Abstract
Chemokine receptor cross-desensitization provides an important mechanism to regulate immune cell recruitment at sites of inflammation. We previously reported that the mycobacterial cell wall glycophospholipid lipoarabinomannan (ManLAM) could induce human peripheral blood T cell chemotaxis. Therefore, we examined the ability of ManLAM to desensitize T cells to other chemoattractants as a potential mechanism for impaired T cell homing and delayed lung recruitment during mycobacterial infection. We found that ManLAM pretreatment inhibited in vitro migration of naïve human or mouse T cells to the lymph node egress signal sphingosine-1-phosphate (S1P). Intratracheal administration of ManLAM in mice resulted in significant increases in T cells, primarily CCR5+ (Th1) cells, in lung draining lymph nodes. To investigate the selective CCR5 effect, mouse T cells were differentiated into Th1 or Th2 populations in vitro and their ability to migrate to S1P with or without ManLAM pretreatment was analyzed. ManLAM pretreatment of Th1 populations inhibited S1P-induced migration, but had no effect on Th2 cell S1P-directed migration suggesting a differential effect by S1P on the two subsets. The PI3K/AKT inhibitor Ly294002 inhibited S1P-directed migration by Th1 cells, whereas the ERK inhibitor U0126 inhibited Th2 cell S1P-directed migration. These observations demonstrate that S1P-induced migratory responses in Th1 and Th2 lymphocytes occurs via different signaling pathways, and suggests further that the production of ManLAM during MTB infection may function to sequester Th1 cells in lung-draining lymph nodes thereby delaying their recruitment to the lung.
INTRODUCTION
Initiation of adaptive immune responses most often occurs in tissue-draining lymph nodes where lymphocytes can become activated, mature, and acquire effector functions before returning to the site of injury or infection. Normal T lymphocyte migration to lymph nodes requires binding to high endothelial venules and a chemokine gradient of the CCR7 ligands CCL19 and CCL21 (1). Following interactions with antigen presenting cells in the node, T cells become activated and upregulate the sphingosine-1-phosphate receptor 1 (S1P1) 3 (2). A sphingosine-1-phosphate (S1P) concentration gradient then facilitates T cell egress from the nodes and their entrance back into the circulation, thus allowing them to migrate to the affected tissues where they exert their effector functions (3, 4). Enhanced migration to CCR7 ligands or abrogation of migration to S1P would promote a disproportionate accumulation of T cells in lymph tissues, resulting in a reduction of primed effector cells entering the circulation and tissues. This mechanism is thought to account for the immunosuppressive effects of drugs that induce lymphadenopathy by down regulating S1P1 such as FTY720, which has been shown to be therapeutically effective in transplant and multiple sclerosis clinical trials (5–7).
Induction of T lymphocyte responses against pulmonary Mycobacterium tuberculosis (MTB) infection are initiated in lung draining lymph nodes (8). Following activation, T cells exit the lymph node and migrate to the infected lung (8). However, it has been noted that T cell responses against MTB are delayed as compared to other pulmonary pathogens. Several hypotheses have been provided to explain this delayed T cell response, including inhibition of antigen presenting cell maturation by MTB, and altered kinetics of leukocyte migration to and from the lung tissue (9). Our lab has previously shown that a component of the MTB cell wall, mannose-capped lipoarabinomannan (ManLAM) is able to direct T cell migration. Due to the growing body of evidence that chemokine receptor cross-desensitization accounts for another layer of regulation of leukocyte recruitment, we investigated the ability of ManLAM to desensitize T lymphocytes to chemoattractants involved in migration to and from lymph tissue.
In vivo infection studies in mice and humans have demonstrated that the development of anti-ManLAM antibodies appears to be beneficial, as the levels correlate with decreased dissemination, lower bacterial loads, prolonged survival and better disease outcomes (10, 11). This is likely due to the prevention of immunomodulatory effects that ManLAM can exert on host cells. During MTB infection, ManLAM is secreted from infected host macrophages and dendritic cells in the form of lipid bodies (12). Once secreted, ManLAM can interact with host cell surface receptors such as C-type lectins or the mannose receptor (13–15). Alternatively, ManLAM can incorporate directly into lipid rafts on peripheral blood mononuclear cells (16). ManLAM’s interactions with host cell receptors and membranes results in altered cellular signaling and responses. This is thought to be achieved through a steric inhibition mechanism, or through direct binding of host proteins to the acyl tails of ManLAM itself, which resemble mammalian PIP3 (17–19). A recent study demonstrated ManLAM’s ability to inhibit CD4+ T cell activation via inhibition of p56lck phosphorylation and signaling from the T cell receptor (20). Furthermore, ManLAM stimulation prevents phagolysosomal fusion in MTB infected macrophages via a phosphatidylinositol 3 kinase dependent pathway (PI3K) (21, 22). Mechanistically, S1P-directed migration in endothelial cells and T lymphocytes has previously been shown to rely upon PI3K/AKT signaling pathways (23–25).
In this study we investigated the ability of ManLAM to desensitize human and mouse T lymphocytes to CCL21 and S1P directed chemotaxis in vitro. Our data demonstrates that ManLAM exposure desensitizes both human and mouse T cells to S1P stimulation. We further identified a preferential effect on the CCR5+ (Th1) subset, which are considered to be a host-protective population during MTB infection. Intratracheal instillation of ManLAM confirmed the selective effect of ManLAM as CCR5+ cells were significantly increased in lung-draining lymph nodes as compared with CCR4+ (Th2) cells. We therefore tested the response of in vitro differentiated mouse Th1 and Th2 cells to S1P directed migration with or without ManLAM pretreatment, and found that only Th1 cell migration was inhibited. We propose that selective inhibition of Th1 cell migration by ManLAM is related to differential signaling pathway induction by S1P in the Th1 versus Th2 cell subset, and further, this data suggests another mechanism by which ManLAM produced during MTB infection can alter the immune response.
MATERIALS AND METHODS
Antigens & Antibodies
Mannose-capped lipoarabinomannan (ManLAM), anti-ManLAM antibody (CS-35, 1:250 titer), whole irradiated H37Rv, and hexamannosylated phosphatidyl inositol (PIM6) were obtained through the Colorado State University and NIH Tuberculosis Vaccine Testing and Research Materials Agreement Contract No. HHSN266200400091C.
Mice
Eight- to twelve-week-old female C57BL/6J for ManLAM experiments (Jax 000664) were housed at the Laboratory Animal Science Center at Boston University School of Medicine, or the Animal Medicine facility at University of Massachusetts Medical School. Mice were administered food and water ad libitum. All experiments were performed in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and were approved by either the Boston University Institutional Animal Care and Use Committee, or the University of Massachusetts Medical Center Institutional Animal Care and Use Committee and Institutional Biosafety Committee, respectively.
