Abstract
Objective
Evidence from pre-clinical studies suggests inhibition of Stearoyl Co-A Desaturase-1 (SCD-1) activity improves insulin sensitivity. Translation of these findings to humans remains less defined. The purpose of this research was to evaluate the association between different measures of SCD-1 activity and incident diabetes in a large, prospective human study.
Methods
In 2738 white participants (aged 45-64 yrs, 47% men) who were free of diabetes at baseline, SCD-1 activity was estimated at baseline by plasma fatty acid ratios in cholesterol esters (SCD16c=16:1n-7/16:0, SCD18c =18:1n-9/18:0) and in phospholipids (SCD16p=16:1n-7/16:0, SCD18p=18:1n-9/18:0). Incident diabetes was ascertained during 3 follow-up visits. Cox proportional hazards regression was used to determine the association between estimated SCD-1 activity and incident diabetes.
Results
During follow-up (mean 8.0 ± SE 2.1 years), 207 (7.6%) participants developed diabetes. After adjusting for age and sex, higher SCD16c, higher SCD16p, and lower SCD18p were significantly associated with incident diabetes. After additional adjustment for education, parental history of diabetes, smoking, dietary intake (carbohydrate, fiber, saturated/monounsaturated/polyunsaturated fat), alcohol use, physical activity, body mass index (BMI), waist-hip ratio, blood pressure, and lipid composition – only SCD16c remained significantly associated with incident diabetes (Hazard Ratio=1.1 linearly across decreasing quintiles, 95% CI 1.01-1.30; p =0.03) which remained nominally associated after adjusting for insulin resistance (p=0.05).
Conclusions
In a large community-based prospective cohort study, the estimate of SCD-1 activity by SCD16c had the strongest association with incident diabetes. Refinement of SCD-1 measurement and replication of its association with incident diabetes in an independent cohort is recommended.
Keywords: fatty acid ratio, Type 2 Diabetes, prospective study
Introduction
Given the epidemic of type 2 diabetes, there is a great clinical need for biomarkers that predict incident diabetes and possibly provide mechanistic insight. Plasma fatty acids have been of particular interest, with free fatty acid levels and fatty acid composition demonstrating an association with incident diabetes. The composition of plasma fatty acids depends on many factors, including fatty acid intake age, sex, exercise, endogenous synthesis and genetic predisposition and of particular relevance to this study - Stearoyl Co-A desaturase (SCD-1).
SCD-1, a delta 9 desaturase, is the rate limiting enzyme in the synthesis of monounsaturated fatty acids and a critical player in lipid synthesis and oxidation. Its preferred substrates are palmitoyl CoA and stearoyl CoA. SCD-1 is found in many lipogenic tissues, specifically the liver, adipose tissue and muscle. In animals, a deficiency in SCD-1 improves insulin sensitivity, likely via increased lipid oxidation, improved skeletal muscle insulin signaling, and enhanced hepatic AMP-Kinase activity. Thus, inhibition of SCD-1 activity may be a potential mechanism for treatment of obesity and diabetes. In recent animal studies (mice, rat), the in vivo inhibition of SCD-1 expression through injection of an antisense oligonucleotide prevented diet-induced obesity and improved hepatic insulin resistance.
In humans, the role of SCD-1 in humans is less well defined. The expression of hepatic SCD-1 mRNA correlates with the product-precursor ratio in hepatic tissue ; in turn, this ratio correlates with the product-precursor ratio in serum fatty acids. Large scale human studies commonly estimate SCD-1 activity by using the product-precursor ratio of palmitoleic acid (16:1n-7)/palmitic acid (16:0) or oleic acid (18:1n-9)/stearic acid (18:0) in either plasma cholesterol esters, plasma phospholipids or erythrocyte phospholipids.
This variability in the estimation of SCD-1 activity in human studies defines the rationale for the current study. The composition of plasma phospholipids is more variable than the composition of plasma cholesterol esters. In terms of specific fatty acids, oleic acid (18:1n-9) is more variable than palmitoleic acid (16:1n-7), palmitic acid (16:0) or stearic acid (18:0). In large scale human studies, the most precise estimate of SCD-1 activity should theoretically be derived from fatty acid ratios in plasma cholesterol esters, particularly palmitoleic acid (16:1n-7)/palmitic acid (16:0). Yet a direct comparison between common estimates of SCD-1 activity and their association with incident diabetes in a prospective human study has not been made.
We used a prospective population-based cohort, the Atherosclerosis Risk in Communities Study (ARIC) study, to determine whether common estimates of SCD-1 activity – as represented by the plasma product-precursor ratio of palmitoleic acid (16:1n-7)/palmitic acid (16:0) or oleic acid (18:1n-9)/stearic acid (18:0) in plasma phospholipids and plasma cholesterol esters – are associated with incident diabetes. In ARIC, fatty acid composition was measured in baseline plasma samples. Three follow-up examinations were conducted over 9 years to ascertain incident diabetes. We hypothesized that higher estimated SCD-1 activity, particularly derived from the palmitoleic acid (16:1n-7)/palmitic acid (16:0) ratio in plasma cholesterol esters, would be associated with higher rates of incident diabetes.
