 diabetes mellitus | 5 (7) | 4 (5) | >.99f |
| Fibromyalgia | 29 (38) | 8 (11) | <.001 |
| Irritable bowel syndrome | 35 (46) | 23 (31) | .05 |
| Temporomandibular joint syndrome | 14 (18) | 5 (7) | .03 |
| Hypertension | 23 (30) | 13 (17) | .06 |
| Coronary artery disease | 5 (7) | 4 (5) | >.99f |
| Anemia | 4 (5) | 6 (8) | .53f |
| Hypothyroidism/hyperthyroidism | 16 (21) | 12 (16) | .42 |
| Sleep conditionsg | |||
| Sleep phase disorder | 3 (4) | 0 | .25f |
| Insomnia | 45 (59) | 35 (47) | .12 |
| Mental health conditionsg | |||
| Depression/major depression | 53 (70) | 32 (43) | <.001 |
| Anxiety disorder | 39 (51) | 31 (41) | .22 |
| Somatoform disorder | 4 (5) | 0 | .12f |
| Dysthymic disorder | 17 (22) | 15 (20) | .72 |
| Chronic fatigue lasting ≥6 mo | 76 (100) | 65 (87) | … |
| Symptomsg | … | ||
| Muscle pain | 74 (97) | 33 (44) | |
| Unrefreshing/nonrestorative sleep | 69 (91) | 40 (53) | |
| Multijoint pain | 69 (91) | 31 (41) | |
| New headaches | 58 (76) | 32 (43) | |
| Sore throat | 43 (57) | 8 (11) | |
| Impaired memory or concentration | 41 (54) | 17 (23) | |
| Postexertional malaise | 27 (36) | 3 (4) | |
| Tender cervical or axillary lymph nodes | 15 (20) | 2 (3) | |
| Fatigue/symptoms affecting daily activities or work | 71/75 (95) | 40/49 (82) | … |
| Duration from first to last symptoms of CFS (y) | 3.9 (4.4) [0.0-31.1] | 2.6 (2.5) [0.0-11.3] | … |
CFS = chronic fatigue syndrome; ISF = insufficient/idiopathic fatigue.
Data are presented as mean (SD) [range] or No. (percentage) of patients.
Diagnosis made using study clinical classification (1994 case definition).
χ2 test unless indicated otherwise.
2-sample t test.
Fisher exact test.
Defined based on whether date field was entered in database.
Comorbid medical and psychiatric conditions were common among both CFS and ISF groups (Table 4). The most common comorbid medical conditions for CFS cases were irritable bowel syndrome (35 patients [46%]), fibromyalgia (29 patients [38%]), and hypertension (23 patients [30%]). Compared with ISF patients, patients with CFS were significantly more likely to have fibromyalgia (P<.001) and temporomandibular joint syndrome (P=.03); irritable bowel syndrome (P=.05) and hypertension (P=.06) were slightly but nonsignificantly more common in the CFS group. Insomnia was the most common sleep problem (45 patients [59%]), and depression (53 patients [70%]) and anxiety (39 patients [51%]) were the most common psychiatric comorbid conditions in CFS cases. Depression was significantly more common in the CFS group than in the ISF group (70% vs 43%; P<.001).
Discussion
We used ICD-9 and H-ICDA codes from the REP in conjunction with medical record review to classify patients with CFS according to international research criteria.1 The codes accurately identified 66% of patients with CFS, which reflects a high level of knowledge and awareness of CFS among health care professionals in Olmsted County. Of note, 70% of patients who met symptom criteria had a current exclusionary condition. The most common exclusionary conditions were obstructive sleep apnea, restless legs syndrome, and substance abuse. These are treatable conditions, which underscores the importance of conducting a complete medical evaluation in patients who present with these symptoms. Our findings suggest that studies classifying CFS only from diagnostic codes likely overestimate prevalence and incidence.
We estimated that 0.071% of residents of Olmsted County had CFS; the incidence was 0.013%. The prevalence and incidence of ISF in this population were similar (0.073% and 0.013%, respectively). The combined incidence rates of about 0.03% for CFS and ISF are lower than previously reported.19 When the 1994 case definition1 was used for prevalence estimations, prevalence estimates ranged from 0.29% to 2.6%, depending on the sex and ethnic distribution of the population studied.11,12,14-18 The wide differences in prevalence estimates are most likely due to study design and methodology, illness definition, and study populations. For example, one study with a higher estimated prevalence used random-digit dialing in the general population,12 whereas another study that initially reported high rates confirmed a much lower estimate when patients with psychiatric disorders were excluded.11 The current study used a retrospective medical record review of all persons in Olmsted County during 1998-2002, and this design is not analogous to prospective studies.
In addition, almost half the patients with CFS or ISF in our current study had at least one nonexclusionary comorbid condition. Particularly notable is the high percentage of somatic disorders, insomnia, and comorbid psychiatric conditions, which suggests physiologic dysregulations that may overlap in these disorders. We also found that psychiatric comorbid conditions (particularly depression and anxiety) were common in patients with both CFS and ISF. The presence of comorbid conditions in CFS and ISF is an important factor for clinicians to consider as they develop and revise management plans. For example, a patient meeting CFS criteria may also have a sleep disorder as a comorbid condition. The sleep disorder requires proper treatment and management wholly apart from the management of CFS and should be routinely evaluated.