Isolation of human peripheral blood T cells
All human cell studies have been approved by the Boston University Institutional Review Board and the National Institutes of Health, and were conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. Primary human T cells from healthy donors were isolated as previously described (26). Briefly, peripheral blood was obtained from healthy donors and the T cells isolated from the buffy coat following Hypaque Ficoll (Amersham Biosciences) density gradient centrifugation and nylon wool adherence (Polysciences). The T cells, 95% CD3+, were cultured in complete M199 (Gibco) until use.
Isolation of murine T cells
Mouse T cells were isolated from mixed spleen and lymph node preparations. Cells were dissociated and red blood cell lysis was performed using Red Blood Cell Lysing Buffer (Sigma-Aldrich) prior to selection with either nylon wool or an Invitrogen Dynal CD4 negative bead isolation kit per the manufacturer’s protocol. Mouse T cells were incubated in RPMI supplemented with 1% BSA, HEPES, and penicillin/streptomycin prior to use in assays (Gibco).
In vitro M. tuberculosis infections
Bone marrow-derived macrophages were generated through culturing bone marrows isolated from C57BL/6J mice in complete RPMI containing 20% L929 culture supernatant for 1 week. 4 x 105 cells plated in plated in 24-well plates were either left in culture (control) or infected with M. tuberculosis Erdman (Trudeau Institute Mycobacterial Culture Collection) at an MOI of 5. Cell culture supernatants were collected 48h post-infection and were sterile filtered and frozen prior to use. Anti-ManLAM antibody (CS-35) was used to preclear either control or MTB infected supernatants at 4°C overnight prior to use in chemotaxis assays. After preclearing, the supernatants were used to pretreat murine T cells prior to use in Transwell assays.
Migration Assays
Boyden chamber assays were conducted as previously described (27). Human T cells were incubated with components from the H37Rv strain of Mycobacterium tuberculosis prior to migration induced by S1P (Avanti Polar Lipids) and CCL21 (R&D Systems). Migration was conducted for 1 hour using an 8μM pore nitrocellulose filter (Neuroprobe) before the filters were fixed and H&E stained. Migration through the filter was compared with baseline migration established with media control. Quantification was accomplished by counting 5 high power fields, and on average 10–15 cells per field were counted for media controls. Migration was expressed as fold over media control.
Transwell assays were conducted using mouse T cells from spleens and lymph nodes. Cells were incubated in RPMI with 1% BSA, HEPES, and penicillin/streptomycin, and then suspended at 1×106 cells/100μl. The cells were incubated in Transwell chambers with 5μm polycarbonate filters for 3.5 hr prior to counting cells in the bottom chamber. Directed migration was established as an increased number of cells as compared to media control wells, which usually contained 10,000–20,000 cells. Flow cytometric analysis of CCR4 and CCR5 expression was performed on pooled cells that migrated to the bottom chambers. Migration was expressed as fold over media control.
Flow cytometry analysis
Cells were Fc-blocked with anti-CD16/32 antibodies (eBioscience, San Diego, CA) for 10min prior to staining with selected antibodies for 30 min on ice. The following anti-mouse antibodies and isotype controls were purchased from eBioscience: CD3-FITC (clone 145-2C11), CD3-PerCP-Cy5.5 (clone 145-2C11), CD4-FITC (clone GK1.5), CD8-PE (clone 53-6.7), CCR5-PE (clone HM-CCR5 (7A4)), CCR7-PE-Cy7 (clone 4B12). Anti-mouse CCR4-APC (clone 2G12) and APC Armenian hamster Ig were purchased from BioLegend. Propidium iodide was purchased from Sigma-Aldrich. Samples were run on a Becton Dickinson LSR II. Data was collected with FACSDiva software version 6.0 (BD Biosciences) and analyzed using FlowJo software v7.2.2 (Treestar, Ashland, OR).
In vivo imaging experiments
Isolated T lymphocytes were labeled with QDot 565 nanocrystals per the manufacturer’s protocol (Invitrogen). Labeling was confirmed by flow cytometry, and 5×106 labeled T cells were injected via the tail vein into recipient 8–12wk old female C57BL/6J mice. Mice were allowed to rest for 1h before PBS or 25μg ManLAM was administered intratracheally. Mice were imaged 24h post-instillation with the Caliper Life Sciences IVIS Spectrum and Living Image software version 3.2 (Hopkinton, Massachusetts) with the following settings: excitation = 430nm, emission = 560nm, F stop = 1, exposure = 30 sec; image depicts counts following background subtraction.
In vivo intratracheal instillation of tuberculosis antigens
Mice were intratracheally instilled with the tuberculosis antigens in a volume of 100μl. Twenty four hours following instillation, the inguinal, cervical, mediastinal, and axillary lymph nodes were isolated and cell counts performed. T cell numbers were determined via flow cytometric analysis of CD3 expression. Cells isolated from lymph nodes were also assessed for CCR4, CCR5 and CCR7 expression.
Th1/Th2 skewing
Mouse T cells were skewed using 3μg/mL plate bound anti-CD3 (eBioscience, clone 17A2) and 3μg/mL soluble anti-CD28 (eBioscience, clone 37.51), and cultured for 3 days in RPMI supplemented with 10% FCS (Atlanta Biologicals), 1X NEAA, HEPES, sodium pyruvate, 2-mercaptoethanol, and penicillin/streptomycin (Gibco). Th1 cells were cultured with 10ng/mL IL-12, 10μg/mL αIL-4 (R&D Systems clone 30340), and Th2 cells were cultured with 10ng/mL IL-4, 10μg/mL αIFN-γ (R&D Systems clone H22). Cells were split on day 3, rested in plain medium 24h prior to harvest, and harvested on day 7–8. Skewing was confirmed by IL-4, IL-5 and IFN-γ ELISA (R&D Systems).
Western blotting
Human or mouse T cells were incubated with ManLAM or vehicle control for 2h. Cells were then washed and stimulated with S1P for 5 minutes prior to lysis with buffer (Cell Signaling Technologies) containing protease inhibitors (Roche) and phosphatase inhibitors (Sigma). Samples were run on a 4–12% Bis-Tris gel (Invitrogen), and transferred to PVDF membranes (Millipore). Membranes were blocked with 4% milk in Tris-buffered saline with 0.1% Tween 20, and probed with the following antibodies from Cell Signaling Technologies per the manufacturer’s protocol: phospho-AKT Ser473, total AKT, phospho-p44/42 MAPK, total p44/42 MAPK, and goat anti-rabbit HRP conjugated secondary antibody. Detection was performed with an ECL reagent kit (GE Healthcare) and films were scanned for densitometry analysis with Image J Software (NIH).
Statistical analyses
All data are presented as the mean ± SEM. Statistical analyses were performed as either Student’s t-test or 2 way ANOVA with Bonferroni post tests (Graph-Pad Prism software, version 5.0 for Windows).