Methods
Participants
The Atherosclerosis Risk in Communities Study (ARIC) is a population based cohort study of 15792 participants (ages 45-64 years) enrolled during 1987-1989 from 4 communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland), with follow-up exams approximately 3 years apart (1990-1992, 1993-1995, 1996-1998). Eligibility for our study was restricted to participants from the Minneapolis field center, the only center that measured plasma fatty acid composition from phospholipid and cholesterol esters. The ARIC study was approved by the Institutional Review Board of each participating center. The current study was specifically approved by the University of Minnesota Institutional Review Board.
Within the Minneapolis cohort (n=4009), 37 participants were nonwhite and excluded from the analysis (due to small numbers). Of the remaining 3972 participants, an additional 1190 participants were excluded because they had one or more of the following at baseline: prevalent or unknown diabetes status (n=432), missing fatty acid measurements (n=37), use of medications (lipid lowering agents and bile acid sequestrants) that may affect the lipid profile (n=906 total participants, n=721 participants not previously excluded) and fasting less than 8 hours before the blood draw (n=85 total participants, n=44 not previously excluded). After applying the exclusion criteria, 2738 participants were available for analysis.
Measurement of Plasma Fatty Acids
Plasma collection and fatty acid composition measurements have been previously described. Briefly, fasting blood was collected in EDTA, centrifuged and frozen at −70 degrees Celsius until analysis approximately 2 years later. The lipid extract was obtained by exposing plasma to methanol and chloroform under a nitrogen atmosphere. The cholesterol ester and phospholipid fractions were separated using a silica gel plate (Silica Gel H: Analtech, Newark DE, USA) and two stage mobile phase development with 80:20:1 (by volume) and 40:60:1 (by volume) solvents of petroleum ether, diethyl ether, and glacial acetic acid. Dichlorofluorescein was used to visualize the phospholipid, cholesterol ester, triacylglycerol, and free fatty acid bands under ultraviolet light. The phospholipid and cholesterol esters were scraped into separate test tubes and converted into methyl esters of fatty acids by boron trifluoride catalysis. The methyl esters were separated and measured using gas chromatography (model 5890 Hewlett Packard, Avondale, PA, USA) equipped with a glass capillary column (J&W Scientific, Folsom CA, USA) and a flame ionization detector. Each fatty acid peak was identified by comparison to synthetic standards of known fatty acid composition. The relative amount was quantified by integrating the area under the specific peak and dividing the results by the total area for all fatty acids. Short-term repeatability (reliability coefficient: 0.5 to 0.93 for plasma cholesterol esters, 0.31 to 0.89 for plasma phospholipids) and long-term repeatability (reliability coefficient: 0.35 to 0.83 for plasma cholesterol esters, 0.30 to 0.81 for plasma phospholipids) of this quantitation technique were previously reported.
Estimation of SCD-1 activity
SCD-1 converts palmitic acid (16:0) to palmitoleic acid (16:1n-7) and stearic acid (18:0) to oleic acid (18:1n-9). Following the precedent set by previous epidemiological studies, we used both plasma phospholipid and cholesterol esters composition to estimate SCD-1 activity. Specifically, we examined 4 different concentration ratios: (i) palmitoleic acid (16:1n-7)/palmitic acid (16:0) in plasma cholesterol esters (SCD16c), (ii) palmitoleic acid (16:1n-7)/palmitic acid (16:0) in plasma phospholipids (SCD16p), (iii) oleic acid (18:1n-9)/stearic acid (18:0) in plasma cholesterol esters (SCD18c), and (iv) oleic acid (18:1n-9)/stearic acid (18:0) in plasma phospholipids (SCD18p). Because the plasma samples were obtained in a fasting state, we assumed that the estimated SCD-1 activity would primarily represent hepatic SCD-1 activity.
Ascertainment of incident diabetes
Incident diabetes was defined by documentation of one or more of the following at any of the 3 follow-up visits for the ARIC study: self-reported physician diagnosis, fasting (≥ 8 hours) serum glucose ≥ to 7.0 mmol/l, non-fasting serum glucose ≥ to 11.1 mmol/l, or use of anti-diabetes medication within the past two weeks. The date of diabetes incidence was estimated by linear interpolation using glucose values at the ascertaining visit and the previous one, as previously described.
Measurement of Covariates
The following covariates, all measured at baseline, were selected as possible confounders in our analyses because of their known association with the plasma lipid profile : age, sex, and education; parental history of diabetes; smoking history (cigarette-years); dietary intake of fiber (soluble and insoluble), carbohydrate, and fat (saturated, monounsaturated, and polyunsaturated); alcohol use; physical activity; body mass index (BMI); waist-hip ratio; blood pressure; and fasting blood levels of glucose, insulin, high density lipoprotein (HDL), low density lipoprotein (LDL), and triglycerides.