Our demographic results regarding educational achievement are different from previous reports in which CFS was observed in patients with lower levels of education.10,18 Our results may reflect the higher educational attainment of Minnesota's population in general.24 Future studies focused on samples of patients with varying educational and socioeconomic status may be useful in further clarifying this issue. Review of our patients' medical records provided important insight into the complex task of accurately diagnosing CFS. Clinicians must consider a myriad of exclusionary and treatable causes of fatigue that must be evaluated before making a CFS diagnosis. Although ISF is not a clinical diagnosis, from a clinical perspective it is a major classification according to the 1994 case definition. Patients with ISF must be observed longitudinally to monitor for development of a treatable cause of fatigue, other medical disorders, or CFS.25 Educating new or inexperienced health care professionals about the diagnosis of CFS would help to identify treatable exclusionary conditions in a large proportion of patients with suspected CFS and to accurately identify and treat patients with CFS.
The major strength of this study was complete clinical record review from essentially the entire population of Olmsted County who used medical services between 1998 and 2002. Patient dossiers provide the opportunity to examine the natural history of illness beginning before onset of illness and to evaluate comorbid conditions and other factors associated with health care use. Clinical record review offers advantages over population surveys, which are considerably more costly and can be subject to both selection and participation bias. However, since population surveys can more accurately estimate the population burden of illnesses like CFS because a large proportion of persons with such illness do not seek medical care,26 this may be a limitation of our study. Additionally, the low percentage of patients with postexertional malaise in our study may reflect the lack of inquiry about this important symptom in routine clinical practice.
Conclusion
We found that CFS and ISF could be identified by medical record review and that the incidence and prevalence of CFS and ISF are relatively low in the Olmsted County population but are within prevalence ranges cited in other studies. One explanation for the low incidence is identification of patients who fulfill diagnostic criteria for a syndrome but who have an underlying exclusionary disease process that is readily identifiable or that is evolving. Such an outcome may be attributed to the current study design in which patients have undergone thorough medical and psychiatric evaluations. The current study identifies questions that should be addressed, such as whether comorbid illnesses contribute to CFS or whether the symptoms and consequences of CFS and ISF simply allow identification of patients whose underlying disease is not yet manifest. Additionally, the sensitivity and specificity of the methodologies we used could have resulted in our prevalence estimates. Careful initial identification of CFS and ISF with use of uniform criteria is essential, and case identification could serve as an important stepping stone to understanding the origins of these syndromic illnesses as well as a possible beacon leading to underlying diagnoses.
Our study highlights the need to carefully consider whether fatigue and other symptoms should be attributed to exclusionary or comorbid conditions (particularly obstructive sleep apnea, advanced age, or psychiatric conditions) rather than CFS itself. In contrast to research endeavors in which disease classification must be stringent, physicians in clinical practice often recognize that patients with these conditions could have a component of CFS. Careful attribution to the correct underlying conditions will ensure appropriate treatment of patients with persistent fatigue who do not actually have CFS or ISF, and for the latter, disease-specific care.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Department of Health and Human Services, the US Food and Drug Administration, the Centers for Disease Control and Prevention, the Center for Translational Science Activities at Mayo Clinic, the National Center for Research Resources, or the National Institutes of Health.
Dr Vincent had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Grant Support: This study was supported in part by grants from the Centers for Disease Control and Prevention (200-2009-M-32507, FP00055021·02), the Rochester Epidemiology Project (R01-AG034676; Principle Investigators: Walter A. Rocca, MD, MPH, and Barbara P. Yawn, MD, MSc), and the Center for Translational Science Activities at Mayo Clinic (this center is funded in part by a grant [RR024150] from the National Center for Research Resources, a component of the National Institutes of Health).
Author Contributions: Ann Vincent, MD—Design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dana J. Brimmer, PhD, MPH—Design and conduct of the study; analysis and interpretation of the data; and preparation, review, or approval of the manuscript. Mary O. Whipple, BA—Design and conduct of the study; collection, management, and preparation, review, or approval of the manuscript. James F. Jones, MD—Design and conduct of the study; interpretation of the data; and preparation, review, or approval of the manuscript. Roumiana Boneva, MD, PhD—Design and conduct of the study; interpretation of the data; and preparation, review, or approval of the manuscript. Brian D. Lahr, MS—Statistical analysis; interpretation of the data; and preparation, review, or approval of the manuscript. Elizabeth Maloney, DrPh, MS—Design and conduct of the study; interpretation of the data; and preparation, review, or approval of the manuscript. Jennifer L. St. Sauver, PhD—Design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. William C. Reeves, MD, MS—Design and conduct of the study; interpretation of the data; and preparation, review, or approval of the manuscript.
Dr Maloney is now with the Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology, Division of Epidemiology II, US Food and Drug Administration, Silver Spring, MD. Dr Reeves is now with the Division of Behavioral Surveillance, Centers for Disease Control and Prevention, Atlanta, GA.