RESULTS
Human T cells exhibit decreased migratory responses to sphingosine-1-phosphate following ManLAM treatment
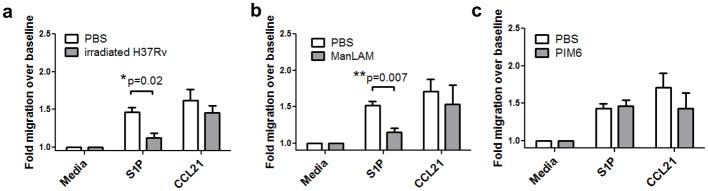
To initially determine whether ManLAM could affect T lymphocyte trafficking to or from lymph tissue, we examined the ability of human T lymphocytes to migrate to a lymph node specific chemokine, CCL21, or to the lymph node egress factor S1P following ManLAM treatment. T cells were isolated from peripheral blood mononuclear cells from healthy human donors, pretreated with whole irradiated H37Rv MTB, ManLAM, another MTB cell wall glycolipid phosphatidylinositol hexamannoside (PIM6) (16), or vehicle for 2 h, washed and placed in Boyden chemotaxis chambers. Boyden assays were performed with human cells due to reproducibility and migratory capacity of the cells, based on prior studies and experience in our laboratory (27). S1P-induced human T cell migration, but not CCL21-induced migration, was significantly inhibited following irradiated H37Rv or ManLAM treatment (Figure 1a & b, *P=0.02, **P=0.007, optimal doses shown, see also Supplementary Figure 1). PIM6 stimulation, used as a glycolipid control had no effect on human T cell chemotaxis following S1P stimulation (Figure 1c). A dose titration curve of ManLAM demonstrated that a concentration of 100ng/mL was the lowest effective concentration of ManLAM that inhibited S1P directed migration (Supplementary Figure 1).
Figure 1. ManLAM pretreatment desensitizes naïve human T cell migration to S1P.
T cells from 3 healthy donors were treated with (A) 1mg/mL whole irradiated H37Rv, (B) 100ng/mL ManLAM or (C) 100ng/mL PIM6 for 2 hours before use in Boyden chamber assays for media control, S1P (10nM), or CCL21 (50ng/ml). Migrating cells were fixed and stained, and number of T cells counted at 400x magnification from at least 10 fields were normalized to media control. irH37Rv inhibited S1P directed migration but not CCL21 directed migration (*P=0.02, Student’s t test). (B) Pretreatment with 100ng/mL ManLAM also blocks S1P directed migration (**P=0.007 Student’s t test). Data in (A & B) were obtained from 3 donors assayed in duplicate (mean ± s.e.m.). (C) 100ng/mL PIM6 had no effect on human T lymphocyte migration induced by S1P (n=2 donors assayed in duplicate, mean ± s.e.m.).
ManLAM pretreatment inhibits S1P-induced AKT phosphorylation in human T cells
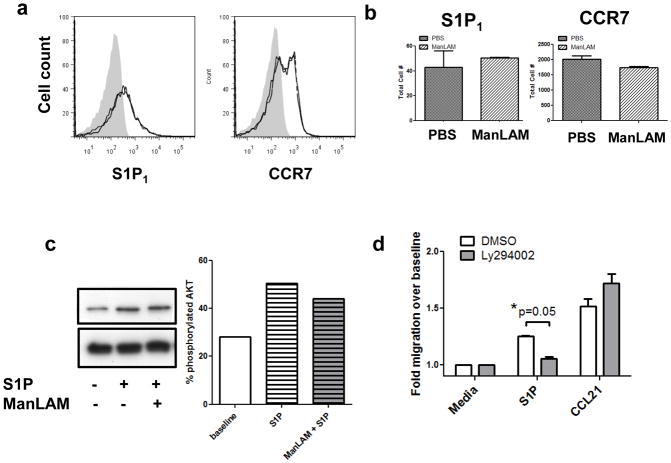
To determine the mechanism for inhibition of S1P-induced migration, surface expression levels of CCR7 and S1P1 were assessed via flow cytometry following ManLAM pretreatment. It was determined that there was no down-modulation of receptors from the cell surface (Figure 2a & b). Since ManLAM had no effect on chemokine receptor expression, we examined signaling pathway activation in the T cells to determine the mechanism of desensitization. Western blotting was performed on cell pellets that had been pretreated with vehicle or ManLAM, then stimulated with S1P for a total of 5 minutes (Figure 2c). Quantification demonstrated a 22% increase in AKT phosphorylation over baseline following S1P stimulation, which was partially blocked by ManLAM pretreatment (16% increase over baseline, representative blot from 2 separate experiments). Next, we wanted to determine if this phosphorylation event was biologically relevant for S1P-induced migration. A recent study demonstrated that S1P/S1P1 signaling in T cells affected Treg cell development and suppressive functions through a PI3K/AKT-dependent migration pathway (23). Another study demonstrated that AKT-mediated phosphorylation of S1P1 is required for endothelial cell migration towards S1P (24). Treatment with the PI3K/AKT inhibitor Ly294002 blocked naïve human T cell migration to S1P (Figure 2d, *P=0.05).
Figure 2. Naïve human T cell migration to S1P requires intact AKT signaling.
(A) Cells pretreated with ManLAM do not modulate surface CCR7 or S1P1 expression as determined by flow cytometry (grey filled histogram = isotype control, black = PBS treated, grey = ManLAM treated; representative image). (B) Quantification of surface receptor expression as in (A) (n=3 donors assayed in duplicate, mean± s.e.m.). (C) Western blotting analysis of human T cells pretreated with ManLAM or PBS for 2h, then stimulated with S1P or vehicle for 5 minutes. Percent phosphorylated AKT was determined by creating a ratio of densitometry of phospho-AKT Ser473 to total AKT. (Representative blot, n=2 separate experiments with 3 donors). (D) Pretreatment with 10μM Ly294002 for 1h inhibits human T cell migration to S1P. (*P=0.05 Student’s t test, n=2 donors assayed in duplicate, mean ± s.e.m.).