Food intake was assessed with the ARIC 66-item, interviewer-administered, food frequency questionnaire – a modified version of Willet’s food frequency questionnaire that also included additional fish categories. Participants were asked how often specific food and beverages were consumed, as well as what type of fat and oil were used in food preparation and at the table. Physical activity was ascertained by the Baecke questionnaire and summarized quantitatively as total activity (work, leisure, and sport; range 3-15) and play activity (leisure and sport; range 2-10). Insulin and glucose were measured as previously reported. Insulin resistance was represented by the homeostatic model assessment (HOMA-IR) and by fasting glucose.
Adjustment for cigarette smoking included both smoking history and cigarette-years, but as this did not change the results in a sensitivity analysis, only cigarette-years was used in the final model. Insulin resistance was adjusted by using fasting glucose or HOMA-IR as a covariate, but as this did not change the results in a sensitivity analysis, only HOMA-IR was used in the final model.
Statistical analysis
All analyses were conducted by using SAS (version 9.2 - SAS institute, Inc, Cary, North Carolina). Baseline characteristics were summarized for participants with and without incident diabetes and compared by using two-sample t-tests or chi-squared tests as appropriate.
Pearson’s and Spearman’s correlation coefficients were computed between the different estimates of SCD-1 activity.
Separately for each measure of SCD-1 activity (after confirming the Cox proportional hazards assumption), a proportional hazards model was used to evaluate the association of SCD-1 activity with incident diabetes. The SCD-1 ratios were somewhat skewed and the four SCD-1 estimates had different measurement scales; because we did not want to constrain the model to a linear association of SCD-1 with log hazard, we instead computed quintiles for each SCD-1 measure to use as our exposure variable in the modeling. Baseline characteristics were summarized for participants in the highest vs. lowest quintiles and compared by using two-sample t-tests or chi-squared tests as appropriate.
We used three models to examine how the associations between estimated SCD-1 activity and incident diabetes changed after adjustment for participant characteristics. Model 1 was adjusted for age and sex. Model 2 additionally adjusted for education (<high school, high school, > high school), parental history of diabetes (absent or present), cigarette smoking (cigarette years), dietary intake (fiber, grams/day; carbohydrate, % of total kcal; saturated fat, % of total kcal; monounsaturated fat, % of total kcal, polyunsaturated fat, % of total kcal), alcohol use (grams/day), physical activity (total of work, leisure and sport activity), BMI (kg/m2), waist-hip ratio, mean arterial pressure (mmHg), HDL (mmol/L), LDL (mmol/L), and triglycerides (mmol/L). Although insulin resistance may be on the casual pathway from SCD-1 activity to diabetes, Model 3 included all of the above characteristics plus insulin resistance, as measured by HOMA-IR. Our goal was to examine whether the association between SCD-1 and incident diabetes might be independent of insulin resistance and to observe the degree of attenuation of the association upon adjustment for a potential mediating factor. We also tested a linear trend (p-trend) across the SCD-1 quintiles in log hazard of diabetes by considering the SCD-1 quintile as a continuous rather than categorical variable. Statistical significance was set at p<0.05.
Results
Baseline characteristics for participants with and without incident diabetes
Of the 2,738 participants (47% men, n=1290), 207 (7.6%) developed diabetes over a mean follow-up period of 8 years (± SE 2.1 years). As shown in Table 1, compared to those without diabetes, participants with incident diabetes were more likely to be men, have less education, have at least one parent with diabetes, and have a positive history of smoking. Participants with incident diabetes also had significantly higher baseline values for all of the following: dietary fiber intake, BMI, waist-hip ratio, mean arterial pressure, fasting glucose, HOMA-IR, triglycerides, plasma saturated fatty acids, % palmitic acid (16:0) in cholesterol esters, % palmitoleic acid (16:1n-7) in cholesterol esters, % stearic acid (18:0) in cholesterol esters, % palmitoleic acid (16:1n-7) in plasma phospholipids, % stearic acid (18:0) in plasma phospholipids, and SCD16c. Lastly, participants with incident diabetes had significantly lower baseline values for sport+leisure activity, HDL, plasma polyunsaturated fatty acids, % oleic acid (18:1n-9) in plasma phospholipids, SCD18c, and SCD18p.
Table 1.