Data Previously Presented: These data were presented in part at the annual meeting of the American Psychiatric Association, Honolulu, HI, May 14-19, 2011.
References
- 1.Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A., International Chronic Fatigue Syndrome Study Group The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Taylor R.R., Jason L.A., Curie C.J. Prognosis of chronic fatigue in a community-based sample. Psychosom Med. 2002;64(2):319–327. doi: 10.1097/00006842-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Jason L.A., Porter N., Hunnell J., Brown A., Rademaker A., Richman J.A. A natural history study of chronic fatigue syndrome. Rehabil Psychol. 2011;56(1):32–42. doi: 10.1037/a0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brimmer D.J., Fridinger F., Lin J.M., Reeves W.C.U.S. healthcare providers' knowledge, attitudes, beliefs, and perceptions concerning chronic fatigue syndrome. BMC Fam Pract. 2010;11:28. doi: 10.1186/1471-2296-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon L., Reeves W.C. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004;164(20):2241–2245. doi: 10.1001/archinte.164.20.2241. [DOI] [PubMed] [Google Scholar]
- 6.Afari N., Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160(2):221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 7.Reyes M., Gary H.E., Jr, Dobbins J.G. Surveillance for chronic fatigue syndrome—four U.S. cities, September 1989 through August 1993. MMWR CDC Surveill Summ. 1997;46(2):1–13. [PubMed] [Google Scholar]
- 8.Lawrie S.M., Pelosi A.J. Chronic fatigue syndrome in the community: prevalence and associations. Br J Psychiatry. 1995;166(6):793–797. doi: 10.1192/bjp.166.6.793. [DOI] [PubMed] [Google Scholar]
- 9.Steele L., Dobbins J.G., Fukuda K. The epidemiology of chronic fatigue in San Francisco. Am J Med. 1998;105(3A):83S–90S. doi: 10.1016/s0002-9343(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 10.Jason L.A., Richman J.A., Rademaker A.W. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159(18):2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 11.Wessely S., Chalder T., Hirsch S., Wallace P., Wright D. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. 1997;87(9):1449–1455. doi: 10.2105/ajph.87.9.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves W.C., Jones J.F., Maloney E. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Popul Health Metr. 2007;5:5. doi: 10.1186/1478-7954-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jason L.A., Porter N., Brown M. CFS: a review of epidemiology and natural history studies. Bull IACFS ME. 2009;17(3):88–106. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamaguchi M., Kawahito Y., Takeda N., Kato T., Kojima T. Characteristics of chronic fatigue syndrome in a Japanese community population: chronic fatigue syndrome in Japan. Clin Rheumatol. 2011;30(7):895–906. doi: 10.1007/s10067-011-1702-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim C.H., Shin H.C., Won C.W. Prevalence of chronic fatigue and chronic fatigue syndrome in Korea: community-based primary care study. J Korean Med Sci. 2005;20(4):529–534. doi: 10.3346/jkms.2005.20.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindal E., Stefánsson J.G., Bergmann S. The prevalence of chronic fatigue syndrome in Iceland—a national comparison by gender drawing on four different criteria. Nord J Psychiatry. 2002;56(4):273–277. doi: 10.1080/08039480260242769. [published correction appears in Nord J Psychiatry. 2006;60(2):183] [DOI] [PubMed] [Google Scholar]
- 17.Njoku M.G., Jason L.A., Torres-Harding S.R. The prevalence of chronic fatigue syndrome in Nigeria. J Health Psychol. 2007;12(3):461–474. doi: 10.1177/1359105307076233. [DOI] [PubMed] [Google Scholar]
- 18.Reyes M., Nisenbaum R., Hoaglin D.C. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163(13):1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- 19.Melton L.J., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.St Sauver J.L., Grossardt B.R., Yawn B.P., Melton L.J., III, Rocca W.A. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves W.C., Lloyd A., Vernon S.D., International Chronic Fatigue Syndrome Study Group Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. 2003;3(1):25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergstralh E.J., Offord K.P., Chu C.P., Beard C.M., O'Fallon W.M., Melton L.J., III . April 1992. Technical Report Series No. 49: Calculating incidence, prevalence and mortality rates for Olmsted County, Minnesota: an update. Department of Health Science Research, Mayo Clinic, Rochester, MN. [Google Scholar]
- 24.Minnesota Office of Higher Education Educational attainment data. http://www.ohe.state.mn.us/mPg.cfm?pageID=1873 Minnesota Office of Higher Education website. Accessed March 1, 2012.
- 25.Nisenbaum R., Jones J.F., Unger E.R., Reyes M., Reeves W.C. A population-based study of the clinical course of chronic fatigue syndrome. Health Qual Life Outcomes. 2003;1:49. doi: 10.1186/1477-7525-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J.M., Brimmer D.J., Boneva R.S., Jones J.F., Reeves W.C. Barriers to healthcare utilization in fatiguing illness: a population-based study in Georgia. BMC Health Serv Res. 2009;9:13. doi: 10.1186/1472-6963-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