ManLAM treatment induces decreased mouse T cell migratory responses to sphingosine-1-phosphate
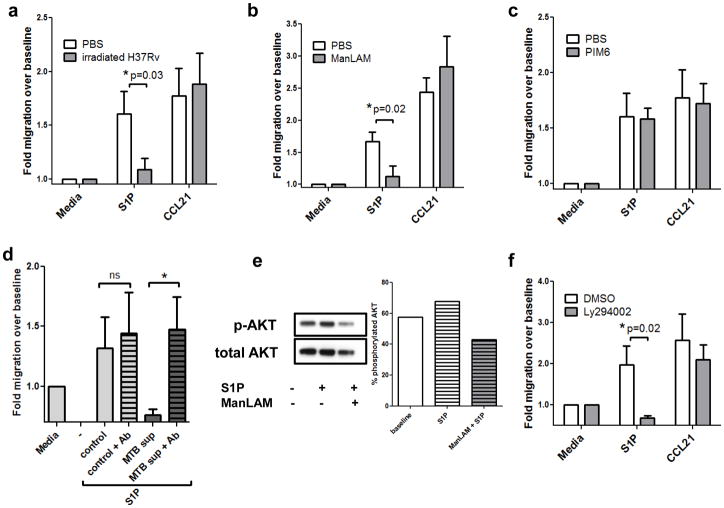
In order to determine whether the effects of ManLAM could be investigated in a murine model, we next examined the ability of ManLAM to affect in vitro migration of mouse T cells stimulated by either CCL21 or S1P. T lymphocytes were isolated from spleen and lymph nodes of 8–12 week old female C57BL/6J mice, and were pretreated with whole irradiated H37Rv MTB for 2h prior to use in Transwell chemotaxis assays (28). As with human cells, mouse naïve T cells that had been treated with irradiated H37Rv or ManLAM demonstrated no significant difference in their responsiveness to CCL21, but did exhibit a significant decrease in their response to S1P as compared to vehicle treated cells (*P=0.03 and 0.02, respectively; Figure 3a & b). The same concentration of PIM6, another MTB glycolipid, had no effect on S1P or CCL21-induced migratory responses (Figure 3c).
Figure 3. Migratory responses of mouse T lymphocytes after exposure to tuberculosis antigens.
Mouse T cells were pre-incubated with (A) 1mg/mL whole irradiated H37Rv, (B) 100ng/mL ManLAM or (C) 100ng/mL PIM6 for 2 hours. Cells were washed and placed in Transwell chambers and stimulated for migration induced by media control, S1P (10nM), or CCL21 (50ng/ml). The assay was terminated after 3.5 hours, and migration expressed as fold change over baseline (media control expressed as 1). Data in panels A–C are from three independent experiments performed in triplicate (mean ± s.e.m., *P=0.03 and *P=0.02, Student’s t test). (D) Culture supernatants from control or MTB-infected mouse bone marrow-derived macrophages were harvested 48h post-infection. Supernatants were precleared with CS35 anti-ManLAM antibodies at 4°C overnight. Primary mouse T cells were pretreated with the supernatants for 1.5h prior to Transwell assay. The data depicted shows migration over baseline to S1P at 10nM. (Duplicate wells from 2 separate experiments; mean ± s.e.m.,*P<0.05, Student’s t test). (E) Western blotting analysis of mouse T cells pretreated with ManLAM or PBS for 2h, then stimulated with S1P or vehicle for 5 minutes. Percent phosphorylated AKT was determined by creating a ratio of densitometry of phospho-AKT Ser473 to total AKT. (Representative blot, n=2 separate experiments). (F) Mouse T lymphocytes were pretreated with 50μM of the PI3K/AKT inhibitor Ly294002 for 1h prior to Transwell migration. S1P migration was significantly inhibited, whereas migration to CCL21 was not significantly affected (n=3 experiments performed in triplicate,*P=0.02, Student’s t test).
To further test the specificity of the effect of ManLAM on S1P-induced migration, we examined the ability of an anti-ManLAM antibody to potentially neutralize the ManLAM effect. For these studies, T cell migration to S1P was assessed following pretreatment with culture supernatants from MTB Erdman-infected mouse bone marrow-derived macrophages (BMDMs). BMDMs were cultured (control) or infected with an MOI of 5 MTB Erdman (MTB sup) for 48h prior to harvest of supernatants. Supernatants were precleared with CS-35 anti-ManLAM antibody overnight at 4°C, before pretreating mouse T cells for 1.5h at 37°C. T cells were then washed and subjected to a Transwell assay. CS-35 alone did not affect mouse T cell migration to S1P (Figure 3d). As expected, MTB supernatant blocked S1P-directed migration; however, supernatant that had been precleared to remove ManLAM had no effect (Figure 3d, *P=0.05). To determine if AKT was also involved in mouse T cell migratory responses to S1P, Western blots were performed as in Figure 2c. As with human T cells, ManLAM pretreatment inhibited AKT phosphorylation in mouse T cells (Figure 3e, representative blot from 2 separate experiments). Ly294002 treatment blocked mouse T cell S1P directed migration, indicating that PI3K/AKT activation is a necessary pathway for S1P chemotaxis in naïve mouse T cells (Figure 3d, *P=0.02).
In vivo instillation of ManLAM in C57BL/6J mice results in retention of T cells in lung draining lymph nodes
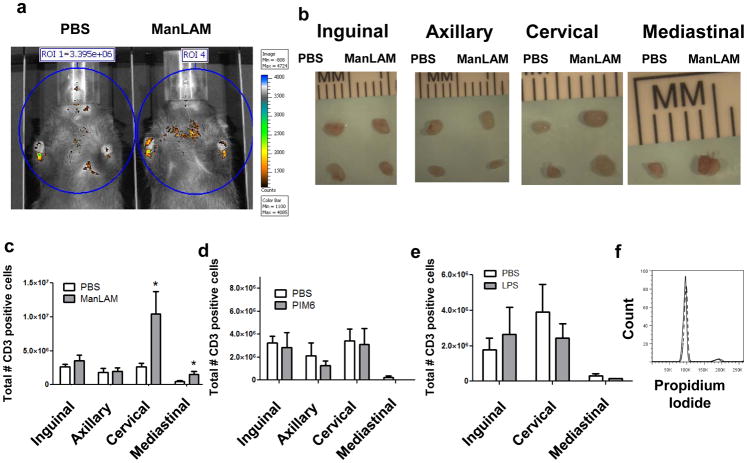
Based on the in vitro data, we hypothesized that ManLAM inhibition of S1P directed migration would result in trapping of T cells in lymph nodes. Therefore, we investigated the ability of ManLAM to alter in vivo T cell homing patterns in mice. T cells from lymph nodes and spleens of donor mice were labeled with Invitrogen 565 QDot nanocrystals. Five million labeled cells were injected via the tail vein into 8–12 week old recipient female C57BL/6J mice and were tracked using whole body imaging. After 1h, the mice were intratracheally (I.T.) administered ManLAM or vehicle. A dose of 25μg of ManLAM was chosen to correspond to doses used in mouse antigen challenge models, and to correspond with levels of ManLAM detected in sputum during live MTB infection (29, 30). Homing patterns and lymph node accumulation of labeled cells 24h post-instillation were determined with the IVIS Spectrum. Based on these heat map images, it was determined that ManLAM instilled I.T. induced accumulation of T cells in areas of the chest which likely corresponded with the mediastinal and cervical lymph nodes (Figure 4a). To more accurately localize the recruited cells, mice were sacrificed and the lymph nodes were excised. The cervical and mediastinal lymph nodes were visibly enlarged whereas the inguinal and axillary nodes had no detectable difference (Figure 4b).
Figure 4. In vivo instillation of MTB ManLAM results in retention of T cells in lung draining lymph nodes.