Baseline characteristics of participants with and without incident diabetes in the Minneapolis cohort of the ARIC study
| No diabetes (n=2531) |
Incident Diabetes (n=207) |
||
|---|---|---|---|
|
| |||
| Mean (SE) | Mean (SE) | P value |
|
| Age (years) | 53.3 (0.1) | 53.7 (0.4) | 0.3 |
| Sex (male) | 45.6% | 65.2% | <0.01 |
| Education (<high school) | 5.0% | 9.2% | 0.04 |
| Positive parental history of diabetes (%) | 18.8% | 30.0% | <0.01 |
| Positive hx of smoking (%) | 60.9% | 72.9% | <0.01 |
| Cigarette years smoked | 303.0 (7.6) | 418.3 (29) | <0.01 |
| Diet-fiber intake (grams) | 16.1 (0.1) | 17.2 (0.6) | 0.04 |
| Diet-saturated fat intake (%kcal) | 12.6 (0.1) | 12.6 (0.2) | 0.9 |
| Diet -monounsaturated fat intake (%kcal) | 13.1 (0.06) | 12.3 (0.2) | 0.5 |
| Diet polyunsaturated fat intake (%kcal) | 5.1 (0.03) | 5.2 (0.1) | 0.7 |
| Diet-carbohydrate intake (%kcal) | 46.4 (0.2) | 45.7 | (0.6) 0.2 |
| Alcohol intake (g/day) | 8.2 (0.3) | 9.3 (1.1) | 0.3 |
| Total activity [3 (low) to 15 (high)]a | 7.4 (0.03) | 7.3 (0.1) | 0.3 |
| Sport+leisure activity [2 (low) to 10 (high)]a | 5.2 (0.02) | 4.9 (0.08) | <0.01 |
| BMI (kg/m2) | 26.1(0.1) | 30.4 (0.3) | <0.01 |
| Waist- hip ratio | 0.9 (0.002) | 1.0 (0.005) | <0.01 |
| Mean arterial pressure (mmHg) | 87.1 (0.2) | 92.7 (0.7) | <0.01 |
| Fasting glucose (mmol/l) | 5.4 (0.01) | 6.0 (0.03) | <0.01 |
| HOMA-IR | 2.1 (0.03) | 4.0 (0.2) | <0.01 |
| LDL (mmol/L) | 3.5 (0.02) | 3.6 (0.07) | 0.3 |
| HDL (mmol/L) | 1.4 (0.01) | 1.1 (0.03) | <0.01 |
| Triglycerides (mmol/L) | 1.3 (0.01) | 1.7 (0.1) | <0.01 |
| Quantity in plasma (% of total) | |||
| Saturated fatty acids | 33.0 (0.03) | 33.7(0.1) | <0.01 |
| Monounsaturated fatty acids | 13.9 (0.04) | 14.1(0.1) | 0.27 |
| Polyunsaturated fatty acids | 53.1 (0.05) | 52.3 (0.2) | <0.01 |
| Quantity in plasma cholesterol esters (% of total) |
|||
| Palmitic acid (16:0) | 9.9 (0.02) | 10.3 (0.06) | <0.01 |
| Palmitoleic acid (16:1n-7) | 2.5 (0.02) | 2.8 (0.08) | <0.01 |
| Stearic acid (18:0) | 0.89 (0.004) | 0.94 (0.01) | <0.01 |
| Oleic acid (18:1n-9) | 15.9 (0.04) | 16.2 (0.13) | 0.09 |
| Quantity in plasma phospholipids (% of total) |
|||
| Palmitic acid (16:0) | 25.3 (0.03) | 25.7 (0.01) | 0.09 |
| Palmitoleic acid (16:1n-7) | 0.63 (0.003) | 0.66 (0.01) | 0.02 |
| Stearic acid (18:0) | 13.2 (0.02) | 13.6 (0.09) | <0.01 |
| Oleic acid (18:1n-9) | 8.6 (0.02) | 8.5 (0.08) | 0.04 |
| Estimates of SCD-1 activity | |||
| SCD16c | 0.2 (0.002) | 0.3 (0.007) | <0.01 |
| SCD16p | 0.02 (0.0001) | 0.03 (0.0004) | 0.1 |
| SCD18c | 18.6 (0.1) | 17.8 (0.2) | 0.01 |
| SCD18p | 0.7(0.002) | 0.6 (0.008) | <0.01 |
Data presented as mean ± SE
P values calculated using two-sample t-test for continuous variables and using the chi-squared test for categorical variables
Self reported activity was quantified using a standardized survey
Correlation between estimatedSCD-1 activity ratios
The four estimated SCD-1 activity ratios correlated positively and significantly (p<0.001) with each other. Correlations with SCD16c were 0.78 (SCD16p), 0.66 (SCD18c) and 0.55 (SCD18p). Correlations with SCD16p were 0.48 (SCD18c) and 0.58 (SCD18p). Correlation of SCD18c with SCD18p was 0.64.