(A) In Vivo Imaging of 5×106 QDot 565 labeled T lymphocytes 24h after intratracheal administration of PBS (left) or 25μg ManLAM (right) in 8–12wk old female C57BL/6J mice (representative image, n=3 per group). (B) 40x image of inguinal, axillary, cervical and mediastinal lymph nodes isolated from PBS treated (left) or ManLAM treated (right) mice 24h post-instillation (representative images from 2 experiments). (C) Average number of CD3+ T cells per lymph node 24h post-instillation, n=6 mice per group from two separate experiments (mean ± s.e.m., *P=0.04 and *P=0.03 respectively, Student’s t test). (D) PIM6 or (E) LPS treatment does not induce sequestration-associated lymphadenopathy 24h post-instillation, n=4 or 5 mice per group from two separate experiments (mean ± s.e.m.). (F) Cell cycle analysis of the T cells from the cervical lymph nodes of PBS (solid) or ManLAM (dashed) treated mice as determined by propidium iodide staining. The lack of change from control mice indicates that the cells in the lung draining lymph nodes following ManLAM treatment do not have an increased proliferative index (representative plot from two independent experiments).
To more accurately quantify cell numbers and to assess the rate of accumulation, T cell kinetics studies were performed to determine onset and extent of the effect of a single bolus of ManLAM on T cell homing to secondary lymph tissue. Inguinal, axillary, cervical and mediastinal lymph nodes were harvested from mice 12, 24, and 48h following instillation of ManLAM or vehicle. Total T cell numbers were determined via CD3 staining, and maximal accumulation was observed at 24h post-instillation (Figure 4c, only the 24h timepoint is shown). There was a threefold increase in T lymphocytes in the cervical lymph nodes and a two-fold increase in T cells in mediastinal lymph nodes of mice that received ManLAM as compared to mice that received vehicle 24h following treatment (*P=0.04 and 0.03, respectively, Figure 4c). In contrast, there was no significant difference in the number of cells present in either the inguinal or axillary lymph nodes (Figure 4c), suggesting that I.T. administration of ManLAM altered homing patterns of cells only within lymph nodes that directly drain the lungs. Cell numbers from animals treated with PIM6 were not significantly different from vehicle treated animals (Figure 4d). In addition, mice were instilled with LPS. LPS is a likely contaminant, and it has been shown to have effects on recruitment and or activation of immune cells in the lungs (31, 32). As shown in figure 4e, instillation of LPS induced a slight decrease in the number of T cells in lung draining lymph nodes, though this was not statistically significant.
The increase in T cell numbers in the lymph nodes could be a result of increased proliferation. To ascertain whether or not the increase in T cells in lung draining lymph nodes following ManLAM instillation was as a result of T cell proliferation, cell samples from ManLAM and vehicle treated mice were stained with propidium iodide and were subjected to cell cycle analysis. As shown in figure 4f, there was no difference in proliferative profiles of cells isolated from vehicle or ManLAM treated animals, indicating that ManLAM was not inducing proliferation of these cell populations. This data agrees with other published studies demonstrating that ManLAM does not have a proliferative effect and in many cases inhibits T cell proliferation (33).
LAMs from other mycobacterial species
We also tested LAMs from other mycobacterial strains to determine if the effects we observed were unique to MTB. M. smegmatis LAM was assayed and found to inhibit T cell migration to both S1P and CCL21 (Supplementary Figure 2a). Similar to ManLAM, LAM from M. leprae inhibited T cell migration to S1P to the same extent as MTB ManLAM (Supplementary Figure 2a), as well as having no effect on CCL21 induced migration.
We also tested LAM from M. leprae and M. smegmatis in the in vivo I.T. model. LAM from M. smegmatis was unable to induce an effect on the number of T cells in the lymph nodes (Supplementary Figure 2c), whereas LAM from M. leprae did induce a significant increase in cervical lymph node T cells, but the effect was not as large as that seen with MTB ManLAM (*P=0.04, Supplementary Figure 2d, 2 fold increase in cervical lymph node T cells following M. leprae LAM treatment versus up to 4 fold increase in cervical lymph node T cells following M. tuberculosis LAM treatment). While M. smegmatis LAM was capable of inhibiting S1P-induced migration, its inhibitory effect on CCL21-induced migration may have reduced the number of T cells in vivo that were recruited to the lymph nodes following intratracheal administration. Therefore, the equal numbers of T cells may result from the balance of both a net reduction of newly recruited cells and egressing cells, thereby resulting in the little increase in detectable lymphadenopathy.
ManLAM preferentially inhibits CCR5+T cell migration to S1P in vitro and in vivo
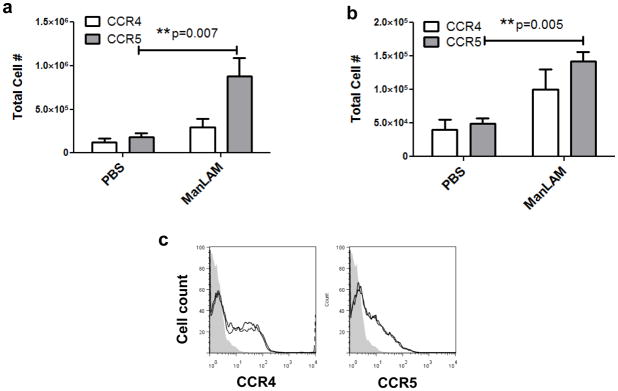
To better understand the inhibitory effect of ManLAM, we assessed the phenotype of lymph node sequestered cells following in vivo ManLAM treatment. Cells were dissociated from lymph tissue of mice that had been treated intratracheally with PBS or ManLAM for 24h, and were assessed for expression of CCR4 (predominantly expressed by Th2 cells) and CCR5 (predominantly expressed by Th1 cells) by flow cytometry. The ratios of CCR5+ to CCR4+ (Th1:Th2) cells isolated from cervical and mediastinal lymph nodes of mice that had received ManLAM were higher than that of mice that received vehicle, with statistically significant increases in total CCR5+ but not CCR4+ cell numbers accumulating in lymph nodes (*P=0.007 and 0.005, Figure 5a & b; see also Supplementary Figure 2d & e). This data indicates that ManLAM exposure in the lung results in preferential sequestration of CCR5+ cells in lung-draining lymph nodes.
Figure 5. ManLAM preferentially sequesters CCR5+ (Th1) cells in lymph nodes.
(A) CCR5:CCR4 ratios of T lymphocytes isolated from cervical lymph nodes of mice that received intratracheal ManLAM or PBS. Average Th1:Th2 ratio was 1.6:1 for PBS treatment and 2.62:1 for ManLAM treatment, n=7 mice per group (mean ± s.e.m., **P=0.007 Student’s t test). (B) CCR5:CCR4 ratios of T cells from mediastinal lymph nodes. Average Th1:Th2 ratio was 1.21:1 for PBS treatment and 1.42:1 for ManLAM treatment, n=3 mice per group (mean ± s.e.m., **P=0.005 Student’s t test). (C) ManLAM treatment does not alter chemokine receptor expression. Mouse T cells were incubated in vitro with ManLAM for 24h and analyzed via flow cytometry. There was no difference between PBS (black) and ManLAM (grey) histograms (representative from 2 separate experiments; filled grey histogram = isotype).
To determine whether changes in the percentage of CCR5+ cells could be attributed exclusively to altered migration patterns, or whether ManLAM stimulation could induce alteration in surface expression of CCR4 or CCR5, T cells were incubated in vitro with ManLAM for 2h, 5.5h and 24h prior to surface receptor analysis (these timepoints correspond with pretreatment, migration assays, and in vivo ManLAM administration, respectively). For all concentrations and time points investigated there was no significant change in expression of CCR4 or CCR5 (Figure 5c representative data shown). These findings indicate that the significant reduction in CCR5+ cell numbers responding to S1P was due to direct effects on migration and not on the alteration of CCR5 expression.
We also examined total cell numbers (Supplementary Figure 2b & c) and ratios of CCR5+ to CCR4+ (Th1 to Th2) cells (Supplementary Figure 2d & e) from mice that had been administered LAM from M. smegmatis or M. leprae and found no significant increases. This indicates that the preferential sequestration of CCR5+ (Th1) cells in lymph nodes is a selective effect for MTB ManLAM.
Th1 and Th2 cells require different signaling pathways to migrate towards S1P
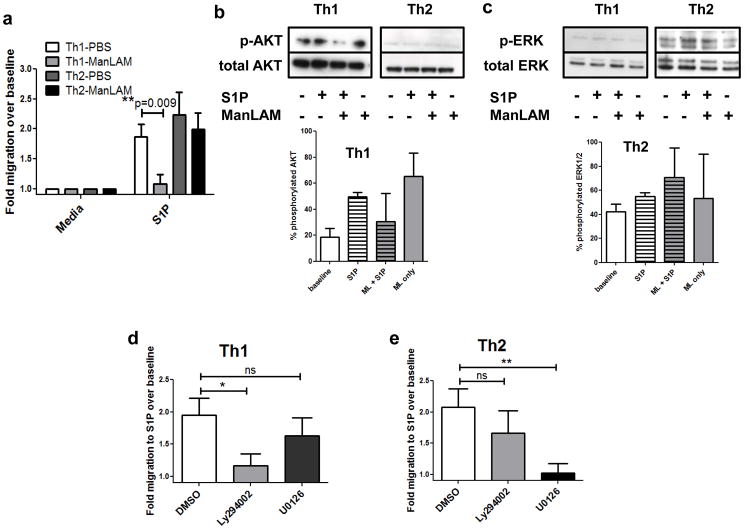
The in vivo migration studies indicated that ManLAM was having a greater effect on CCR5+ than CCR4+ cell S1P migration. To more directly investigate this we analyzed S1P directed migration in Th1 versus Th2 cell populations. Naïve cells were skewed in vitro under Th1 or Th2 polarizing conditions, and subset purity was confirmed using Th1/Th2 cytokine analysis by ELISA. Skewed cell populations were pretreated with ManLAM or vehicle, washed and placed in Transwell chambers. ManLAM pretreatment was able to inhibit Th1 cell migration to S1P but not Th2 cell migration (Figure 6a, **P=0.009). These data are consistent with reports showing that ManLAM stimulation can enhance Th2 cytokine production but diminish Th1 cytokine production (34).
Figure 6. ManLAM inhibits Th1, but not Th2, cell migration induced by S1P because of differential signaling requirements.
(A) Spleen and lymph node T cells were isolated from 7–12wk old C57BL/6J female mice and skewed in vitro before treatment and Transwell assay. There was a statistically significant decrease in Th1 cell migration to 10nM S1P following 100ng/mL ManLAM treatment for 2h, and no significant difference in Th2 cell migration (n=3 experiments performed in triplicate, mean ± s.e.m., **P=0.009 Student’s t test). (B & C) Western blotting analysis of Th1 and Th2 cells following ManLAM pretreatment and S1P stimulation for (B) phosphorylated AKT or (C) phosphorylated ERK. Insets depict % phosphorylated protein averaged from blots from 2 separate experiments. (D) Th1 and (E) Th2 cell migration to 10nM S1P following a 1h treatment with DMSO vehicle, 10μM PI3K inhibitor Ly294002, or 10μM MEK1/2 inhibitor U0126. S1P-directed Th1 cell migration was inhibited by Ly294002 and S1P-directed Th2 cell migration was inhibited by U0126 pretreatment (n=3 separate experiments performed in triplicate, mean ± s.e.m., *P=0.02 and **P=0.005 Student’s t test).
ManLAM has been shown to preferentially desensitize the AKT pathway (18, 19), thereby providing a potential mechanism for the selective inhibitory effect of ManLAM on Th1 cell migration. To examine differences in AKT pathway activation, Western blotting was performed on cell pellets of Th1 and Th2 cells that had been pretreated with PBS or ManLAM, followed by stimulation with S1P (Figure 6b & c, representative blots shown with average % phosphorylated protein from 2 separate experiments). Strikingly, Th1 cells exhibited basal phospho-AKT whereas Th2 cells exhibited basal phospho-ERK. ManLAM pretreatment alone induced an increase in phospho-AKT over baseline, but prevented cells from achieving the same extent of AKT phosphorylation as control cells stimulated with S1P (Figure 6b). Conversely, ManLAM pretreatment resulted in enhanced ERK phosphorylation as compared with S1P stimulation alone in Th2 cells, indicating that the cells were still responsive to the stimulus following ManLAM pretreatment (Figure 6c).
We next investigated the functionality of the AKT and ERK pathways, which have both been shown to be important for T cell chemotaxis, in Th1 and Th2 cell migration induced by S1P. As shown in Figure 6d & e, the AKT inhibitor Ly294002 significantly inhibited Th1 migration to S1P while the ERK inhibitor U0126 significantly inhibited Th2 migration to S1P, with neither inhibitor significantly affecting the migratory response of the other T cell subset (*P=0.02 and **P=0.005). Taken together, these data indicate that S1P-induced migration of Th1 cells requires activation of the AKT pathway, while S1P-induced migration of Th2 cells is primarily dependent on the ERK pathway. Further, MTB ManLAM preferentially affects the AKT pathway, thereby inhibiting S1P-induced migration of Th1 cells.