Associations between estimated SCD-1 activity ratios and baseline risk factors for type 2 diabetes
SCD16c was positively but weakly correlated with HOMA-IR (r=0.1, p<0.01), fasting glucose (r=0.06, p<0.01), BMI (r=0.12, p<0.01) and waist-hip ratio (r=0.08, p<0.01). SCD16p positively but weakly correlated only with HOMA-IR (r=0.06, p <0.01) and BMI (r=0.06, p<0.01). In contrast, both SCD18c and SCD18p correlated negatively with HOMA-IR (r= −0.24and −0.17, respectively, p<0.01 for both), fasting glucose (r= −0.06 and −0.12, p<0.01 for both), BMI (r= −0.16 and −0.22, p<0.01 for both) and waist-hip ratio (r= −0.14 and −0.11, p<0.01 for both). Non-parametric Spearman correlations showed similar results (not shown).
Table 2 compares the mean values of potentially confounding variables between participants whose SCD16c activity ratios were in the highest quintile (range: 0.3-1.2) versus lowest quintile (range: 0.08-0.17) (Note: Corresponding data for SCD16p, SCD18c and SCD18p activity ratios are described in the supplemental materials). Participants with SCD16c values in the highest (vs. lowest) quintile were significantly (p<0.05) more likely to be older, female, and less educated; to have a positive history of smoking; to have higher baseline values for alcohol intake, BMI, waist-hip ratio, mean arterial pressure, fasting glucose, HOMA-IR, HDL, and triglycerides; and to have lower baseline values for fiber intake, monounsaturated fat intake, polyunsaturated fat intake, carbohydrate intake, and physical activity.
Table 2.
Baseline characteristics of participants by extreme quintilesof SCD16c in the Minneapolis cohort of the ARIC population
| Lowest quintile (n=547) | Highest quintile (n=547) |
||
|---|---|---|---|
|
|
|||
| Mean (SE) | Mean (SE) | P value | |
| SCD16c (range) | 0.08-0.17 | 0.3-1.2 | |
| Age (years) | 52.9 (0.2) | 53.8 (0.2) | 0.01 |
| Sex (male) | 60.5% | 35.8% | <0.01 |
| Education (<high school) | 5.0% | 6.0% | <0.01 |
| Positive parental history of diabetes (%) | 19.6% | 23.4% | 0.35 |
| Positive history of smoking | 54.1% | 72.9% | <0.01 |
| Cigarette years smoked | 246.4 (15.2) | 434.2 (18.2) | <0.01 |
| Diet-fiber intake (grams) | 17.4 (0.3) | 15.2 (0.3) | <0.01 |
| Diet-saturated fat (%kcal) | 12.3 (0.1) | 12.3 (0.1) | 0.94 |
| Diet- monounsaturated fat (% kcal) | 13.5 (0.1) | 12.4 (0.1) | <0.01 |
| Diet-polyunsaturated fat (% kcal) | 5.6 (0.1) | 4.6 (0.1) | <0.01 |
| Diet-carbohydrate (%kcal) | 47.5 (0.3) | 44.5 (0.4) | <0.01 |
| Alcohol intake (g/day) | 3.8 (0.3) | 16.8 (0.9) | <0.01 |
| Total activity [3 (low) to 15 (high)] a | 7.7 (0.05) | 7.1 (0.06) | <0.01 |
| Sport+leisure activity [2 (low) to 10 (high)]a | 5.4 (0.05) | 4.94(0.05) | <0.01 |
| BMI (kg/m2) | 25.4 (0.1) | 27.3 (0.2) | <0.01 |
| Waist - hip ratio | 0.90 (0.004) | 0.92 (0.003) | <0.01 |
| Mean arterial pressure | 85.6 (0.42) | 90.3 (0.46) | <0.01 |
| Fasting glucose (mmol/L) | 5.4 (0.02) | 5.6 (0.02) | <0.01 |
| HOMA-IR | 2.0 (0.05) | 2.6 (0.08) | <0.01 |
| HDL (mmol/L) | 1.3 (0.02) | 1.5 (0.02) | <0.01 |
| LDL (mmol/L) | 3.4 (0.04) | 3.5 (0.04) | 0.47 |
| Triglycerides (mmol/L) | 1.1 (0.02) | 1.6 (0.04) | <0.01 |
Data presented as mean ± SE
P values calculated using two-sample t-test for continuous variables and using the chi-squared test for categorical variables
Self reported activity was quantified using a standardized survey
Association between estimated SCD-1 activity ratios and incident diabetes
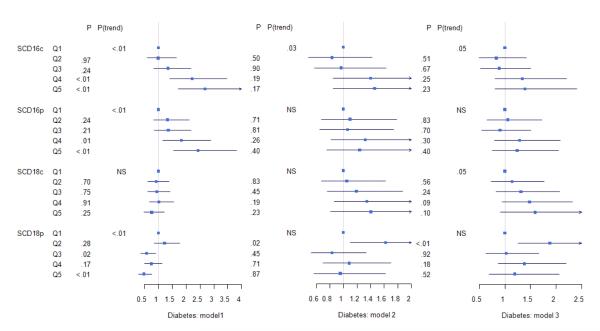
In Model 1 (age and sex adjusted), higher SCD16c and SCD16p were significantly associated with incident diabetes (p<0.01), including a significant linear trend across the quintiles (p trend<0.01). In contrast, lower SCD18p was significantly associated with incident diabetes (Figure 1) (p<0.01), including a significant negative linear trend (p trend <0.01).