DISCUSSION
In this study we demonstrate that a MTB virulence factor, ManLAM, is capable of inhibiting T cell migration to the lymph tissue egress signal S1P. This was accomplished by interference with PI3K/AKT pathway activation. Th1 populations were preferentially affected due to their dependence on AKT activation for S1P induced migration. It is possible that the effect of ManLAM on T cells could delay and potentially reduce initiation of Th1 effector responses in lung tissue, where even transient delays in initiation of adaptive immunity could contribute to long-term impairment for control of infection (8, 35, 36). Further, these findings demonstrate a potential mechanism by which MTB could foster a favorable infection environment, and suggest that therapeutics that target ManLAM, such as benzothiazinones, may significantly alter Th1 cell skewing and recruitment to the lung thereby reducing the bacterial burden (37).
We have identified that naïve and Th1 cell S1P-induced migration requires the AKT pathway, and that ManLAM pretreatment is able to inhibit S1P-directed migration of these populations. The selective effect of ManLAM on Th1 cells seems to rely on their propensity to use the AKT pathway as opposed to the ERK pathway: we found that Th1 cells have basal levels of phospho-AKT that are absent in Th2 populations, and conversely Th2 cells have basal phospho-ERK that is absent in Th1 populations. This data corroborates previous studies that have shown distinct signaling events in human Th1 and Th2 cells (38), and that AKT and ERK contribute to Th1 and Th2 differentiation, respectively (39, 40). Additional studies are required to examine in more detail the mechanisms by which ManLAM desensitizes the AKT pathway.
A number of chemoattractants have been reported to induce desensitization of cells to subsequent migratory stimuli. Examples of this would be cross-desensitization of CCR5 by IL-16 and MIP-1β (41), CD4-iduced desensitization of CXCR3 and CCR5 following IL-16 stimulation (42), and desensitization of CXCR2 and C5aR by fMLP stimulation (43, 44). ManLAM has previously been shown to induce T cell migration (45) and we now demonstrate that pretreatment with ManLAM renders T cells refractory to S1P stimulation. Most of the chemokine receptor cross-desensitization studies involved receptor mediated desensitization, and ManLAM has been shown to interact with some cell surface receptors including C-type lectins and the mannose receptor (13–15). Future studies could determine whether or not ManLAM interacts with a receptor on the surface of T cells that could mediate this effect. Another potential mechanism is the direct incorporation of ManLAM into the membranes of PBMCs: Ilangumaran et. al. showed that ManLAM preferentially incorporates into lipid rafts where AKT activation following S1P1 stimulation normally occurs (16). Our data suggest that ManLAM can selectively inhibit the AKT pathway without abrogating ERK signaling. ERK signaling has been shown to be caveolae independent and therefore potentially less affected by membrane integration by ManLAM (46). This difference may explain why ManLAM preferentially inhibits the PI3K pathway; however, additional studies are required to determine the mechanism by which ManLAM binds to T cells and initiates the inhibitory effect on S1P receptor signaling.
Our studies did not address effects of ManLAM on other S1P receptors expressed by other immune cells. Of note, there are 5 different S1P receptors that are differentially expressed on immune cells (47). Our studies focused on T lymphocytes (48), which express S1P1 andS1P4 as do B lymphocytes; however, mast cells, macrophages and dendritic cells express S1P2 and ManLAM stimulation either through membrane integration or receptor interaction could affect cellular responses. Further examination of this phenotype could reveal preferential use of different S1P receptors on different leukocytes (49). It has also been shown that MTB inhibits sphingosine kinase activity, the enzyme responsible for production of S1P (50, 51). It is possible that reduced production of S1P in vivo, a consequence of MTB infection, could contribute to the phenotype we observe.
Previous studies have shown that 100ng/mL of ManLAM represents the lower end of a dose response curve used to inhibit CD4+ T cell activation and cytokine secretion (20, 34). In addition, Human MTB patient sputum has been shown to contain 1 ng/mL to 1 μg/mL of ManLAM (30), though the precise concentration in lymph nodes is unknown. It has been reported that ManLAM incorporates into lipid rafts on the surface of PBMCs as rapidly as 30 min, with maximal incorporation occurring within several hours (16). Concentrations and timepoints used in this study correspond well with these studies, supporting the concept that ManLAM can alter the immune response in the nanogram range and within a short period of time. During the course of a live infection, it is likely that ManLAM is constantly secreted by infected macrophages, thus potentiating the sequestration of cells for an extended period of time. The spread of infection to the lymph nodes and subsequent ManLAM secretion within the confines of the lymph tissue could further contribute to inhibition of Th1 cell egress with reduced immune responses in the lung. Interestingly, MTB infected CCR5−/− mice demonstrate increased lymphocytic infiltrates in their lungs and increased proinflammatory cytokine production (52), which would be consistent with ManLAM’s ability to efficiently and selectively inhibit CCR5+/Th1 cell subsets but not CCR4+/Th2 subsets.
There is evidence that indicates a shift in Th1/Th2 cytokines during MTB infection. Infected mice initially produce both IFNγ and IL-4 in the lungs, but IFNγ levels decrease while IL-4 levels increase over the course of infection (53). This finding is consistent with our data indicating that ManLAM production would alter normal Th1 cell homing patterns. ManLAM has also been shown to promote secretion of Th2 related cytokines while simultaneously decreasing release of Th1 cytokines from T lymphocytes (34). As IFNγ production by Th1 cells is considered to be host-protective, our data would support the hypothesis that shedding of ManLAM during MTB infection promotes an environment that is more favorable for infection, and suggests further that antibiotics targeting ManLAM, such as benzothiazinones, may significantly alter Th1 cell skewing in the lung thereby reducing the bacterial burden (37). It is likely that live MTB produce other factors that are immunoregulatory. Our data indicate that shedding of ManLAM represents a major component for induction of lymphadenopathy with selectivity for CCR5+ T cells; however, additional studies are required to more fully understand how bacterial products can modify the immune response and promote the infection process.
Supplementary Material
Acknowledgments
We would like to thank Nathalie Ayahi for assistance with Th1/Th2 skewing methods, Matthew Jones for assistance with Western blotting methods, Matthew Blahna for assistance with propidium iodide staining methods, Marina Tuzova for assistance with cell culture, and Elizabeth Duffy for assistance with ELISAs for assessing Th1/Th2 skewing.
All TB materials were obtained through collaboration with Colorado State University under the NIH, NIAID Contract No. HHSN266200400091C entitled “Tuberculosis Vaccine Testing and Research Materials.”
All flow cytometric data were acquired using equipment maintained by the Boston University Medical Campus and University of Massachusetts Worcester Flow Cytometry Core Facilities.
Whole body animal imaging was acquired using equipment maintained by the Boston University Medical Campus Animal Imaging Core Facilities.
Footnotes
This work was supported by National Institutes of Health Grants RO1 HL081149 (to H.K.) and RO1 CA122737 (to W.W.C.). J.R. was supported by a Fellowship from National Science Foundation Grant 0538608.