Figure 1.
Hazard ratios for association of SCD-1 quintiles with incident diabetes. Model 1: Adjusted for age and sex. Model 2 is additionally adjusted for risk factors associated with diabetes including: parental history of diabetes (absent or present), cigarette smoking (cigarette years), mean arterial pressure (mmHg), physical activity (total of work, leisure and sport activity), dietary fiber intake (grams/day), carbohydrate intake (% of total kcal), saturated fat intake (% of total kcal), monounsaturated fat intake (% of total kcal), polyunsaturated fat intake (% of total kcal), HDL (mmol/l), LDL (mmol), triglycerides (mmol/L) alcohol use (grams/day), education (<high school, high school, > high school), Waist-hip ratio, and BMI (kg/m2). Model 3 is additionally adjusted for HOMA-IR. Quintile 1(lowest level of SCD-1 ratio) is the common reference group. P trend measured the linear relationship across quintile levels with incident diabetes. Statistical significance was set at p<=0.05 for all analyses.
The linear association across SCD16c quintiles with incident diabetes (Hazard Ratio=1.1 linearly across decreasing quintiles, 95% CI 1.01-1.30; p=0.03) persisted after adjusting for additional risk factors in Model 2 and remained nominally associated (p trend =0.05) after adjusting for insulin resistance in Model 3. The associations between SCD16p and SCD18p with incident diabetes (Model 1) were completely attenuated after adjustment for other diabetes risk factors (Model 2, Model 3). For SCD18c, a nominal association (p trend =0.05) was observed between SCD18c quintiles and incident diabetes only in Model 3.
Discussion
The main finding of this study is that among the four different estimates of SCD-1 activity that we examined, the ratio of palmitoleic acid (16:1n-7)/palmitic acid (16:0) in plasma cholesterol esters (SCD16c) had the strongest association with incident diabetes. This association persisted after adjusting for many known risk factors. These findings suggest that SCD-1 activity may be a novel risk factor for incident diabetes and, given the available technology, is best estimated by SCD16c in large cohort studies.
The observed association between estimated SCD-1 activity and incident diabetes in this study supports and extends the findings from previous research. Our observed values for SCD16c, SCD16p, and SCD18p are comparable to previous literature. Previously, SCD-1 activity, as estimated by SCD16c, has been associated with reduced insulin sensitivity and the metabolic syndrome. In a case-control study, SCD-1 activity as estimated by SCD16p or SCD18p was associated with diabetes, but this study involved an older population (mean age 64 years) and used a narrower definition of diabetes (ascertained by self-report, use of diabetes medication, or linkage to medical records). Other studies have shown an association between SCD16p and diabetes, whereas results for the association of SCD18p with diabetes were mixed.
Compared to prior work, our study is strengthened by many features, most notably its use of a prospective cohort of men and women to directly address the association between multiple measures of estimate SCD-1 activity and incident diabetes. The resources of the ARIC study were uniquely suited to examine this association. First, previous analysis confirmed the correlation between dietary fat composition and plasma fatty acid composition and the short-term and long term repeatability of the plasma fatty acid measurements. Second, the use of an older cohort (age group 45-64 at baseline) with 8 years of follow up ensured a reasonable event rate of incident diabetes. Third, the diagnosis of diabetes was confirmed through objective and laboratory criteria rather than by self-report or record linkage.
The findings from this study suggest that estimated SCD-1 activity may be a risk factor for incident diabetes in humans. The mechanism by which SCD-1 might contribute to the pathogenesis of incident diabetes and the extent to which this may translate to humans remain under active investigation. SCD-1 deficient mice have reduced body fat and increased insulin sensitivity, and are protected from the metabolic effects of a high fat diet; these effects are likely due to increased lipid oxidation, reduced lipid synthesis, and enhanced insulin signaling in the muscle, white adipose tissue, liver, and heart. Whether these changes are due to SCD-1 itself or its product, particularly palmitoleic acid (16:1n-7), remains unknown. A mouse model of increased plasma palmitoleic acid (16:1n-7) had increased skeletal muscle insulin sensitivity and was protected from the development of hepatic steatosis. In humans, however, high plasma levels of palmitoleic acid (16:1n-7) have been associated with diabetes and insulin resistance. The metabolic consequences of SCD-1 overexpression may be tissue-specific. Skeletal muscle SCD-1 protein levels and mRNA levels are higher in trained athletes compared with BMI-matched sedentary controls. Interventional studies using thiazolidinediones in humans have shown improved skeletal muscle insulin sensitivity correlating with increased skeletal muscle SCD-1 activity, as measured by mRNA expression and alterations in muscle lipid saturation. These differences highlight the need for further exploration of the role of SCD-1 in humans.
From a clinical standpoint, there is intense interest in SCD-1 inhibition as a potential treatment for diabetes and therefore a great need to easily estimate SCD-1 activity in large prospective studies. In previous large scale human studies, SCD-1 activity was estimated by using SCD16c, SCD16p, SCD18c and SCD18p, but no direct comparison with regards to the outcome of incident diabetes were performed. In our study, estimation of SCD-1 activity in humans by SCD16c was most consistent with SCD-1 as a risk factor for diabetes; moreover, this finding is consistent with results from animal studies (mice, rats) showing that reduction of in vivo SCD-1 expression reduces diet-induced obesity and improves hepatic insulin resistance. Even so, we acknowledge that the estimate of SCD-1 by SCD16c remains limited and further refinement in the measurement of SCD-1 activity in humans will be needed.
Several limitations of our study should be considered. The timing of the onset of diabetes was uncertain and ascertained by interpolation between visits. Estimating the SCD-1 activity from baseline measurements at the same time as the lipoprotein profile, HOMA-IR, and fasting glucose measurements prevented us from making any inferences about cause-effect relationships. Dietary intake, alcohol intake, smoking, and physical activity may affect plasma fatty levels to varying degrees depending on the specific lipid, although we did adjust for these covariates in our analyses. Analytical variation may affect reproducibility, obscuring changes in SCD16p due to low levels of 16:1n-7 in plasma phospholipids compared with levels in plasma cholesterol esters. Although the estimated SCD-1 activity from serum fatty acid ratios has been shown to approximate liver SCD-1 activity, the plasma product-precursor ratio may not completely represent SCD-1 activity, as lecithin-cholesterol acyltransferase activity may affect plasma cholesterol ester composition. A better estimate of hepatic SCD-1 expression may involve measuring fatty acid ratios of very low density lipoproteins or hepatic tissue, which was not available for this study. Adjustment for baseline insulin resistance by using HOMA-IR (Model 3) could lead to overmodeling, as insulin resistance is considered to be on the causal pathway for diabetes. Yet the finding of a significant association between SCD16c and incident diabetes (Model 2) – an association that remained after adjusting for HOMA-IR (Model 3) – suggests that the relationship between SCD16 and incident diabetes may involve a mechanism independent of insulin resistance.
Conclusion
In a large, population-based cohort study of middle-aged individuals, we found that of several common estimates of SCD-1 activity, estimation of SCD-1 activity by SCD16c had the strongest association with incident diabetes. This association was attenuated but persistent when adjusting for known risk factors for type 2 diabetes. These results support a possible role for SCD-1 in the development of diabetes in humans. Our findings should be validated in an independent cohort and further studies to elucidate the underlying mechanism are warranted.
Supplementary Material
Acknowledgements
The authors thank Ching Ping Hong, Division of Epidemiology and Community Health, University of Minnesota, for providing the ARIC data for analysis. The authors acknowledge the assistance of Dr. Anne Marie Weber-Main, Department of Medicine, University of Minnesota, for her critical review and editing of manuscript drafts. The authors thank the staff and participants of the ARIC study for their important contributions.
Funding The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- 16:0
palmitic acid
- 16:1n-7
palmitoleic acid
- 18:0
stearic acid
- 18:1n-9
oleic acid
- SCD-1
Stearoyl Co-A desaturase-1
- SCD16c
16:1n-7/16:0 ratio in plasma cholesterol esters
- SCD16p
16:1n-7/16:0 ratio in plasma phospholipids
- SCD18c
18:1n-9/18:0 ratio in plasma cholesterol esters
- SCD18p
18:1n-9/18:0 ratio in plasma phospholipids
Footnotes
Disclosure Statement There are no conflicts of interest or duality of interest reported by any of the authors associated with this manuscript.