Abbreviations used in this paper: S1P, sphingosine-1-phosphate; S1P1, sphingosine-1-phosphate receptor 1; MTB, Mycobacterium tuberculosis; ManLAM, mannose-capped lipoarabinomannan; AraLAM, arabinose-capped lipoarabinomannan; PIM6, hexamannosylated phosphatidyl inositol
COMPETING FINANCIAL INTERESTS
The authors have no financial conflict of interest.
References
- 1.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 3.Pham THM, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 Receptor Signaling Overrides Retention Mediated by Gai-Coupled Receptors to Promote T Cell Egress. Immunity. 2007;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, Vanrenterghem Y. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2004;77:1826–1833. [PubMed] [Google Scholar]
- 7.Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 8.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello AM, Kumar A, Narayan V, Akbar MS, Ahmed S, Abou-Zeid C, Rook GA, Stanford J, Moreno C. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg. 1992;86:686–692. doi: 10.1016/0035-9203(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 12.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 13.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 15.Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages. J Biol Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- 16.Ilangumaran S, Arni S, Poincelet M, Theler JM, Brennan PJ, Nasirud D, Hoessli DC. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- 17.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strohmeier GR, Fenton MJ. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1999;1:709–717. doi: 10.1016/s1286-4579(99)80072-0. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 20.Mahon RN, Rojas RE, Fulton SA, Franko JL, Harding CV, Boom WH. Mycobacterium tuberculosis cell wall glycolipids directly inhibit CD4+ T-cell activation by interfering with proximal T-cell-receptor signaling. Infect Immun. 2009;77:4574–4583. doi: 10.1128/IAI.00222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 26.McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, Cruikshank W. Preferential migration of T regulatory cells induced by IL-16. J Immunol. 2007;179:6439–6445. doi: 10.4049/jimmunol.179.10.6439. [DOI] [PubMed] [Google Scholar]
- 27.Green DS, Center DM, Cruikshank WW. Human immunodeficiency virus type 1 gp120 reprogramming of CD4+ T-cell migration provides a mechanism for lymphadenopathy. J Virol. 2009;83:5765–5772. doi: 10.1128/JVI.00130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juffermans NP, Verbon A, Belisle JT, Hill PJ, Speelman P, van Deventer SJ, van der Poll T. Mycobacterial lipoarabinomannan induces an inflammatory response in the mouse lung. A role for interleukin-1. Am J Respir Crit Care Med. 2000;162:486–489. doi: 10.1164/ajrccm.162.2.9911009. [DOI] [PubMed] [Google Scholar]
- 30.Pereira Arias-Bouda LM, Nguyen LN, Ho LM, Kuijper S, Jansen HM, Kolk AH. Development of antigen detection assay for diagnosis of tuberculosis using sputum samples. J Clin Microbiol. 2000;38:2278–2283. doi: 10.1128/jcm.38.6.2278-2283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrara G, Bleck B, Richeldi L, Reibman J, Fabbri LM, Rom WN, Condos R. Mycobacterium tuberculosis induces CCL18 expression in human macrophages. Scand J Immunol. 2008;68:668–674. doi: 10.1111/j.1365-3083.2008.02182.x. [DOI] [PubMed] [Google Scholar]
- 32.Rajashree P, Supriya P, Das SD. Differential migration of human monocyte-derived dendritic cells after infection with prevalent clinical strains of Mycobacterium tuberculosis. Immunobiology. 2008;213:567–575. doi: 10.1016/j.imbio.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Moreno C, Mehlert A, Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988;74:206–210. [PMC free article] [PubMed] [Google Scholar]
- 34.Shabaana AK, Kulangara K, Semac I, Parel Y, Ilangumaran S, Dharmalingam K, Chizzolini C, Hoessli DC. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signalling. Cell Mol Life Sci. 2005;62:179–187. doi: 10.1007/s00018-004-4404-5. [DOI] [PubMed] [Google Scholar]
- 35.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makarov V, Manina G, Mikusova K, Mollmann U, Ryabova O, Saint-Joanis B, Dhar N, Pasca MR, Buroni S, Lucarelli AP, Milano A, De Rossi E, Belanova M, Bobovska A, Dianiskova P, Kordulakova J, Sala C, Fullam E, Schneider P, McKinney JD, Brodin P, Christophe T, Waddell S, Butcher P, Albrethsen J, Rosenkrands I, Brosch R, Nandi V, Bharath S, Gaonkar S, Shandil RK, Balasubramanian V, Balganesh T, Tyagi S, Grosset J, Riccardi G, Cole ST. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science. 2009;324:801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannier S, Bitegye C, Demotz S. Early events of TCR signaling are distinct in human Th1 and Th2 cells. J Immunol. 2002;169:1904–1911. doi: 10.4049/jimmunol.169.4.1904. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280:29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 40.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 41.Mashikian MV, Ryan TC, Seman A, Brazer W, Center DM, Cruikshank WW. Reciprocal desensitization of CCR5 and CD4 is mediated by IL-16 and macrophage-inflammatory protein-1 beta, respectively. J Immunol. 1999;163:3123–3130. [PubMed] [Google Scholar]
- 42.Rahangdale S, Morgan R, Heijens C, Ryan TC, Yamasaki H, Bentley E, Sullivan E, Center DM, Cruikshank WW. Chemokine receptor CXCR3 desensitization by IL-16/CD4 signaling is dependent on CCR5 and intact membrane cholesterol. J Immunol. 2006;176:2337–2345. doi: 10.4049/jimmunol.176.4.2337. [DOI] [PubMed] [Google Scholar]
- 43.Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–23836. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- 44.Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 45.Berman JS, Blumenthal RL, Kornfeld H, Cook JA, Cruikshank WW, Vermeulen MW, Chatterjee D, Belisle JT, Fenton MJ. Chemotactic activity of mycobacterial lipoarabinomannans for human blood T lymphocytes in vitro. J Immunol. 1996;156:3828–3835. [PubMed] [Google Scholar]
- 46.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954–11963. doi: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 48.Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 49.Tahaa TA, Argravesb Kelly M, Obeid Lina M. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochimica et Biophysica Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Malik ZA, Thompson CR, Hashimi S, Porter B, Iyer SS, Kusner DJ. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J Immunol. 2003;170:2811–2815. doi: 10.4049/jimmunol.170.6.2811. [DOI] [PubMed] [Google Scholar]
- 51.Thompson CR, Iyer SS, Melrose N, VanOosten R, Johnson K, Pitson SM, Obeid LM, Kusner DJ. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: inhibition of SK1 translocation by Mycobacterium tuberculosis. J Immunol. 2005;174:3551–3561. doi: 10.4049/jimmunol.174.6.3551. [DOI] [PubMed] [Google Scholar]
- 52.Algood HM, Flynn JL. CCR5-deficient mice control Mycobacterium tuberculosis infection despite increased pulmonary lymphocytic infiltration. J Immunol. 2004;173:3287–3296. doi: 10.4049/jimmunol.173.5.3287. [DOI] [PubMed] [Google Scholar]
- 53.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.