Contribution statement LSC designed the research. JHE, RCH, DJC, JSP provided essential data for analysis. LSC, SL, LEE analyzed the data. LSC, SL and LEE wrote the manuscript. LSC, SL, LEE, ERS, JHE, RCH, DJC, LMS and JSP contributed to the discussion and reviewed/edited the manuscript. LSC has primary responsibility for the final content. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest reported
References
- 1.Pankow JS, Duncan BB, Schmidt MI, et al. Fasting plasma free fatty acids and risk of type 2 diabetes - The Atherosclerosis Risk in Communities study. Diabetes Care. 2004;27(1):77–82. doi: 10.2337/diacare.27.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Folsom AR, Zheng ZJ, et al. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. American Journal of Clinical Nutrition. 2003;78(1):91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Folsom AR, Shahar E, et al. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. American Journal of Clinical Nutrition. 1995;62(3):564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 4.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in Lipid Research. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Progress in Lipid Research. 2004;43(2):91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 6.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Current Opinion in Lipidology. 2008;19(3):248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. Journal of Clinical Investigation. 2007;117(6):1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntambi JM, Miyazaki M, Stoehr JP, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman SM, Dobrzyn A, Dobrzyn P, et al. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):11110–11115. doi: 10.1073/pnas.1934571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrzyn P, Dobrzyn A, Miyazaki M, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das SK, Chakrabarti R. Antiobesity therapy: Emerging drugs and targets. Current Medicinal Chemistry. 2006;13(12):1429–1460. doi: 10.2174/092986706776872880. [DOI] [PubMed] [Google Scholar]
- 12.Jiang GQ, Li ZH, Liu F, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. Journal of Clinical Investigation. 2005;115(4):1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez-Juarez R, Pocai A, Mulas C, et al. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. Journal of Clinical Investigation. 2006;116(6):1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter A, Cegan A, Wagner S, et al. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clinical Chemistry. 2009;55(12):2113–2120. doi: 10.1373/clinchem.2009.127274. [DOI] [PubMed] [Google Scholar]
- 15.Kotronen A, Seppanen-Laakso T, Westerbacka J, et al. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity. 2010;18(5):937–944. doi: 10.1038/oby.2009.326. [DOI] [PubMed] [Google Scholar]
- 16.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48(10):1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 17.Riserus U, Arlov J, Berglund L. Long-term predictors of insulin resistance: Role of lifestyle and metabolic factors in middle-aged men. Diabetes Care. 2007;30(11):2928–2933. doi: 10.2337/dc07-0360. [DOI] [PubMed] [Google Scholar]
- 18.Zhou YE, Egeland GM, Meltzer SJ, et al. The association of desaturase 9 and plasma fatty acid composition with insulin resistance-associated factors in female adolescents. Metabolism-Clinical and Experimental. 2009;58(2):158–166. doi: 10.1016/j.metabol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Patel PS, Sharp SJ, Jansen E, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. American Journal of Clinical Nutrition. 2010;92(5):1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 20.Kroger J, Zietemann V, Enzenbach C, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. American Journal of Clinical Nutrition. 2011;93(1):127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Folsom AR, Eckfeldt JH, et al. Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters. American Journal of Clinical Nutrition. 1995;62(3):572–578. doi: 10.1093/ajcn/62.3.572. [DOI] [PubMed] [Google Scholar]
- 22.Williams OD. The Atherosclerosis Risk in Communities (ARIC) Study - Design and Objectives. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 23.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes - The Atherosclerosis Risk in Communities Study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 24.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. Journal of Lipid Research. 1964;5(4):600–608. [PubMed] [Google Scholar]
- 25.Flowers MT. The delta9 fatty acid desaturation index as a predictor of metabolic disease. Clinical Chemistry. 2009;55(12):2071–2073. doi: 10.1373/clinchem.2009.135152. [DOI] [PubMed] [Google Scholar]
- 26.Investigators The ARIC The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and Validity of a Semiquantitative Food Frequency Questionnaire. American Journal of Epidemiology. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Szklo M, Stevens J, et al. A Prospective Study of Coronary Heart Disease in Relation to Fasting Insulin, Glucose, and Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1997;20(6):935–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Saggiani F, Targher G, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity - Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Van Woudenbergh GJ, Kuijsten A, Van der Kallen CJ, et al. Comparison of fatty acid proportions in serum cholesteryl esters among people with different glucose tolerance status: The CoDAM study. Nutrition Metabolism and Cardiovascular Diseases. 2012;22(2):133–140. doi: 10.1016/j.numecd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Hodge AM, English DR, O’Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. American Journal of Clinical Nutrition. 2007;86(1):189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 33.Flowers JB, Rabaglia ME, Schueler KL, et al. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56(5):1228–1239. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 34.Cao HM, Gerhold K, Mayers JR, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Cao HM, King IB, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. American Journal of Clinical Nutrition. 2010;92(6):1350–1358. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amati F, Dube JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60(10):2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergman BC, Perreault L, Hunerdosse DM, et al. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. Journal of Applied Physiology. 2010;108(5):1134–1141. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao-Borengasser A, Rassouli N, Varma V, et al. Stearoyl-Coenzyme A Desaturase 1 Gene Expression Increases after Pioglitazone Treatment and Is Associated with Peroxisomal Proliferator-Activated Receptor-gamma Responsiveness. Journal of Clinical Endocrinology & Metabolism. 2008;93(11):4431–4439. doi: 10.1210/jc.2008-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mai K, Andres J, Bobbert T, et al. Rosiglitazone increases fatty acid Δ9-desaturation and decreases elongase activity index in human skeletal muscle in vivo. Metabolism. 2012;61(1):108–116. doi: 10.1016/j.metabol.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Oballa RM, Belair L, Black WC, et al. Development of a Liver-Targeted Stearoyl-CoA Desaturase (SCD) Inhibitor (MK-8245) to Establish a Therapeutic Window for the Treatment of Diabetes and Dyslipidemia. Journal of Medicinal Chemistry. 2011;54(14):5082–5096. doi: 10.1021/jm200319u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.